- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Social Recognition Memory Test in Rodents
Published: Vol 6, Iss 9, May 5, 2016 DOI: 10.21769/BioProtoc.1804 Views: 16484
Reviewed by: Soyun KimTifany DesprezPascal Fossat

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2607 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1645 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2585 Views
Abstract
Social recognition memory is essential for the establishment and maintenance of a rodent colony. Recognition memory is important for social hierarchy, mate and offspring recognition, and interspecies recognition. Interspecies recognition is vital for recognizing frequent visitors to the animal’s habitat and whether or not the visitors pose a threat to the animals or colony (Macbeth et al., 2009; Noack et al., 2010). Here, we describe a protocol which effectively and reproducibly measures the social recognition for a juvenile male, a female, a mouse of another strain, and a rat. This task relies on the animal’s innate tendency to explore a novel social partner and decrease the exploration of a known familiar social partner (Thor et al., 1982). A significant decrease in the exploration of a partner from the training session to the recall session demonstrates a memory of the social partner. Also, we describe a social recognition procedure, the habituation-dishabituation paradigm that closely mimics typical short, frequent interactions between animals in a colony (Dantzer et al., 1987; Winslow and Camacho, 1995). Further, olfaction is a key component of social recognition, to test olfaction see Jacobs et al. (2016). In this protocol, we use transgenic NR2A overexpression mice to demonstrate how an impairment in social recognition memory may appear.
Materials and Reagents
- Adult (3-8 months old) transgenic, knockout or treatment group mice (we use NR2A transgenic mice) and wild type mice (referred to as “subject mouse”)
- Juvenile (approximately 6-week old) male mice on the same genetic background
- Juvenile male mice on a different genetic background of a different color
- Approximately 3-month old adult female mice on the same genetic background
- Juvenile male rats
- 70% ethanol solution
Equipment
- Clean mouse cage identical to the home cage (standard mouse cage dimensions are 11 in x 7.5 in x 5 in), one for each subject mouse tested, change between sessions
- Clean rat home cage (standard rat housing cage dimensions are 22 in x 12.5 in x 8 in), one for each subject rat tested, change between sessions
- Clear Plexiglas to cover the cage top
- Small wire enclosure (for a female mouse), small wire pencil cup can be used (see Figure 1)
- Large wire mesh enclosure (for a juvenile male rat), made in-house from Plexiglas and wire mesh (Thermo Fisher Scientific, catalog number: AA42777HZ ) (see Figure 2)
- Digital recording camera
- Laboratory timer
- Stopwatch for recording investigation times
Procedure
- Experimental setup
- Experimental space should be quiet and dimly lit to avoid distraction from sounds and increased anxiety level from bright lights.
- The experimental area is surrounded by a back curtain with the experimenter outside of the curtain to avoid distraction from movements.
- A digital recording camera is mounted overhead and connected to a computer for recording the experiment.
- Social recognition experiments have two phases, a training phase and a recall phase. If the subject mouse does not spend at least 25 sec investigating the stimulus animal in the training phase the subject animal should be excluded for that experiment (Kogan et al., 2000). Insufficient exploration time in the training phase may lead to inconclusive or incorrect results.
- The subject mouse is said to be exploring the stimulus if the subject mouse is facing the stimulus and within 1 cm of the stimulus or following closely (≤ 1 cm) behind (Kogan et al., 2000).
- Experimental space should be quiet and dimly lit to avoid distraction from sounds and increased anxiety level from bright lights.
- Social recognition in mice
Social recognition of a conspecific juvenile male, or a juvenile male of a different strain- Training phase
- Subject mice (transgenic mice and their wildtype littermates) should be group housed (2-5 mice) for best results as single housing mouse can lead to decreased social recognition memory (Kogan et al., 2000).
- Place the subject mouse into a clean plastic cage identical to the home cage for 30 min prior to testing to allow the animal to habituate to the cage.
- Place a juvenile male mouse (the stimulus mouse) into the cage with the subject mouse for 5 min.
- Allow the mice to explore each other, but if the mice begin to fight they should be separated immediately to avoid injury.
- After 5 min, both mice are placed back into their home cage.
- Subject mice (transgenic mice and their wildtype littermates) should be group housed (2-5 mice) for best results as single housing mouse can lead to decreased social recognition memory (Kogan et al., 2000).
- Recall phase
- For the recall phase, split the subject mice into two groups. One group will be placed with a novel juvenile male and the second group will be placed with the same juvenile male as in the training phase.
