- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Heterologous Production and Anaerobic Purification of His- and StrepII-tagged Recombinant Proteins
Published: Vol 3, Iss 17, Sep 5, 2013 DOI: 10.21769/BioProtoc.881 Views: 16201
Reviewed by: Ru Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assay for Phytaspase-mediated Peptide Precursor Cleavage Using Synthetic Oligopeptide Substrates
Sven Reichardt [...] Andreas Schaller
Feb 5, 2023 1795 Views

A Simple Sonication Method to Isolate the Chloroplast Lumen in Arabidopsis thaliana
Jingfang Hao and Alizée Malnoë
Aug 5, 2023 2285 Views
Abstract
This protocol describes the heterologous expression and purification of proteins related to anoxic hydrogen production of Chlamydomonas reinhardtii (Noth et al., 2013). For this, the bacterial expression hosts Escherichia coli BL21 (DE3) ΔiscR (Akhtar MK et al., 2008) and Clostridium acetobutylicum ATCC 824 are used, which are grown either aerobic or anaerobic with glucose. Two standard chromatographic methods for purification were applied using His- and StrepII-tagged proteins (Figure 1). All procedures have been performed in an anaerobic tent to avoid the access of oxygen.
Keywords: Fermentation 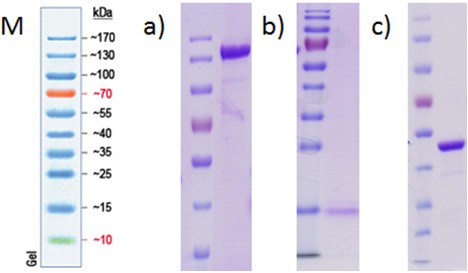
Figure 1. Coomassie stained SDS-PAGE of purified, heterologously expressed proteins from C. reinhardtii. M: MW marker PageRuler Prestained Protein Ladder 10-170 kDa; a) purified PFR1 loaded onto a 10% SDS-polyacrylamidgel; b) purified [2Fe2S] ferredoxin (PetF) loaded onto a 15% SDS-polyacrylamidgel; c) purifiedhydrogenase (HydA1) loaded onto a 10% SDS-polyacrylamidgel. Different amounts of protein are loaded onto each gel.
Materials and Reagents
- Expression vector (pASK-IBA)
- LB medium (Lennox) (Carl Roth)
- Vogel Bonner minimal medium (homemade) (Vogel HJ et al., 1956)
- Thiamin hydrochlorid (Carl Roth)
- Resazurin (Riedel-de Haën)
- Immidazole (Alfa Aesar)
- Escherichia coli BL21 (DE3) ΔiscR
- Clostridium acetobutylicum ATCC 824
- Ni Sepharose 6 Fast Flow (GE Healthcare)
- Strep-Tactin Superflow (IBA Gmb)
- Ampicillin
- Anhydrotetracycline
- Glucose
- Sodium dithionite (laboratory reagent grade > 85%)
- Avidin (Affiland)
- Strep tactin
- Glycerol
- d-desthiobiotin (≥ 98%, TLC)
- Thiamine pyrophosphate
- PageRuler Prestained Protein Ladder 10-170 kDa (Thermo Fisher Scientific, catalog number: 26616 )
- 0.1 M Tris buffer (pH 8) (see Recipes)
- Pre-equilibrated gravity flow Ni-NTA (see Recipes)
Equipment
- Airtight vial
- Sonicator: Branson Sonifier 250 (Branson)
- Ultracentrifuge
- Anaerobic tent (1% H2, 99% N2) (Toepffer Lab Systems)
- 0.2 μm pore size sterile filter (Sarstedt AG & Co.)
- NanoDrop (Paqlab, Germany)
- Batch fermenter (Infors HT, CH)
Procedure
- Anaerobic expression of pyruvate: ferredoxin oxidoreductase (Noth et al., 2013)
- Electroporation (Sambrook et al., 2006) of 100 μl E. coli BL21 (DE3) ΔiscR (Akhtar et al., 2008) with ~100 ng expression vector (pASK-IBA).
- Inoculation of 200 ml LB and aerobic growth of a preculture over night at 37 °C (180 rpm).
- Inoculation of 4 L Vogel Bonner medium (8 x 500 ml; 2,000 ml Erlenmeyer flasks) supplemented with 100 μg/ml ampicillin, 50 μM thiamin hydrochlorid and 0.2 μM resazurin using 15 ml preculture each.
