- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Olfactory Recognition Memory Test in Mice
Published: Vol 6, Iss 9, May 5, 2016 DOI: 10.21769/BioProtoc.1803 Views: 9848
Reviewed by: Soyun KimTifany DesprezPascal Fossat

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2607 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1645 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2585 Views
Abstract
Olfactory memory is an ethologically relevant task that relies on a mouse’s innate ability to use olfaction to forage for food (Zou et al., 2015), and identify safe foods. Although many of the same brain areas involved in other forms of memory are also involved in olfactory memory, the mechanisms are different (Sanchez-Andrade et al., 2005; Tong et al., 2014). Here, we describe one way to test olfactory memory in mice. The protocol described can be used to test long-term memory (memory which requires de novo protein synthesis) or short term memory by adjusting the delay time between the training session and the recall session (Freedman et al., 2013) and has been designed to mimic the single presentation of the social recognition paradigm. This paradigm relies on the mouse’s innate tendency to investigate a novel scent more than a familiar scent. Transgenic NR2A overexpression mice are known to have impaired long-term olfactory memory, but intact short-term memory, and are used here to demonstrate how one form of impaired olfactory memory may appear. Other genetically or chemically manipulated mice may be used in place of the transgenic mice used here.
Materials and Reagents
- Filter paper (Bio-Rad Laboratories, catalog number: 1703967 )
- Pipet or syringe (BD, catalog number: 309625 )
- Low odor epoxy or glue, or tape to seal the scent cup
- Adult 3-8 months old mice, for the testing group (transgenic NR2A overexpression mice) and wild type mice for the control group (referred to as “subject mouse”)
- 70% ethanol solution
- Fresh or canned, non-expired 100% juice samples [pineapple (Dole), orange (Tropicana), 100% lemon and 100% lime juices] (see Figure 1)
Equipment
- Clean mouse cages identical to the home cage (7.5 in x 11 in x 5 in), one for each subject mouse per phase
- Clear Plexiglas to cover the cage top (so the mice cannot climb out)
- Small plastic container with a lid (i.e., a plastic petri dish, 35 mm x 10 mm) (Thermo Fisher Scientific, catalog number: FB0875711YZ , or other similar replacement)
- Drill with small (1 mm) drill bit or 18 g needle (BD, catalog number: 305196 ) for making holes in top of scent cup
- Digital recording camera
- Laboratory timer
- Stopwatch for recording investigation times

Figure 1. Experimental set-up for the olfactory recognition task. For the olfactory recognition task, a selection of juices (pineapple, orange, lemon and lime), a small Petri dish, a syringe and an empty clean mouse cage are used.
Procedure
- Experimental setup
- Experimental space should be quiet and dimly lit (75 to 100 lux) to avoid distraction from sounds and increased anxiety level from bright lights.
- The experimental area is surrounded by a back curtain with the experimenter outside of the curtain to avoid distraction from movements.
- A digital recording camera is mounted overhead and connected to a computer for recording the experiment.
- The olfactory recognition experiment has two phases, a training phase and a recall phase. Long-term memory can be tested by delaying the recall phase for 24 h. Short-term memory can be tested by employing a short delay time between the two phases, such as one hour.
- Create the scent cup by drilling four or five small holes in the lid of the container. Place a piece of the filter paper inside the container and seal the container using low odor glue or epoxy, or tape.
- Experimental space should be quiet and dimly lit (75 to 100 lux) to avoid distraction from sounds and increased anxiety level from bright lights.
- Olfactory recognition
- Training phase
- Place the subject mouse into a clean cage, identical to the home cage, with the scent cup for 30 min prior to testing to allow the animal to habituate to the cage and the scent cup.
- Briefly remove the scent cup, place 0.10 ml of fruit juice on the filter paper inside the scent cup with a syringe or pipet and place the scent cup back into the cage. For guidance on placement within the cage see Figure 2.
- Allow the mouse to freely explore the scent, after 5 min, place the mouse back into its home cage.
- Place the subject mouse into a clean cage, identical to the home cage, with the scent cup for 30 min prior to testing to allow the animal to habituate to the cage and the scent cup.
- Recall phase
- For the recall phase, split the subject mice into two groups. One group will be paired with a novel fruit juice (this is the control group), the novel juice should be explored to a similar extent as the juice in the training phase. This control group demonstrates that the decrease in exploration is not due to fatiguing effects. The second group will be paired with the same fruit juice as in the training phase (this is the experimental group).
