- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Estimation of Wound Tissue Neutrophil and Macrophage Accumulation by Measuring Myeloperoxidase (MPO) and N-Acetyl-β-D-glucosaminidase (NAG) Activities
Published: Vol 5, Iss 22, Nov 20, 2015 DOI: 10.21769/BioProtoc.1662 Views: 14186
Reviewed by: Ivan ZanoniAchille BroggiMarco Di Gioia

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Quantification of Macrophage Cellular Ferrous Iron (Fe2+) Content using a Highly Specific Fluorescent Probe in a Plate-Reader
Philipp Grubwieser [...] Christa Pfeifhofer-Obermair
Feb 5, 2024 2212 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3543 Views
Utilizing EdU to Track Leukocyte Recruitment to the Brain
Zoie K. Lipfert [...] David P. Sullivan
Dec 5, 2025 1556 Views
Abstract
The inflammatory response is essential to the reestablishment of cutaneous homeostasis following injury. In this context, leukocytes arrive at the wound site and orchestrate essential events in the wound healing process. Therefore, the quantification of specific subsets of inflammatory cells in the wound tissue is of considerable interest. The current protocol focus on a quantitative index of neutrophils and macrophages accumulation within skin lesions by measuring the specific activity of the marker enzymes Myeloperoxidase (MPO) and N-acetyl-β-D-glucosaminidase (NAG), respectively. MPO is present in high levels in the azurophilic granules of neutrophils and NAG in lysosomes of activated macrophages. These methods allow the indirect estimation of the abundance of neutrophils and macrophages accumulated into the skin.
Materials and Reagents
- 2 ml microcentrifuge tubes (Eppendorf)
- Circular biopsy punch (ABC, catalog number: 0418 )
- 96-well microplates (Thermo Fisher Scientific, FisherbrandTM, catalog number: 21-377-203 )
- 3% Hydrogen peroxide (H2O2) (Sigma-Aldrich, catalog number: H1009 )
- 3, 3’-5, 5’-tetramethylbenzidine (TMB) (Sigma-Aldrich, catalog number: T2885 )
- 4-nitrophenyl-N-acetyl-β-D-glucosaminide (Sigma-Aldrich, catalog number: N9376 )
- Citric acid (Sigma-Aldrich, catalog number: 251275 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 472301 )
- Glucose Solution (Sigma-Aldrich, catalog number: G8644 )
- Sulfuric acid (H2SO4) (Sigma-Aldrich, catalog number: 258105 )
- Hexadecyltrimethylammonium bromide (HTAB) (Sigma-Aldrich, catalog number: H9151 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P0662 )
- Liquid nitrogen
- Ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA) (Sigma-Aldrich, catalog number: V000114 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: 255793 )
- Sodium phosphate tribasic dodecahydrate (Na3PO4) (Sigma-Aldrich, catalog number: 222003 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 221465 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Phosphate buffered saline (PBS) (10x, pH 7.2) (see Recipes)
- PBS (1x) (see Recipes)
- Buffer 1 (see Recipes)
- Buffer 2 (see Recipes)
- H2O2 (0.002% v/v) (Sigma-Aldrich, catalog number: 7722-84-1 ) (see Recipes)
- MPO substrate solution (see Recipes)
- Saline-Triton buffer (see Recipes)
- Phosphate-citrate buffer (see Recipes)
- NAG substrate solution (see Recipes)
- Glycine buffer (see Recipes)
Equipment
- Freezer (-20 °C)
- Freezer (-80 °C)
- High Precision Laboratory Balance (Shimadzu Corporation, catalog number: ATX -224 )
- Micropipettes
- Microplate Spectrophotometer Reader (Molecular Devices, model: SpectraMax )
- Refrigerated centrifuge (Eppendorf, model: 5810R )
- Tissue homogenizer (Thermo Fisher Scientific, catalog number: PowerGen 125 )
- Vortex Mixer (VELP Scientifica, catalog number: F202A0171 )
Procedure
- Harvesting wound tissue
- The wounds are harvested after animal euthanasia. Anesthetic overdose is the preferred method for euthanasia. Avoid cervical dislocation or decapitation methods.