- Place the subject mouse into a clean plastic cage identical to the home cage for 30 min prior to testing. Reusing the same cage can skew the result as the subject mouse may have left its scent in the bedding of the cage.
- Place the appropriate juvenile male stimulus mouse into the cage with the subject mouse for 5 min.
- Allow the subject mouse to explore the stimulus mouse, but if the mice begin to fight they should be separated immediately to avoid injury.
- After 5 min, both mice are placed back into their home cage.
- For the recall phase, split the subject mice into two groups. One group will be placed with a novel juvenile male and the second group will be placed with the same juvenile male as in the training phase.
Social recognition of a conspecific female- Training phase
- Place the female mouse into the round wire enclosure in a clean cage for ten min two times the day before testing to reduce stress and fear in the female mouse (for required equipment see Figure 1).

Figure 1. Experimental set-up for the female social recognition task. A. For the female social recognition task, a small wire enclosure and an empty clean mouse cage are used. B. This is an enlarged picture of the small wire enclosure used for the female mice. - Place the subject mouse into a clean plastic cage identical to the home cage with the small wire enclosure for 30 min prior to testing to allow the animal to habituate to the cage and the wire enclosure.
- Briefly, lift the wire enclosure and place a female mouse into the wire enclosure, and allow the subject mouse to explore the wire enclosure containing the female mouse for 5 min.
- After 5 min, place both mice back into their home cage.
- Place the female mouse into the round wire enclosure in a clean cage for ten min two times the day before testing to reduce stress and fear in the female mouse (for required equipment see Figure 1).
- Recall phase
- For the recall phase, split the subject mice into two groups. One group will be placed with a novel female and the second group will be placed with the same female as in the training phase.
- Place the subject mouse into a clean cage, identical to the home cage, with the wire enclosure in it, for 30 min prior to testing.
- Briefly, lift the wire enclosure and place the appropriate female mouse into the wire enclosure, and allow the subject mouse to explore the wire enclosure containing the female mouse for 5 min.
- After 5 min, both mice are placed back into their respective home cages.
- For the recall phase, split the subject mice into two groups. One group will be placed with a novel female and the second group will be placed with the same female as in the training phase.
Social recognition of a mouse for a rodent of another species- Training phase
- Place the rat into the wire mesh enclosure in a clean rat cage for ten minutes two times the day before testing to reduce stress and fear in the rat (for required equipment see Figure 2).
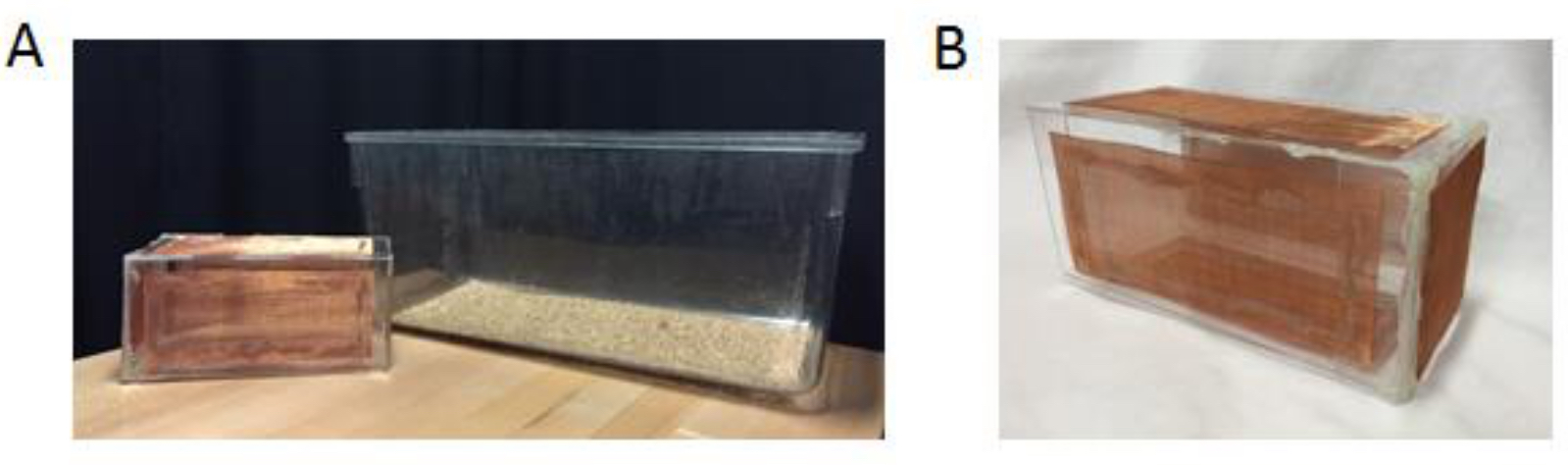
Figure 2. Experimental set-up for the rat social recognition task. A. For the rat social recognition task, a large wire enclosure and an empty clean rat cage are used. B. This is an enlarged picture of the large wire enclosure used for the rat. - Place the subject mouse into a clean plastic rat cage with the wire mesh enclosure for 30 min prior to testing to allow the mouse to habituate to the cage and the wire enclosure.
