- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of CD8 and CD4 T Cell Responses in Mouse Lungs
Published: Vol 4, Iss 6, Mar 20, 2014 DOI: 10.21769/BioProtoc.1083 Views: 17605
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2531 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3993 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3551 Views
Abstract
Study of the adaptive immune response to a viral challenge in an animal model often includes analysis of the T cell response. Here we discuss in detail the methods that are used to characterize the CD8 and CD4 T cell response following viral challenge in the lung.
Materials and Reagents
- Mice (NCI) (BALB/c or C57BL/6, 4 weeks to 18+ months)
- Isofluorane (USP inhalation vapour, liquid) ( NDC: 57319-559-06 )
- Dulbecco’s modified eagle medium high glucose (DMEM) (Life Technologies, Gibco®, catalog number: 11965092 )
- Ketaset III ketamine HCl injection (USP 100 mg/ml) (DEA Schedule II Drug) (NDC: 0856-2013-01 )
- 100 mg/ml AnaSed injection xylazine (Lloyd Laboratories, NADA number: 139-236 )
- 0.9% sodium chloride irrigation (USP) (Baxter, catalog number: 2F7124 )
- 1x Dulbecco’s phosphate buffered saline (DPBS) (Life Technologies, Gibco®, catalog number: 14190-144 )
- Collagenase D (Roche Diagnostics, catalog number: 11088882001 )
- DNase I (Roche Diagnostics, catalog number: 10104159001 )
- Hank’s balanced salt solution (HBSS) (Life Technologies, Gibco®, catalog number: 14025 )
- L-Glutamine (200 mM) (Life Technologies, Gibco®, catalog number: 25030-081 )
- Hepes (1 M) (Life Technologies, Gibco®, catalog number: 15630-080 )
- Penicillin streptomycin (Life Technologies, Gibco®, catalog number: 15140-122 )
- RPMI medium 1640 (Life Technologies, Gibco®, catalog number: 11875-093 )
- 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250-100ML )
- BD Golgi PlugTM (BD, catalog number: 554722 )
- Normal rat serum (Life Technologies, InvitrogenTM, catalog number: 10710C )
- FITC Anti-mouse CD8a clone 53-6.7 (0.5 mg/ml) (BD, catalog number: 553031 )
- PerCP-cyanine5.5 Anti-mouse CD4 clone RM4-5 (eBioscience, catalog number: 45-0042-82 )
- Fetal bovine serum (FBS) (Atlanta Biologicals, catalog number: S11150 )
- Sodium azide (AMRESCO, catalog number: 0639 )
- BD Cytofix/CytopermTM fixation and permeabilization solution (BD, catalog number: 554722)
- BD Perm/WashTM buffer (BD, catalog number: 554722)
- APC Anti-mouse IFNγ clone XMG1.2 (eBioscience, catalog number: 17-7311-82 )
- Ketamine solution (see Recipes)
- Digestion buffer (see Recipes)
- RP10 (see Recipes)
- FACS buffer (see Recipes)
- Cell surface staining mixture (see Recipes)
- Intracellular staining mixture (see Recipes)
Equipment
- Dessicator (Narang Medical, catalog number: P37.1517P )
- Precision glide needle (25 G x 5/8) (BD, catalog number: 305122 )
- Gauze sponges (4 x 4 inch) (Pro Advantage® by NDC, catalog number: P157118 )
- 1 ml syringe (BD, catalog number: 309659 )
- 3 ml syringe (BD, catalog number: 309657 )
- Cell strainer (70 µm nylon) (BD, catalog number: 352350 )
- Tissue culture dishes (60 x 15 mm) (BD Biosciences, Falcon®, catalog number: 353002 )
- 96 well cell culture cluster (round bottom with Lid) (Corning, Costar®, catalog number: 3799 )
- 5 ml polystyrene round bottom tube (BD Biosciences, Falcon®, catalog number: 352054 )
- Absorbent pads (Covidien, catalog number: 949 )
- 10 ml syringe (BD, catalog number: 309604 )
- 12 well cell culture cluster (flat bottom with lid) (Corning, Costar®, catalog number: 3513 )
- 1 ml graduate transfer pipette (Research Products International, catalog number: 147501-1S )
- 15 ml screw cap tube conical (Sarstedt AG, catalog number: 62.554.002 )
- 50 ml screw cap tube conical (Sarstedt AG, catalog number: 62.547.004 )
- Biosafety hood
- Spray bottle with 70% ethanol
- Surgical scissors
- Polystyrene foam
- Pipetman p10
- Pipetman p200
- Pipetman p1000
- CO2 incubator
- Pipet aid
- Small metal weighing spatula
- Tweezers
- Vortexer
- Shaker rotisserie
- Refrigerated tabletop centrifuge
- Hemocytometer
- Flow cytometer
Procedure
- Infection
- Infect mice as described in Fett et al. (2014).
- Infect mice as described in Fett et al. (2014).
- Perfusion and removal of lungs
- Euthanize mice by intraperitoneal injection of 300 µl of ketamine/xylazine solution. Secure the mouse in the palm of the hand and hold it horizontally, then insert the needle at a shallow angle tangential to the mouse and slowly dispense ketamine/xylazine into the peritoneal cavity.
- When the mouse is fully anesthetized, immobilize it on a piece of polystyrene foam by pinning each limb with a 25 G x 5/8 needle.