- First, remove the back skin of the mice and carefully remove the wound tissue with the aid of a circular biopsy punch (with a slightly larger diameter than the one employed to wounding) to collect entire wound area (plus about 2 mm surrounding skin tissue) (Figures 1A-D).
- Place the fresh samples (harvested tissue) in 2 ml microcentrifuge tubes and immediately dip in liquid nitrogen.
- Store the harvested tissue at -80 °C until use.
- To the excisional wound healing model, see Moreira et al. (2015).
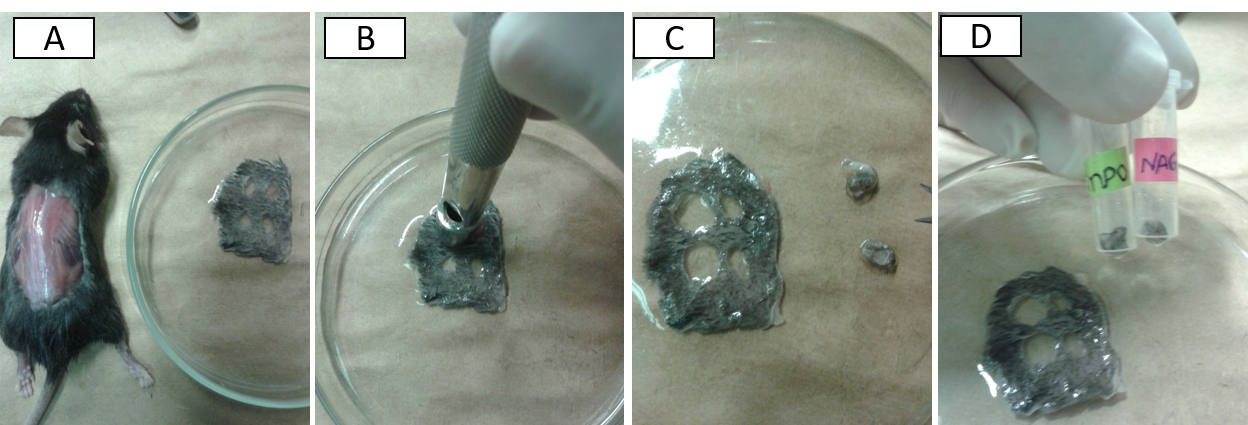
Figure 1. Harvesting wound tissues for analysis. After euthanasia, remove the dorsal wounded skin of mice (A). Place the larger circular punch over the skin lesion, press down, and slowly rotate to remove a circular piece of skin (B-C). Collect for NAG and MPO analyses (D) by immediately dipping the tissue-containing microcentrifuge tubes in liquid nitrogen.
- The wounds are harvested after animal euthanasia. Anesthetic overdose is the preferred method for euthanasia. Avoid cervical dislocation or decapitation methods.
- MPO enzyme isolation and assay (Figure 2)
- Weigh and homogenize the tissue in ice-cold Buffer 1 (100 mg tissue/1 ml buffer), using a powered high speed tissue homogenizer (Ultra-turrax or equivalent). Homogenizing procedures during enzyme isolation must be performed on ice.
- Centrifuge at 10,000 x g for 10 min at 4 °C.
- Discard supernatant and submit the pellet to hypotonic lysis as follows: Resuspend the pellet in 1 ml of 0.2% NaCl for 30 sec, and then add 1.0 ml of a 1.6% NaCl/5% glucose solution.
- Centrifuge at 10,000 x g for 10 min at 4 °C.
- Discard supernatant and resuspend the pellet in Buffer 2 (100 mg tissue/1 ml buffer).
- Vortex and then freeze-thaw three times using liquid nitrogen. The thawing step can be performed under running tap water.
- Centrifuged at 10,000 x g for 15 min at 4 °C.
- Save the supernatants for the enzymatic assay (if the MPO assay is not carried out immediately, the supernatants can be stored at -20 °C until used).
- Dilute samples in Buffer 2 at RT (we suggest 1:3, but the dilution should be adjusted experimentally).