- Place a rat securely into the wire mesh enclosure, and the enclosure into the cage with the adult mouse for 5 min.
- Monitor the rat and mouse pair to ensure that the rat is not able to escape the enclosure for the safety of the mouse.
- After 5 min, place the mouse and the rat back into their respective home cages.
- Place the rat into the wire mesh enclosure in a clean rat cage for ten minutes two times the day before testing to reduce stress and fear in the rat (for required equipment see Figure 2).
- Recall phase
- For the recall phase, split the subject mice into two groups. One group will be placed with a novel rat (this is the control group) and the second group will be placed with the same rat as in the training phase (this is the experimental group).
- Place the subject mouse into a clean plastic rat cage with the wire enclosure in it, for 30 min prior to testing.
- Place the appropriate rat into the cage with the adult mouse for 5 min allowing the male mouse to explore the rat in its enclosure.
- After 5 min, both animals are placed back into their home cages.
- For the recall phase, split the subject mice into two groups. One group will be placed with a novel rat (this is the control group) and the second group will be placed with the same rat as in the training phase (this is the experimental group).
- Training phase
- Habituation-dishabituation in mice
This paradigm mimics the short, frequent interactions that mice may experience traveling throughout the colony (Dantzer et al., 1987; Winslow and Camacho, 1995). It consists of five short exploration sessions each with a one minute duration.- Place the subject mouse into a clean plastic cage identical to the home cage for 30 min prior to testing to allow the animal to habituate to the cage.
- Place a novel juvenile male conspecific into the cage with the subject mouse for 1 min. After one minute remove the stimulus mouse and place it into a holding cage.
- After a ten-minute delay, place the same stimulus mouse back into the cage with the subject mouse. Repeat this two more times for a total of 4 exploratory sessions.
- After the fourth ten-minute delay, the fifth exploratory session, place a novel juvenile conspecific into the cage with the subject mouse. After one minute remove the stimulus mouse from the cage.
- Place the subject mouse into a clean plastic cage identical to the home cage for 30 min prior to testing to allow the animal to habituate to the cage.
- Data analysis
- Using a stopwatch, record the amount of time that the subject mouse spends investigating the stimulus animal. Investigation of the stimulus animal is defined as the subject mouse being within one centimeter of the subject mouse with its head directed toward the other animal, touching or licking the other animal (Figure 3, Video 1). Recently, Arakawa et al. (2014) have also developed an automated software that can be used to detect social interaction patterns, if this method is preferred. The software was shown to produce similar results to human observation but may not be applicable in all instances (Arakawa et al., 2014).