- Aerobic growth at 37 °C and 180 rpm until the culture reaches the anaerobic phase at A600 of 0.6. At that point, the redox indicator resazurin within the medium turns from blue to pink.
- Each 2 L of culture are induced by adding 0.2 μg/ml anhydrotetracycline and transferred into sterile 2 L Schott flasks containing 50 ml 20% glucose (5 g/L).
- Protein expression is carried out over night at 8 °C without stirring.
- Cells are anaerobically harvested by centrifugation for 20 min at 7,500 x g, resuspended in Tris-HCl (pH 8.0), 10% glycerol and stored at -20 °C until purification.
- Electroporation (Sambrook et al., 2006) of 100 μl E. coli BL21 (DE3) ΔiscR (Akhtar et al., 2008) with ~100 ng expression vector (pASK-IBA).
- Anaerobic purification of pyruvate: ferredoxin oxidoreductase (His-tag)
- For purification the pellet (2 L of culture) is thawed at room temperature and lysed by sonication while keeping the cells cooled on ice.
Note: Five times for 30 sec; output, 25; Branson Sonifier 250.
- Sedimentation of cell debris at 200,000 x g for 60 min and 4 °C in an ultracentrifuge.
- The soluble fraction is filtered using a pore size of 0.2 μm to get rid of unwanted material which clogs the column.
- Then, the sample is loaded on a pre-equilibrated (100 mM Tris-HCl, pH 8.0, 10 mM imidazole, 0.5 mM thiamine pyrophosphate) gravity flow Ni-NTA fast-flow column with a bed volume of 4 ml.
- Protein purification is achieved via increasing the imidazole concentration from 10 to 20 mM during washing each with 40 ml buffer.
- The His-tagged PFR1 protein is eluted from the column with 10 ml buffer containing 100 mM imidazole. Nine elution fractions each 1.1 ml are collected.
- The protein concentration of the brownish main elution fractions 3 and 4 are immediately determined using A280.
- For purification the pellet (2 L of culture) is thawed at room temperature and lysed by sonication while keeping the cells cooled on ice.
- Aerobic expression of [2Fe2S] ferredoxins (Jacobs et al., 2009; Winkler et al., 2009) with minor changes
- E. coli BL21 (DE3) ΔiscR containing the expression plasmid pASK-IBA7-FDX is grown in Vogel Bonner minimal medium for 4 h after induction at A600 of 0.6.
- Cells are harvested, washed in Tris-HCl (pH 8.0), sedimented again and stored at -20 °C until purification.
- E. coli BL21 (DE3) ΔiscR containing the expression plasmid pASK-IBA7-FDX is grown in Vogel Bonner minimal medium for 4 h after induction at A600 of 0.6.
- Anaerobic expression of HydA1 (Girbal et al., 2005; von Abendroth et al., 2008)
- Expression plasmid containing C. acetobutylicum ATCC 824 strain is grown in CGM-medium and a glucose concentration of 60 g/L anaerobically in a batch fermenter over night at 35-37 °C and 100 rpm.
- Cells are harvested in an anaerobic tent analog to E. coli, resuspended in Tris-HCl (pH 8.0), 10% glycerol containing 10 mM sodium dithionite and stored at -20 °C until purification.
- Expression plasmid containing C. acetobutylicum ATCC 824 strain is grown in CGM-medium and a glucose concentration of 60 g/L anaerobically in a batch fermenter over night at 35-37 °C and 100 rpm.
- Anaerobic expression of bacterial 2[4Fe4S] ferredoxin analog to HydA1 (Girbal et al., 2005; von Abendroth et al., 2008, Noth et al., 2013)
- Expression plasmid containing C. acetobutylicum ATCC 824 strain is grown in CGM-medium and a glucose concentration of 60 g/L anaerobically in a batch fermenter over night at 35-37 °C and 100 rpm.
- Cells are harvested in an anaerobic tent analog to E. coli, resuspended in Tris-HCl (pH 8.0), 10% glycerol containing 10 mM sodium dithionite and stored at -20 °C until purification.
- Expression plasmid containing C. acetobutylicum ATCC 824 strain is grown in CGM-medium and a glucose concentration of 60 g/L anaerobically in a batch fermenter over night at 35-37 °C and 100 rpm.
- Anaerobic purification of StrepII-tagged proteins (C-E)
- All buffers used contain 2 mM sodium dithionite.
- For purification the cell pellet is thawed at room temperature and lysed by sonication while keeping the cells cooled on ice.