- To assay, add 25 μl of diluted supernatants samples (or Buffer 2, used as blank) per well to 96-well microplate. All samples should be run in duplicate.
- Add 25 μl of MPO substrate solution.
- Incubate for 5 min at 37 °C.
- Add 25 μl of 0.002% H2O2.
- Incubate for 5 min at 37 °C.
- Finally, stop reaction with 25 μl of 1 M H2SO4.
- Read at 450 nm.
- Calculate the relative activity of MPO as follow: (sample absorbance spectrum - blank absorbance spectrum) x sample dilution factor. Express results as relative units (MPO activity/100 mg) that denote activity of MPO per 100 mg of tissue.
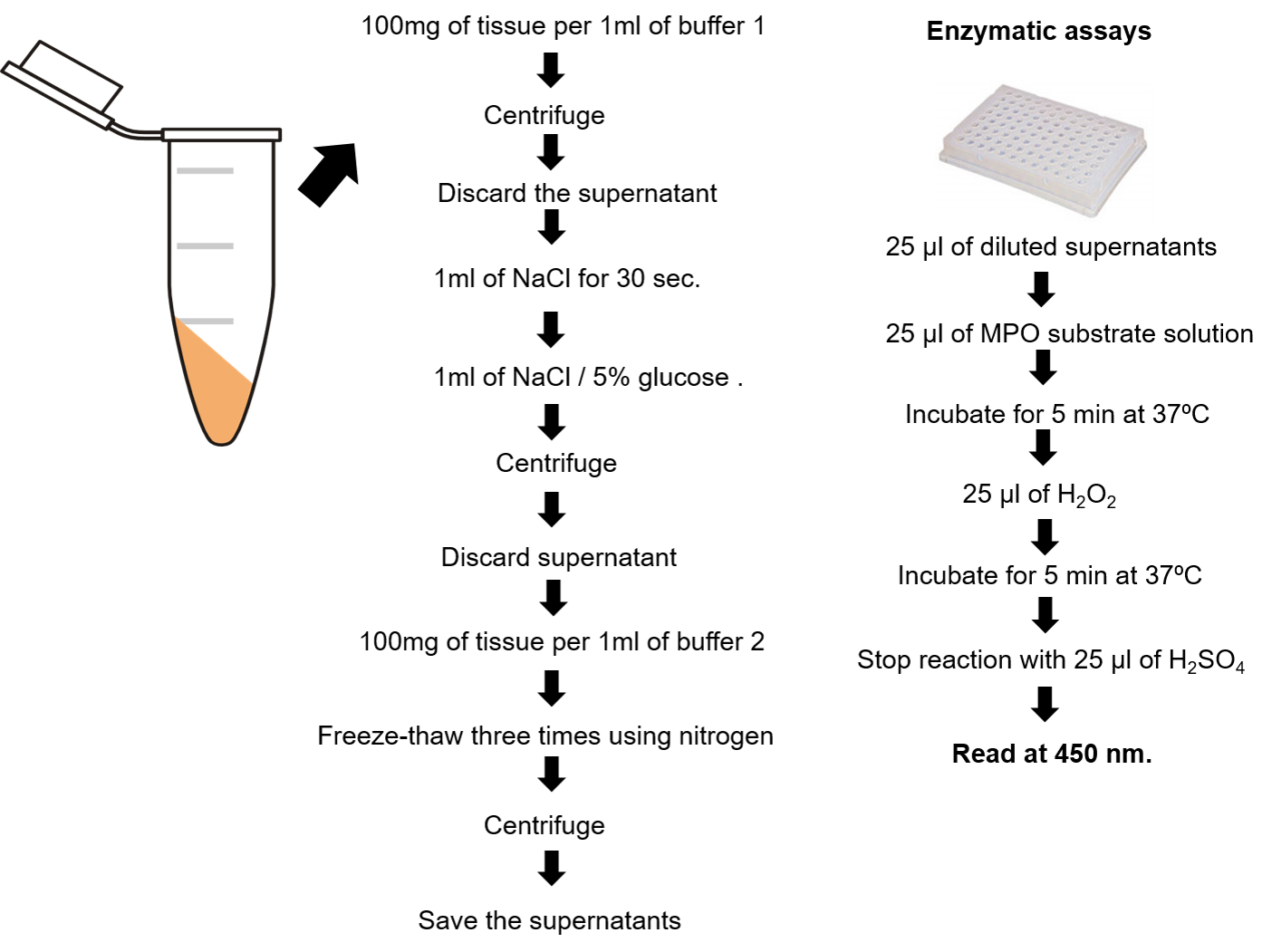
Figure 2. MPO assay procedure summary
- Weigh and homogenize the tissue in ice-cold Buffer 1 (100 mg tissue/1 ml buffer), using a powered high speed tissue homogenizer (Ultra-turrax or equivalent). Homogenizing procedures during enzyme isolation must be performed on ice.