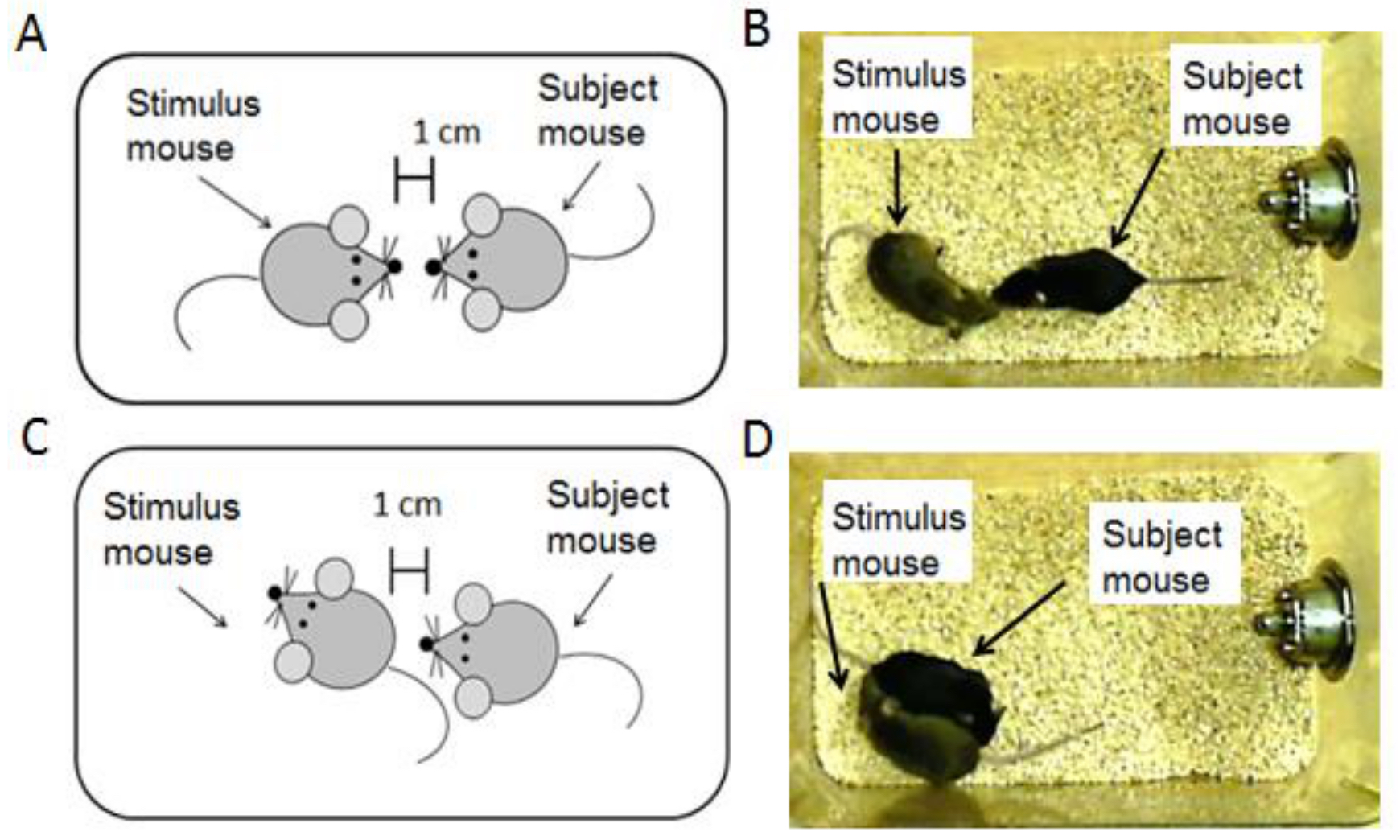
Figure 3. Data analysis for the social recognition task. This diagram illustrates the investigation criteria of the subject mouse. For both examples, the subject mouse must be within 1 cm of the stimulus animal with its face directed toward the stimulus. A. This panel illustrates an example of the subject mouse investigating the facial region of the stimulus mouse. B. This is a picture of the subject mouse investigating the facial region of the stimulus mouse. C. This panel illustrates an example of the subject mouse investigating the ano-genital region of the stimulus mouse. D. In this picture, the stimulus mouse is investigating the ano-genital region of the stimulus mouse.Video 1. Sample video clip for the social recognition task - Record the time in seconds.
- Group the animals according to the genotype, sex, or treatment.
- Average the exploration times and determine the standard error.
- Plot the data using the standard error measurements as the error bars.
- Differences between the training session and the recall session can be determined using a Student’s T-test. The animal is said to have formed a memory of the stimulus animal if there is a significant decrease in the amount of time spent investigating the familiar stimulus animal from the training session to the recall session. Differences among groups can be determined by an ANOVA.
- Using a stopwatch, record the amount of time that the subject mouse spends investigating the stimulus animal. Investigation of the stimulus animal is defined as the subject mouse being within one centimeter of the subject mouse with its head directed toward the other animal, touching or licking the other animal (Figure 3, Video 1). Recently, Arakawa et al. (2014) have also developed an automated software that can be used to detect social interaction patterns, if this method is preferred. The software was shown to produce similar results to human observation but may not be applicable in all instances (Arakawa et al., 2014).