- Place the subject mouse into a clean mouse cage, containing a scent cup for 30 min prior to testing.
- Briefly remove the scent cup, place 0.10 ml of the appropriate fruit juice into the scent cup and return the scent cup back into the cage with the subject mouse for 5 min. For guidance on placement within the cage see Figure 2.
- After 5 min, the subject mouse is placed back into the home cage.
- For the recall phase, split the subject mice into two groups. One group will be paired with a novel fruit juice (this is the control group), the novel juice should be explored to a similar extent as the juice in the training phase. This control group demonstrates that the decrease in exploration is not due to fatiguing effects. The second group will be paired with the same fruit juice as in the training phase (this is the experimental group).
- Training phase
- Data analysis
- Using a stopwatch, have a blind experimenter record the amount of time that the mouse spends investigating the scent. Investigation of the scent is defined as the mouse being within one centimeter of the scent cup with its head directed toward the scent cup (Figure 2, Video 1). Alternatively, there are several video tracking software systems that can be used (i.e., BIOBSERVE software), however if bedding is used in the testing cage, as in this protocol, the scent cup may move. If automated tracking software is used, it would be best to secure the scent cup to the cage floor.
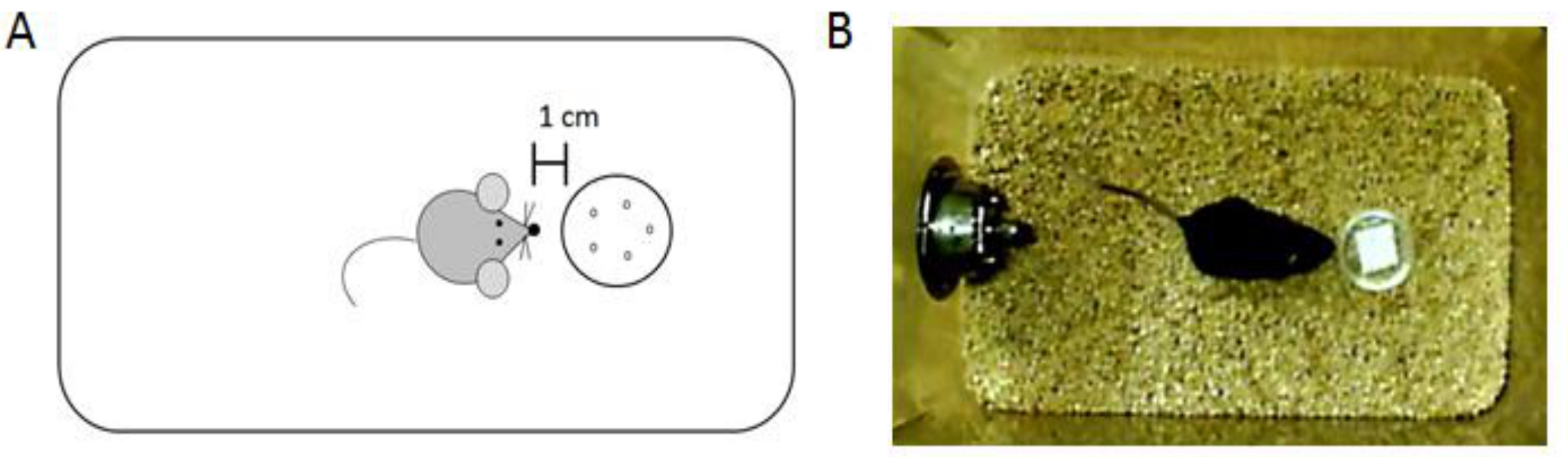
Figure 2. Data analysis for the olfactory recognition task. A. Diagram illustrating the investigation criteria of the scent. B. Picture of a subject mouse investigating a scent.Video 1. Video clip of the olfactory recognition task - Record the time that the animal spends investigating the scent cup in seconds.
- Group the animals according to the genotype, sex, or treatment group.
- Average the exploration times and determine the standard error.
- Plot the data using the standard error measurements as the error bars.
- Differences between the training session and the recall session can be determined using a Student’s t-test. The animal is said to have formed a memory of the scent if there is a significant decrease in the amount of time spent investigating the familiar scent from the training session to the recall session. Differences among groups can be determined by an ANOVA.
