- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pyruvate:ferredoxin Oxidoreductase (PFR1) Activity Assays Using Methyl Viologen as Artificial Electron Acceptor
Published: Vol 3, Iss 17, Sep 5, 2013 DOI: 10.21769/BioProtoc.882 Views: 12896
Reviewed by: Ru Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assay of the Carboxylase Activity of Rubisco from Chlamydomonas reinhardtii
Hemanth P. K. Sudhani [...] Joaquín Moreno
Dec 5, 2015 10408 Views

Determination of Recombinant Mannitol-1-phosphatase Activity from Ectocarpus sp.
Agnès Groisillier and Thierry Tonon
Aug 20, 2016 9069 Views

Determination of Recombinant Mannitol-1-phosphate Dehydrogenase Activity from Ectocarpus sp.
Agnès Groisillier and Thierry Tonon
Nov 5, 2016 8905 Views
Abstract
Here we describe the activity measurements of heterologous expressed pyruvate:ferredoxin oxidoreductase (Noth et al., 2013) (Noth et al.,2013) from Chlamydomonas reinhardtii. This enzyme catalyzes the reversible reaction (I) from pyruvate to acetyl CoA and CO2 generating low potential electrons which are in vivo transferred to ferredoxin.![]()
In this assay we use methyl viologen as artificial electron acceptor which turns into dark violet (ε604 = 13.6 mM-1 cm-1) (Mayhew, 1978) in its reduced state (Figure 1).
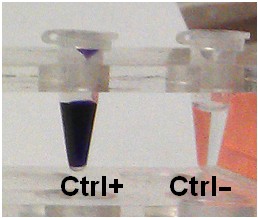
Figure 1. Activity assay using pyruvate, coenzyme A and methyl viologen (Ctrl+). In the absence of pyruvate, no methyl viologen reduction occurs (Ctr-).
Materials and Reagents
Note: All Reagents are dissolved freshly in an anaerobic tent.
- Purified pyruvate: ferredoxin oxidoreductase (PFR1)
- Sodium pyruvate (≥ 99%, Stock 500 mM) (Sigma-Aldrich)
- Sodium coenzyme A (≥ 85%, Stock 20 mM) (Sigma-Aldrich)
- Thiamine pyrophosphate (≥ 95%, Stock 250 mM) (Sigma-Aldrich)
- Methyl viologen (98%, Stock 1 M) (Sigma-Aldrich)
- Dithioerythriol (≥ 99%, Stock 100 mM) (Carl Roth)
- 0.1 M Tris-HCl buffer (pH 8.0) (see Recipes)
Equipment
- Anaerobic tent (1% H2, 99% N2) (Toepffer Lab Systems)
- 96 well plate reader (Beckman Coulter, catalog number: Paradigm1113 )
- PC running Multimode analysis software (Beckman Coulter)
- NanoDrop (Paqlab)
Procedure
- Protein concentration of heterologous expressed and purified PFR1 from 2 L of cell culture is measured at A280nm using NanoDrop. (ref bio-protocol: Heterologous Production and Anaerobic Purification of His- and StrepII-tagged Recombinant Proteins).
- All reduction assays are performed under anaerobic atmosphere (1% H2, 99% N2) at room temperature.
- The reaction mixture contains 10 mM sodium pyruvate, 2 mM sodium coenzyme A, 5 mM thiamine pyrophosphate, 10 mM methyl viologen and 16 mM dithioerythritol in 0.1 mM Tris-HCl (pH 8).
- To start catalysis a final concentration of 1.4 μM PFR1 is added to the reaction mixture and absorbance (A604, 96 well plate reader) can be monitored time resolved every 30 seconds until saturation is reached.
- To determine enzyme activity the molar extinction coefficient ε604 = 13.6 mM-1 cm-1 (Mayhew, 1978) can be used applying Lambert Beer Law (Eq.I). One unit was defined as the conversion of 1 mol of pyruvate or CoA and the reduction of 2 mol of methyl viologen, respectively, per minute.
Eλ = ελ . c . d (Eq.I)
Eλ: extinction
ελ: extinction coefficient
c: concentration
d: layer thickness
Recipes
- 0.1 M Tris-HCl buffer (pH 8.0) (1,000 ml)
Mix 12.114 g of Tris base with 800 ml dH2O
Adjust pH to 8 with HCl
Add ddH2O to 1,000 ml
Autoclave for 20 minutes at 121 °C
Store at 4 °C
Acknowledgments
Kinetics for enzyme dependent methyl viologen reduction is adapted from Zeikus et al. (1977). Research on the pyruvate:ferredoxin oxidoreductase from C. reinhardtii was scientifically supported by Anja Hemschemeier and Thomas Happe.
References
- Mayhew, S. G. (1978). The redox potential of dithionite and SO-2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur J Biochem 85(2): 535-547.
- Noth, J., Krawietz, D., Hemschemeier, A. and Happe, T. (2013). Pyruvate:ferredoxin oxidoreductase is coupled to light-independent hydrogen production in Chlamydomonas reinhardtii. J Biol Chem 288(6): 4368-4377.
- Noth, J. (2013). Heterologous production and anaerobic purification of His- and StrepII-tagged recombinant proteins. Bio-protocol 3(17): e881.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Noth, J. (2013). Pyruvate:ferredoxin Oxidoreductase (PFR1) Activity Assays Using Methyl Viologen as Artificial Electron Acceptor. Bio-protocol 3(17): e882. DOI: 10.21769/BioProtoc.882.
- Noth, J., Krawietz, D., Hemschemeier, A. and Happe, T. (2013). Pyruvate:ferredoxin oxidoreductase is coupled to light-independent hydrogen production in Chlamydomonas reinhardtii. J Biol Chem 288(6): 4368-4377.
Category
Plant Science > Phycology > Protein > Activity
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