Note: Five times for 30 sec; output, 25; Branson Sonifier 250.
- Sedimentation of cell debris at 200,000 x g for 60 min and 4 °C in an ultracentrifuge.
- Supernatant (40 ml) is incubated for 1 hour with 3.5 mg Avidin (Stock 50 mg.ml-1) at 4 °C.
- The soluble fraction is filtered using a poresize of 0.2 μm to get rid of biotinylated, complexed proteins and unwanted material which clogs the column.
- Then, the filtered solution is loaded on a Tris-HCl (pH 8.0) equilibrated 2 ml strep tactin gravity flow column.
- The unbound proteins are washed from the column using 80 ml Tris-HCl (pH 8.0).
- Elution is performed with 10 ml Tris-HCl (pH 8.0), d-desthiobiotin (0.8 mg/ml) in fractions of 1 ml.
- All buffers used contain 2 mM sodium dithionite.
Recipes
- 0.1 M Tris buffer (pH 8) (1,000 ml)
Mix 12.114 g of Tris base with 800 ml dH2O
Add 100 ml Glycerol
pH to 8 with HCl
Add ddH2O to 1,000 ml
Autoclave for 20 min at 121 °C
Store at 4 °C
- Pre-equilibrated gravity flow Ni-NTA
0.1 mM Tris-HCl (pH 8)
10 mM imidazole
0.5 mM thiamine pyrophosphate
Acknowledgments
Aerobic expression of [2Fe2S] ferredoxins was adapted from Jacobs et al. (2009). Anaerobic expression and purification of the 2[4Fe4S] bacterial type ferredoxin was done according to the previously published isolation of [FeFe]-Hydrogenase HydA1 from Chlamydomonas reinhardtii by Girbal et al. (2005) and von Abendroth et al. (2008), which is also presented here. Research on the pyruvate:ferredoxin oxidoreductase from C. reinhardtii was scientifically supported by Anja Hemschemeier and Thomas Happe.
References
- Noth, J., Krawietz, D., Hemschemeier, A. and Happe, T. (2013). Pyruvate:ferredoxin oxidoreductase is coupled to light-independent hydrogen production in Chlamydomonas reinhardtii. J Biol Chem 288(6): 4368-4377.
- Akhtar, M. K. and Jones, P. R. (2008). Deletion of iscR stimulates recombinant clostridial Fe-Fe hydrogenase activity and H2-accumulation in Escherichia coli BL21(DE3). Appl Microbiol Biotechnol 78(5): 853-862.
- Girbal, L., von Abendroth, G., Winkler, M., Benton, P. M., Meynial-Salles, I., Croux, C., Peters, J. W., Happe, T. and Soucaille, P. (2005). Homologous and heterologous overexpression in Clostridium acetobutylicum and characterization of purified clostridial and algal Fe-only hydrogenases with high specific activities. Appl Environ Microbiol 71(5): 2777-2781.
- Jacobs, J., Pudollek, S., Hemschemeier, A. and Happe, T. (2009). A novel, anaerobically induced ferredoxin in Chlamydomonas reinhardtii. FEBS Lett 583(2): 325-329.
- Sambrook, J. and Russell, D. W. (2006). Transformation of E. coli by Electroporation. CSH Protoc 2006(1).
- Vogel, H. J. and Bonner, D. M. (1956). Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218(1): 97-106.
- von Abendroth, G., Stripp, S., Silakov, A., Croux, C., Soucaille, P., Girbal, L. and Happe, T. (2008). Optimized over-expression of [FeFe] hydrogenases with high specific activity in Clostridium acetobutylicum. Inter J Hydrogen Energy 33(21): 6076-6081.
- Winkler, M., Kuhlgert, S., Hippler, M. and Happe, T. (2009). Characterization of the key step for light-driven hydrogen evolution in green algae. J Biol Chem 284(52): 36620-36627.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Noth, J. (2013). Heterologous Production and Anaerobic Purification of His- and StrepII-tagged Recombinant Proteins . Bio-protocol 3(17): e881. DOI: 10.21769/BioProtoc.881.
- Noth, J., Krawietz, D., Hemschemeier, A. and Happe, T. (2013). Pyruvate:ferredoxin oxidoreductase is coupled to light-independent hydrogen production in Chlamydomonas reinhardtii. J Biol Chem 288(6): 4368-4377.
Category
Plant Science > Phycology > Protein > Expression
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