- Using a stopwatch, have a blind experimenter record the amount of time that the mouse spends investigating the scent. Investigation of the scent is defined as the mouse being within one centimeter of the scent cup with its head directed toward the scent cup (Figure 2, Video 1). Alternatively, there are several video tracking software systems that can be used (i.e., BIOBSERVE software), however if bedding is used in the testing cage, as in this protocol, the scent cup may move. If automated tracking software is used, it would be best to secure the scent cup to the cage floor.
Representative data
Figure 3 is representative data for a 24-h olfactory recognition memory task. In this task, the mice were introduced to a fruit scent in the first round and were allowed to investigate the fruit scent for 5 min. The mice were then split into two groups; one group will be presented with the identical scent, the other group will be presented with a novel scent. The wild type mice are able to learn and recall the familiar scent, as evident by the significant decrease (as determined by a Student’s t-test) in the exploration of the familiar scent in the recall phase. The NR2A mice (used here to demonstrate impaired olfactory memory) are unable to recall the familiar scent in the 24 h recall phase and did not significantly reduce the exploration times of the familiar scent. 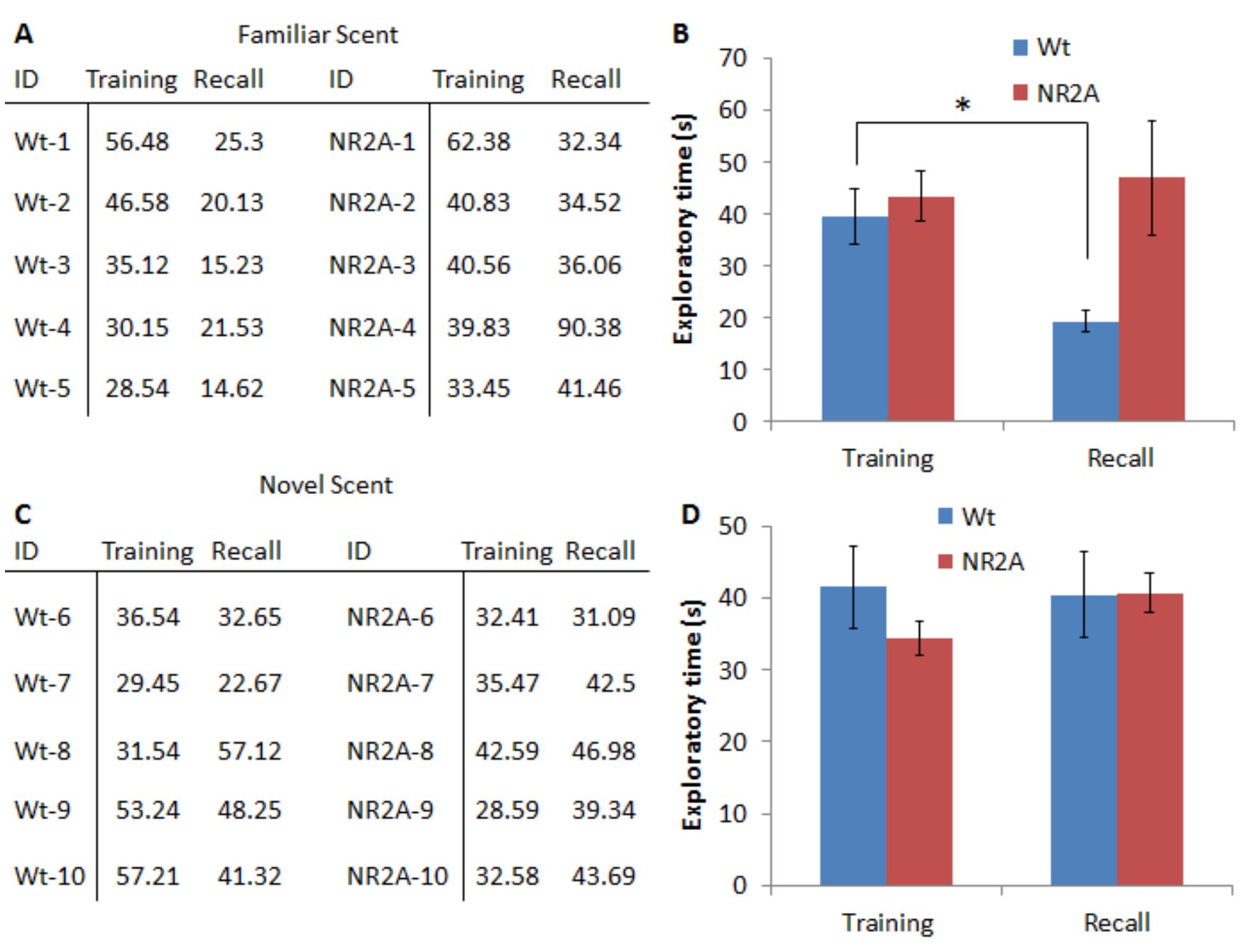
Figure 3. Representative data for a 24 h long-term olfactory recognition task. A. Representative exploration times, in seconds, of the juice for both the wild type and the NR2A transgenic mice. The identical scent was used in both sessions. B. Graphical representation of the averages of the exploration times. C. Representative exploration times, in seconds, of the juices for both the wild type and the NR2A transgenic mice. A novel juice was presented in the recall session. D. Graphical representation of the averages of the exploration times with. A novel juice scent was presented in the recall session.