Representative data
- Social recognition task
Figure 4 is an example of representative data collected during a social recognition paradigm with a juvenile male conspecific with a 24-h delay. Figure 4A and 4B are representative data from the group matched with a novel animal in the recall phase; this is the control group of the experiment. The exploration times are recorded in seconds and each group is averaged together and the standard error is calculated. This data is then plotted. The subject animals are expected to spend a similar amount of time investigating the novel animals in the training phase, as well as the novel animal in the recall phase. This indicated that any decreases in exploration times with a familiar animal are due to a memory of the familiar animal. Figure 4C and 4D are representative data from the group paired with the identical mouse in the recall phase. The wild type mice in the example do significantly decrease the exploration of the familiar mouse in the recall phase, indicating a memory of the stimulus animal. This significant decrease indicates that the wildtype mice have formed a memory for the conspecific. The NR2A mice are known to have impaired social recognition memory and do not show a significant decrease in the exploration time of the familiar mouse in the recall phase after a 24-h delay (Jacobs and Tsien, 2014). Significance between the phases is determined by a Student’s T-test and differences among the groups are determined by an ANOVA test.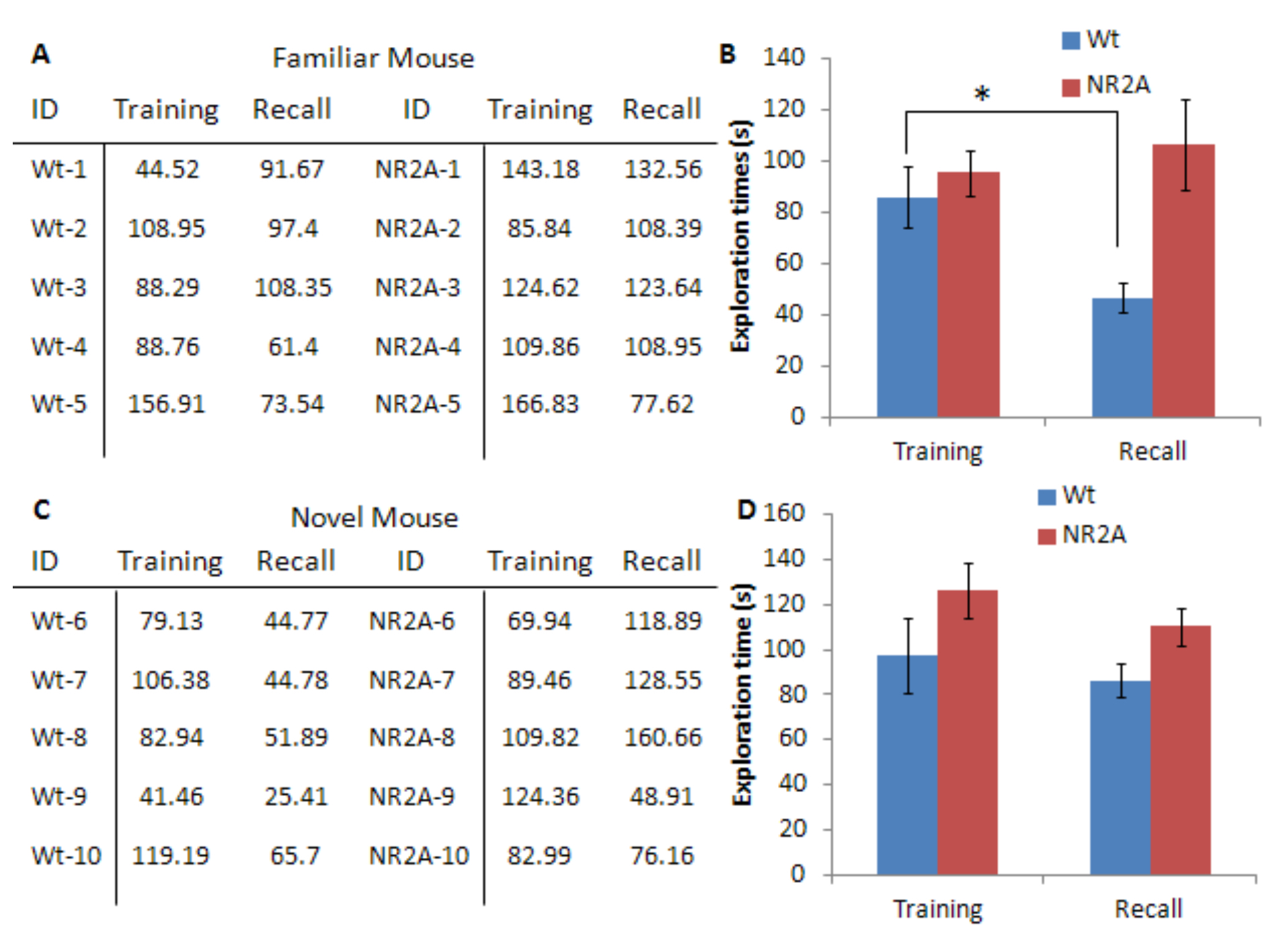
Figure 4. Representative data for the male conspecific recognition. A. Representative exploration times, in seconds, for the juvenile male conspecific task for both the wildtype and the NR2A transgenic mice. The identical juvenile male conspecific was used in both sessions. B. Graphical representation of the averages of the exploration