- NAG enzyme isolation and assay (Figure 3)
- Weigh and homogenize the tissue in ice-cold Saline-Triton buffer (100 mg tissue/1 ml buffer), using a powered high speed tissue homogenizer (Ultra-turrax or equivalent). Homogenizing procedures during enzyme isolation must be performed on ice.
- Centrifuge at 3,000 x g for 10 min at 4 °C.
- Save the supernatant for the enzymatic assay (if the NAG assay is not carried out immediately, the supernatants can be stored at -20 °C until used).
- Dilute assaying samples in phosphate-citrate buffer at RT (we suggest 1:3, but the dilution should be adjusted experimentally).
- To assay, add 25 μl of diluted supernatants samples (or phosphate-citrate buffer, used as blank) per well to 96-well microplate. All samples should be run in duplicate (Figure 4).
- Add 25 μl of NAG substrate solution.
- Incubate for 10 min at 37 °C.
- Finally, stop reaction with 25 μl of 0.2 M Glycine buffer.
- Read at 400 nm.
- Calculate the relative activity of NAG as follow: (Sample absorbance spectrum-blank absorbance spectrum) x sample dilution factor. Express results as relative units (NAG activity/100 mg) that denote activity of NAG per 100 mg of tissue.
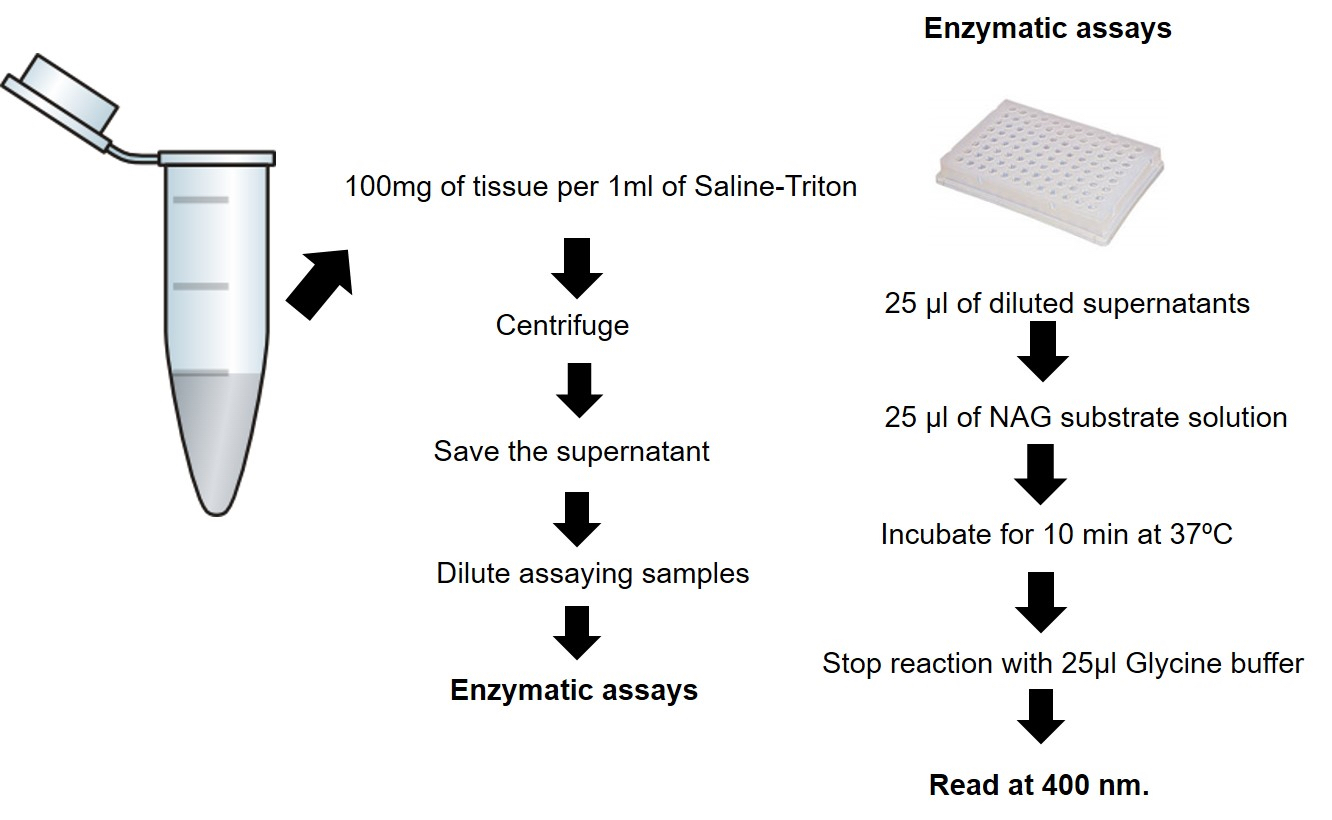
Figure 3. NAG assay procedure summary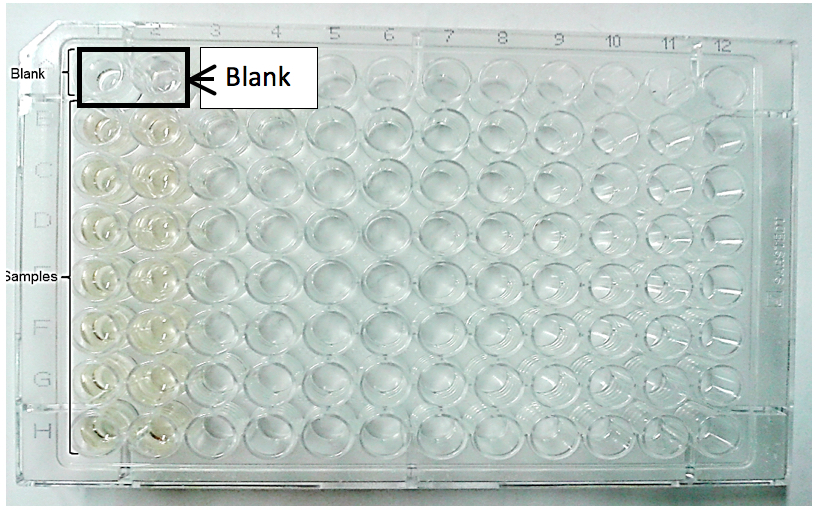
Figure 4. 96-Well NAG/MPO plate layout. Example of blank and sample distribution. All samples should be run in duplicate. For NAG, the blank is phosphate-citrate buffer and for MPO is buffer 2.
- Weigh and homogenize the tissue in ice-cold Saline-Triton buffer (100 mg tissue/1 ml buffer), using a powered high speed tissue homogenizer (Ultra-turrax or equivalent). Homogenizing procedures during enzyme isolation must be performed on ice.
Notes
- The frozen supernatants saved for the enzymatic assays must be thawed at 4 °C and kept on ice during handling.
- Use a suitable ultra-permanent ink resistant pen that can resist to the severities of freezing and thawing at liquid nitrogen temperatures to properly identify the microcentrifuge tubes that will be used for the MPO enzyme isolation procedure.