Notes
- Experimental setup should be in a slightly darkened, quiet room as mice often show fear of brightly lit area and can be startled by sudden loud noises or movements (animal may freeze or avoid venturing away from the walls in response to fearful events) (Liu et al., 2014). Similarly, the animals should be habituated to the testing area (i.e., Ten minutes a day for 3 days prior to testing if standard houses cages are not used) and the empty scent cup (as described) to avoid the animals displaying neophobia (Deacon, 2006).
- If the mice display fear during the task, as a result of not being habituated to the caging or the scent cup, the investigation times will be reduced skewing the results. However, using the described measures, the animal should be habituated and not display signs of fear. If enclosures identical to the home cages are not used, longer habituation times may be required.
- Scent cups can be made to be reusable (i.e., with a screw-top cap). If reusable cups are use, clean the scent cups thoroughly between scents, sessions and animals to avoid scent from other animals contaminating the scent cup and skewing the results. Disposable scent cups can also be sealed with a small bead of low-odor glue or epoxy, or tape. Allow sufficient drying time for the glue or epoxy to avoid a strong scent.
- After the training session, the delay used before the recall session determined the type of memory tested. To test short term memory, use a short delay, such as 1 h. Short-term memory is memory which does not require protein synthesis. Twenty-four hours can be used to test long-term memory as 24 h memory has been found to require de novo protein synthesis.
- The number of animals used will depend on the strength of the manipulation to the subject animals. For many behavioral paradigms it is best to use n ≥ 10 for each group to ensure accuracy.
Acknowledgments
This work was supported by funds from the National Institute of Mental Health (MH060236), National Institute on Aging (AG024022, AG034663 & AG025918), USAMRA00002, and Georgia Research Alliance (all to JZT). This protocol was adapted from (Jacobs and Tsien, 2014; Jacobs et al., 2015).
References
- Deacon, R. M. (2006). Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc 1(2): 936-946.
- Freedman, K. G., Radhakrishna, S., Escanilla, O. and Linster, C. (2013). Duration and specificity of olfactory nonassociative memory. Chem Senses 38(4): 369-375.
- Jacobs, S. A. and Tsien, J. Z. (2014). Overexpression of the NR2A subunit in the forebrain impairs long-term social recognition and non-social olfactory memory. Genes Brain Behav 13(4): 376-384.
- Jacobs, S., Wei, W., Wang, D. and Tsien, J. Z. (2015). Importance of the GluN2B carboxy-terminal domain for enhancement of social memories. Learn Mem 22(8): 401-410.
- Liu, J., Wei, W., Kuang, H., Tsien, J. Z. and Zhao, F. (2014). Heart rate and heart rate variability assessment identifies individual differences in fear response magnitudes to earthquake, free fall, and air puff in mice. PLoS One 9(3): e93270.
- Sanchez-Andrade, G., James, B. M. and Kendrick, K. M. (2005). Neural encoding of olfactory recognition memory. J Reprod Dev 51(5): 547-558.
- Tong, M. T., Peace, S. T. and Cleland, T. A. (2014). Properties and mechanisms of olfactory learning and memory. Front Behav Neurosci 8: 238.
- Zou, J., Wang, W., Pan, Y. W., Lu, S. and Xia, Z. (2015). Methods to measure olfactory behavior in mice. Curr Protoc Toxicol 63: 11 18 11-21.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jacobs, S. A., Huang, F., Tsien, J. Z. and Wei, W. (2016). Olfactory Recognition Memory Test in Mice. Bio-protocol 6(9): e1803. DOI: 10.21769/BioProtoc.1803.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