times with SEM error bars. C. Representative exploration times, in seconds, for the juvenile male conspecific task for both the wildtype and the NR2A transgenic mice. A novel juvenile male conspecific was presented in the recall session. D. Graphical representation of the averages of the exploration times with SEM error bars. A novel juvenile conspecific was presented in the recall session. - Habituation-Dishabituation in mice
Figure 5 is an example of representative data collected during a habituation-dishabituation paradigm of social recognition. In this paradigm, the subject mouse is introduced to a conspecific stimulus mouse four times for one minute each followed by a single one minute exploration session with a novel mouse. The final session acts as a control for the expected decrease in exploration of the familiar stimulus over the first four sessions. Figure 5A is a table listening representative data for all five trials. It is expected that the exploration times of trials one through four decreases as the stimulus conspecific becomes familiar to the subject mouse. The data for each exploration by each group is averaged and the standard error is calculated. Figure 5B is the average exploration for each group plotted by trial number. Significance between consecutive trials is determined by a Student’s T-test and differences among groups are determined by an ANOVA test.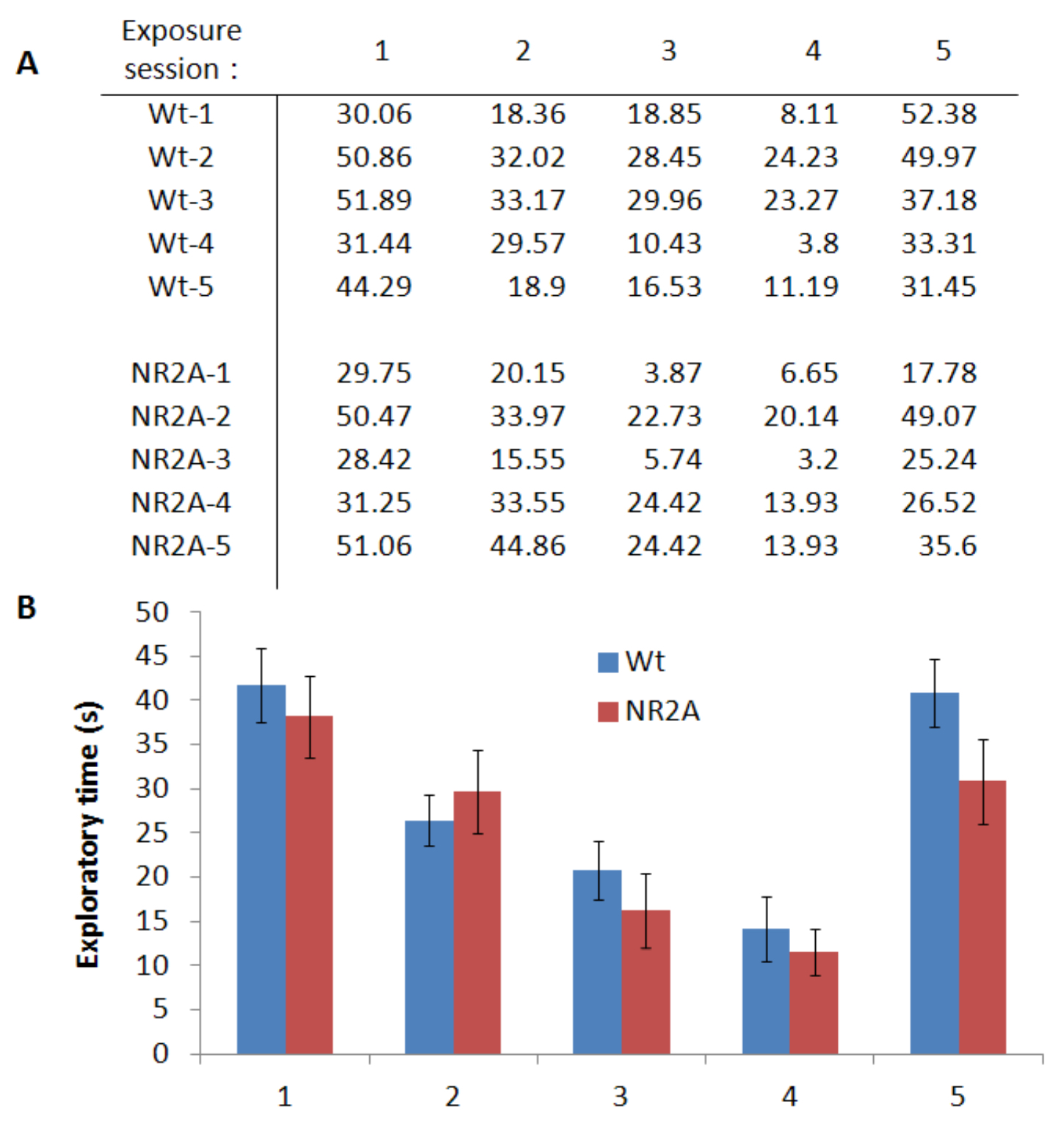
Figure 5. Representative data for the habituation-dishabituation paradigm. A. Representative exploration times, in seconds, for each of the habituation-dishabituation paradigm for both the wildtype and the NR2A transgenic mice. The identical juvenile male conspecific was used in the first four sessions with a novel male conspecific in the fifth session. B. Graphical representative of the averages of the exploration times with SEM error bars.
