- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Skin Wound Healing Model - Excisional Wounding and Assessment of Lesion Area
Published: Vol 5, Iss 22, Nov 20, 2015 DOI: 10.21769/BioProtoc.1661 Views: 24740
Reviewed by: Ivan ZanoniAchille BroggiMarco Di Gioia

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2531 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2553 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3993 Views
Abstract
This protocol focus on the most common surgical mouse model of cutaneous excisional wound healing used to study the cellular and molecular pathways involved in wound repair and regeneration as well as in translational applications such as the evaluation of new therapeutic modalities. This model allows the monitoring of the wound closure and the tissue collection for histological and molecular analyses. Briefly, full skin thickness excisional wounds are created on the dorsum of the mouse as the excision extends through the panniculosus carnosus. Wounds larger and minor diameters are then regularly measured and wound closure rate is calculated based on wound area relative to the original size.
Materials and Reagents
- 5 mm diameter circular biopsy punch (ABC, catalog number: 0418 )
- 10% Ketamine Hydrochloride (Agropecuária Tarumã, catalog number: 8565 )
- 2% Xylazine hydrochloride (Syntec)
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P0662 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: 255793 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- Saline solution (0.9% sodium chloride injectable solutio) (Equiplex)
- dH2O
- 70% alcohol (see Recipes)
- Phosphate buffered saline (PBS) (pH 7.2, 10x) (see Recipes)
- Phosphate buffered saline (PBS) (pH 7.2, 1x) (see Recipes)
Equipment
- Digital caliper (Mitutoyo, catalog number: 573-661 )
- Hair removal machine (Wahl and Toshico)
Procedure
- Creation of skin excisional wounds in mice
- Anesthetize mice as approved in your animal study proposal. We suggest intraperitoneally injecting a mixture of ketamine 100 mg/kg and xylazine 10 mg/kg, diluted in 100 µl of saline solution. It gives about twenty minutes of surgical anesthesia.
- Remove hair from the mice dorsum by using a hair removal machine.
- Prepare the surgical site with an appropriate skin disinfectant. We suggest 70% alcohol.
- Fold and raise the dorsal skin cranially and caudally at midline using the index fingers and thumbs to form a sandwiched skinfold (Figure 1A). Then, place the animal in a lateral position and press down the 5-mm diameter sterile biopsy punch to completely remove the two skin layers (Figure 1B) and create symmetrical full-thickness excisional wounds (Figure 1C).
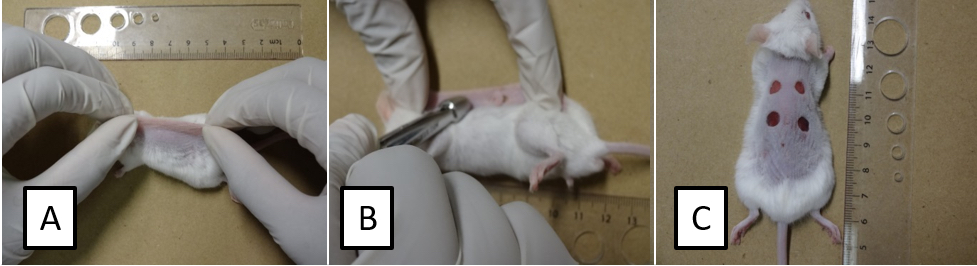
Figure 1. Stepwise skin excisional wounding surgery. Fold and raise the dorsal skin cranially and caudally at midline to form a sandwiched skinfold (A). Place the animal in a lateral position and punch through the folded skin (B) to create symmetrical full-thickness excisional wounds (C). - After surgery, move the animal to a warm area and monitor its recovery from anesthesia. Return the fully recovered animal to its routine housing. Cage individually.
- Anesthetize mice as approved in your animal study proposal. We suggest intraperitoneally injecting a mixture of ketamine 100 mg/kg and xylazine 10 mg/kg, diluted in 100 µl of saline solution. It gives about twenty minutes of surgical anesthesia.
- Wound closure monitoring after surgery
- Animals are anesthetized as described in step A1 and the wound area is assessed every 2-3 days until full closure of the lesions.