Recipes
- Phosphate buffered saline (PBS) (10x, pH 7.2)
NaCl 80 g (137 mM final concentration)
Na2HPO4 11.05 g (or Na2HPO4.12H2O 29 g) (8.01 mM final concentration)
KCl 2 g (2.7 mM final concentration)
KH2PO4 2.1 g (1.5 mM final concentration)
MilliQ water qsp 1,000 ml - PBS (1x)
PBS (10x) 50 ml
MilliQ water 450 ml - Buffer 1 (pH 4.7)
NaCl 5.84 g (0.1 M final concentration)
Na3PO4 3.12 g (0.02 M final concentration)
Na2EDTA 5.58 g (add this only after pH adjustment) (0.015 M final concentration)
MilliQ water qsp 1,000 ml - Buffer 2 (pH 5.4)
Na3PO4 7.8 g (0.05 M final concentration)
HTAB 5 g (add only after pH adjustment) (0.5% p/v final concentration)
MilliQ water qsp 1,000 ml - H2O2 (2.4 mM final concentration)
3% H2O2 7.0 μl
Buffer 2 12 ml - MPO substrate solution (1.6 mM final concentration; protect from light)
TMB 3.845 mg
DMSO 1 ml - Saline-Triton buffer (Triton X-100 0.1% v/v final concentration)
Saline solution (0.9%) - NaCl 9 g in MilliQ water qsp 1,000 ml
Triton X-100 1 ml - Phosphate-citrate buffer (pH 4.5)
Solution A - Citric acid 9.6 g in MilliQ water qsp 500 ml (0.1 M final concentration)
Solution B - Na2HPO4 17.9 g in MilliQ water qsp 500 ml (0.1 M final concentration)
Mix 200 ml of solution A and 310 ml of solution B - NAG Substrate solution (2.24 mM final concentration; protect from light)
p-nitrophenyl-N-acetyl-β-D-glucosaminide 0.767 mg
Phosphate-citrate buffer 1 ml
Vortex and then sonicate the suspension for 10 sec - Glycine buffer (0.2 M final concentration; pH 10.6)
Solution A - Glycine 6.008 g in MilliQ water qsp 100 ml (0.8 M final concentration)
Solution B - NaCl 4.68 g in MilliQ water qsp 100 ml (0.8 M final concentration)
Solution C - NaOH 3.2 g in MilliQ water qsp 100 ml (0.8 M final concentration)
Mix solutions A, B and C
Acknowledgments
This work was supported by Conselho Nacional de Pesquisa/CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES, Fundação de Amparo à Pesquisa de Minas Gerais/FAPEMIG, and Pró-reitoria de Graduação PROGRAD-UFMG, Brazil. CFM holds a PROBIC-FAPEMIG Scientific Initiation scholarship. PCV holds a CAPES PhD scholarship. MSF holds a PROGRAD-UFMG undergraduate scholarship. LSB holds a CNPq Research Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Maria Cecilia Campos Canesso and Thiago Bruno Rezende de Castro for technical support during the setting of the assays.
References
- Bailey, P. J. (1988). Sponge implants as models. Methods Enzymol 162: 327-334.
- Canesso, M. C., Vieira, A. T., Castro, T. B., Schirmer, B. G., Cisalpino, D., Martins, F. S., Rachid, M. A., Nicoli, J. R., Teixeira, M. M. and Barcelos, L. S. (2014). Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol 193(10): 5171-5180.
- Moreira, C. F., Cassini-Vieira, P., da Silva, M. F. and Barcelos, L. S. (2015). Skin wound healing model - excisional wounding and assessment of lesion area. Bio-protocol 5(22): e1661.
- Kuebler, W. M., Abels, C., Schuerer, L. and Goetz, A. E. (1996). Measurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assay. Int J Microcirc Clin Exp 16(2): 89-97.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cassini-Vieira, P., Moreira, C. F., da Silva, M. F. and Barcelos, L. D. S. (2015). Estimation of Wound Tissue Neutrophil and Macrophage Accumulation by Measuring Myeloperoxidase (MPO) and N-Acetyl-β-D-glucosaminidase (NAG) Activities. Bio-protocol 5(22): e1662. DOI: 10.21769/BioProtoc.1662.
Category
Immunology > Immune cell function > Macrophage
Immunology > Immune cell function > Neutrophil
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