Notes
Although the described protocol is relatively simple to perform and involves little specialized equipment, proper execution and attention to detail is essential for correct results and reproducibility.
- Firstly, the experimental set up should be in a slightly darkened, quiet room as mice often show fear of brightly lit area and can be startled by sudden loud noises or movements (Liu et al., 2014).
- Similarly, the animals should be habituated to the empty cage and the empty chambers to avoid the animals displaying neophobia (Deacon, 2006). Do not reuse the same cage as feces and urine from the first partner maybe present in the bedding giving inconsistent or skewed results (Arakawa et al., 2008; Tolokh et al., 2013). Rat cages were used due to the size of the wire mesh enclosure needed for the rat. Be sure to habituate the subject mouse to this testing cage prior to the training protocol.
- The time duration between the train phase and the recall phase vary depending on the type of memory being tested. Typically for short-term memory the time duration is 1 hour or two hours. For long-term memory, typical time durations are 12-24 h.
- Stimulus animal selection is important for several reasons. Juvenile male mice are used to decrease the possibility of the adult subject mouse to exhibit aggressive behaviors (Hlinak and Krejci, 1991; Heinla et al., 2014). Mouse strains can differ in their social aggressiveness [i.e., CD-1 mice (Golden et al., 2011), Tg2576 mice, an Alzheimer’s strain (Alexander et al., 2011), and mice with altered serotonin levels (Takahashi et al., 2012)] and more aggressive strains may need to be separated to avoid fighting (Levine et al., 1979; Serri and Ely, 1984). Additionally, mice that are singly housed, or have been used for breeding may be more aggressive. Fighting during the exploration trials can be dangerous for both the stimulus mouse and the subject mouse. If fighting is observed the trial should be stopped immediately and the animals separated to avoid injury.
- Social recognition may be diminished if the stimulus mouse is on a different strain (Macbeth et al., 2009; Jacobs and Tsien, 2012; Jacobs and Tsien, 2014). The females used as stimulus animals should be on the same genetic background due to the observation that female mice in the wild are more likely to breed with a male from the same genetic background (Macbeth et al., 2009).
- During the social recognition protocol described here, breeding was not to occur. The wire enclosure allows the male mouse to explore the scent of the female without mounting or mating behaviors.
- In the described protocol, a rat was used to investigate cross-species recognition. Juvenile male rats can be used and may be preferred due to the smaller size. The rat enclosure prevents the rat and mouse from having direct interaction as rats and prevent muricide by the rat (Noack et al., 2010). Be sure to thoroughly clean all enclosures between animals and sessions with 70% ethanol or a suitable alternative.
Acknowledgments
This work was supported by funds from the National Institute of Mental Health (MH060236), National Institute on Aging (AG024022, AG034663 & AG025918), USAMRA00002, and Georgia Research Alliance (all to JZT). This protocol was adapted from (Jacobs and Tsien, 2014; Jacobs et al., 2015).
References
- Alexander, G., Hanna, A., Serna, V., Younkin, L., Younkin, S. and Janus, C. (2011). Increased aggression in males in transgenic Tg2576 mouse model of Alzheimer's disease. Behav Brain Res 216(1): 77-83.