- By using a digital caliper, measure the larger and minor diameters of the lesions (Figure 2) and determine the wound area by applying the following formula: (diameter A/2) x (diameter B/2) x π.
- Calculate the percentage of wound closure as follow: [(area of original wound-area of actual wound)/area of original wound] x 100.
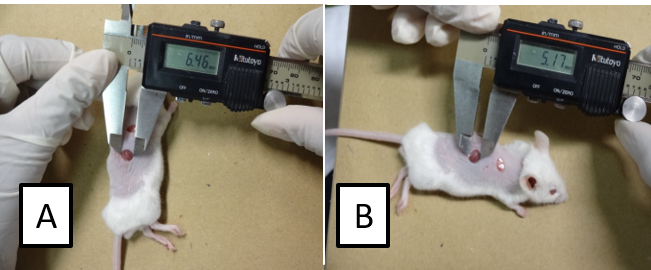
Figure 2. Assessment of the wound area. Measure the larger (A) and the minor (B) diameter of the lesion. Calculate the area as follows: (diameter A/2) x (diameter B/2) x π.
- Animals are anesthetized as described in step A1 and the wound area is assessed every 2-3 days until full closure of the lesions.
Notes
- Create wounds with approximately 5 mm apart.
- Analgesic drugs use depends on the experimental design and the approval by local committee for ethical conduct in the care and use of animals in scientific research. Preemptive use of analgesic drugs is recommended. We suggest Buprenorphine 0.05 mg/kg subcutaneously every 12 h for the first 24 h post-surgery.
- The actual surface area of a full-thickness cutaneous excisional wound becomes slightly larger than its initial size.
- For accuracy and reproducibility of the experiments, the area measurement must be performed by a single person throughout the experimental time-course.
- If the skin is going to be used for histological analysis, after harvesting the wound tissue, lay it on a sandwiched piece of filter paper to avoid tissue folding during the fixation process and place it in a histological cassette for fixation in 10% formalin solution for 24 h. Then, follow for conventional paraffin embedding processing. Otherwise, place the harvested tissue in 1.5 ml microtube and then immediately dip in liquid nitrogen to store the frozen tissue for further tissue analyzes (e.g., biochemical analysis, ELISA, qPCR, etc.). Please, see Cassini-Vieira et al. (2015) for details on how to harvesting wound tissues for analysis.
Recipes
- 70% alcohol solution
700 ml of absolute alcohol
Complete the volume with distillate water to 1,000 ml - Phosphate buffered saline (PBS) (pH 7.2, 10x)
NaCl 80 g
Na2HPO4 11.05 g or Na2HPO4.12H2O 29 g
KCl 2 g
KH2PO4 2.1 g
Add dH2O to 1,000 ml - Phosphate buffered saline (PBS) (pH 7.2, 1x)
50 ml PBS (10x)
450 ml dH2O
Acknowledgments
This work was supported by Conselho Nacional de Pesquisa/CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES, Fundação de Amparo à Pesquisa de Minas Gerais/FAPEMIG, and Pró-reitoria de Graduação PROGRAD-UFMG, Brazil. CFM holds a PROBIC-FAPEMIG Scientific Initiation scholarship. PCV holds a CAPES PhD scholarship. MSF holds a PROGRAD-UFMG undergraduate scholarship. LSB holds a CNPq Research Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Canesso, M. C., Vieira, A. T., Castro, T. B., Schirmer, B. G., Cisalpino, D., Martins, F. S., Rachid, M. A., Nicoli, J. R., Teixeira, M. M. and Barcelos, L. S. (2014). Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol 193(10): 5171-5180.
- Cassini-Vieira, P., Moreira, C. F., da Silva, M. F. and Barcelos, L. S. (2015). Estimation of wound tissue neutrophil and macrophage accumulation by measuring myeloperoxidase (MPO) and N-Acetyl-β-D-glucosaminidase (NAG) activities. Bio-protocol 5(22): e1662.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Moreira, C. F., Cassini-Vieira, P., da Silva, M. F. and Barcelos, L. D. S. (2015). Skin Wound Healing Model - Excisional Wounding and Assessment of Lesion Area. Bio-protocol 5(22): e1661. DOI: 10.21769/BioProtoc.1661.
Category
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