- Arakawa, H., Blanchard, D. C., Arakawa, K., Dunlap, C. and Blanchard, R. J. (2008). Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev 32(7): 1236-1248.
- Arakawa, T., Tanave, A., Ikeuchi, S., Takahashi, A., Kakihara, S., Kimura, S., Sugimoto, H., Asada, N., Shiroishi, T., Tomihara, K., Tsuchiya, T. and Koide, T. (2014). A male-specific QTL for social interaction behavior in mice mapped with automated pattern detection by a hidden Markov model incorporated into newly developed freeware. J Neurosci Methods 234: 127-134.
- Dantzer, R., Bluthe, R. M., Koob, G. F. and Le Moal, M. (1987). Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 91(3): 363-368.
- Deacon, R. M. (2006). Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc 1(2): 936-946.
- Golden, S. A., Covington, H. E., 3rd, Berton, O. and Russo, S. J. (2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6(8): 1183-1191.
- Heinla, I., Leidmaa, E., Visnapuu, T., Philips, M. A. and Vasar, E. (2014). Enrichment and individual housing reinforce the differences in aggressiveness and amphetamine response in 129S6/SvEv and C57BL/6 strains. Behav Brain Res 267: 66-73.
- Hlinak, Z. and Krejci, I. (1991). Social recognition in male rats: age differences and modulation by MIF-I and Alaptide. Physiol Res 40(1): 59-67.
- Jacobs, S. A. and Tsien, J. Z. (2012). Genetic overexpression of NR2B subunit enhances social recognition memory for different strains and species. PLoS One 7(4): e36387.
- Jacobs, S. A. and Tsien, J. Z. (2014). Overexpression of the NR2A subunit in the forebrain impairs long-term social recognition and non-social olfactory memory. Genes Brain Behav 13(4): 376-384.
- Jacobs, S. A., Huang, F., Tsien, F. J. and Wei, W. (2016). Olfactory recognition memory test in mice. Bio-protocol 6(9): e1803.
- Jacobs, S., Wei, W., Wang, D. and Tsien, J. Z. (2015). Importance of the GluN2B carboxy-terminal domain for enhancement of social memories. Learn Mem 22(8): 401-410.
- Kogan, J. H., Frankland, P. W. and Silva, A. J. (2000). Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10(1): 47-56.
- Levine, L., Grossfield, J. and Rockwell, R. F. (1979). Functional relationships between genotypes and environments in behavior. Effects of different kinds of early social experience on interstrain fighting in male mice. J Hered 70(5): 317-320.
- Liu, J., Wei, W., Kuang, H., Tsien, J. Z. and Zhao, F. (2014). Heart rate and heart rate variability assessment identifies individual differences in fear response magnitudes to earthquake, free fall, and air puff in mice. PLoS One 9(3): e93270.
- Macbeth, A. H., Lee, H. J., Edds, J. and Young, W. S., 3rd (2009). Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav 8(5): 558-567.
- Noack, J., Richter, K., Laube, G., Haghgoo, H. A., Veh, R. W. and Engelmann, M. (2010). Different importance of the volatile and non-volatile fractions of an olfactory signature for individual social recognition in rats versus mice and short-term versus long-term memory. Neurobiol Learn Mem 94(4): 568-575.
- Serri, G. A. and Ely, D. L. (1984). A comparative study of aggression related changes in brain serotonin in CBA, C57BL, and DBA mice. Behav Brain Res 12(3): 283-289.
- Takahashi, A., Quadros, I. M., de Almeida, R. M. and Miczek, K. A. (2012). Behavioral and pharmacogenetics of aggressive behavior. Curr Top Behav Neurosci 12: 73-138.
- Thor, D. H., Wainwright, K. L. and Holloway, W. R. (1982). Persistence of attention to a novel conspecific: some developmental variables in laboratory rats. Dev Psychobiol 15(1): 1-8.
- Tolokh, II, Fu, X. and Holy, T. E. (2013). Reliable sex and strain discrimination in the mouse vomeronasal organ and accessory olfactory bulb. J Neurosci 33(34): 13903-13913.
- Winslow, J. T. and Camacho, F. (1995). Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology (Berl) 121(2): 164-172.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jacobs, S. A., Huang, F., Tsien, J. Z. and Wei, W. (2016). Social Recognition Memory Test in Rodents. Bio-protocol 6(9): e1804. DOI: 10.21769/BioProtoc.1804.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Share
Bluesky
X
Copy link