- Use a spray bottle filled with 70% ethanol to wet the fur of the mouse.
- Cut open the abdominal and thoracic cavities with scissors by first making an incision from the lower abdominal to the throat of the animal. Opening the abdominal cavity exposes the underside of the diaphragm. Cut through the diaphragm with scissors and then remove the ribcage, fully exposing the heart and lungs.
- Fill a 10 ml syringe with sterile PBS and attach a 25 G x 5/8 needle. Insert the needle into the left ventricle of the heart and smoothly dispense PBS into the heart. While slowly dispensing PBS into the heart, use a tweezers to dissect the right atria away from the heart allowing blood to drain from circulation. Perfuse the general circulation with 5-7 ml of PBS. Remove the needle from the left ventricle and insert it into the right ventricle to more directly perfuse the lungs with the remaining 3-5 ml of PBS. Lungs without significant disease should perfuse easily.
- Cut the heart away from the lungs and then remove the lungs from the thoracic cavity after cutting the trachea and any remaining connective tissue.
- Place the lungs into the well of a 12 well tissue culture plate. If multiple lungs are to be harvested place lungs in PBS, store plate on ice and then transfer to a clean dry well immediately before processing.
- Mince the lungs into very fine pieces using a scissors.
- Transfer minced lung with a 1 ml transfer pipette to 5 ml of digestion buffer in a 15 ml conical tube.
- Place tubes on a shaker rotisserie and gently rotate at room temperature for 30 min.
- Place a 70 µm cell strainer into a 60 x 15 mm tissue culture dish.
- Transfer digestion buffer and lung tissue to the cell strainer using a 1 ml transfer pipette. Transfer some of the strained buffer into a 50 ml conical tube leaving enough liquid in the tissue culture dish so that the lung tissue on the screen of the cell strainer stays wet. With the flat end of a plunger from a 3 ml syringe gently press and dissociate tissue through strainer using small light movements. Intermittently flush tissue with PBS to move cells through the strainer. Process tissue until there is only connective tissue remaining on the strainer and transfer the remaining digestion buffer to the 50 ml conical tube.
- Spin down lung cells in 50 ml conical tubes for 10 min at 1,300 rpm at room temperature in a bucket tabletop centrifuge.
- Pour off supernatant and resuspend cells in 5 ml of RP10.
- Euthanize mice by intraperitoneal injection of 300 µl of ketamine/xylazine solution. Secure the mouse in the palm of the hand and hold it horizontally, then insert the needle at a shallow angle tangential to the mouse and slowly dispense ketamine/xylazine into the peritoneal cavity.
- Peptide stimulation and cell staining
- Count cells using a hemocytometer. Normal lung cell counts can vary from 3 million to 30 million or more depending on the stage and severity of the infection.
- Spin down cells for 5 min at 1,300 rpm.
- Resuspend cells in RP10 at 1 million cells per 100 µl.
- In a 96 well round bottom plate, add 100 µl of cells to 100 µl of RP10 containing Golgi Plug at a concentration of 2 µg/ml for a final concentration of 1 µg/ml. Wells should contain designated amount of peptide. Make sure to include wells for a no peptide control. In our SARS-CoV-infected samples, we routinely stimulate cells with peptide at a concentration of 1 µM. The SARS-CoV-specific peptides S366 (CD8; HNYKYRYL) and N353 (CD4; VNFNFNGL) are used to identify virus-specific CD8 and CD4 T cells by intracellular cytokine staining (ICS) for interferon gamma (IFN-γ). These two epitopes are H-2d-resticted and therefore recognized in BALB/c mice.
- Incubate cells at 37 °C, 5% CO2 for 5 h. Cells can then be stored overnight at 4 °C.
- Count cells using a hemocytometer. Normal lung cell counts can vary from 3 million to 30 million or more depending on the stage and severity of the infection.
- Cell surface staining
- Spin down cells at 1,000 rpm for 5 min at 4 °C and then remove the supernatant by dumping the plate into a waste bin containing 10% bleach (when working with SARS-CoV) or rapidly flipping the plate onto paper towels.
- Add 100 µl of cell surface staining mixture per well. Mix thoroughly by pipeting each well up and down several times.
- Incubate in the dark for 15 min at 4 °C.
- Add 100 µl of FACS buffer and spin down cells at 1,300 rpm for 5 min at 4 °C. Remove supernatant. Add 100 µl of fixation and permeabilization solution; mix thoroughly by pipeting up and down several times.
- Incubate in the dark for 30 min at 4 °C.
- If working with SARS-CoV, cells can be transferred to a new 96 well round bottom plate and then removed from the BSL3.
- Using a pipetman, pass cells in fixation and permeablilization solution through a 70 µm cell strainer into FACS tubes.
- Resuspend cells in 2 ml of BD Perm/WashTM buffer, then spin down cells at 1,300 rpm for 5 min at 4 °C. Remove supernatant.
- Spin down cells at 1,000 rpm for 5 min at 4 °C and then remove the supernatant by dumping the plate into a waste bin containing 10% bleach (when working with SARS-CoV) or rapidly flipping the plate onto paper towels.
- Intracellular staining
- Resuspend cells in 100 µl of intracellular staining mixture per sample. Mix thoroughly by lightly vortexing tubes.
- Incubate in the dark for 30 min.
- Resuspend cells in at least 1 ml of FACS buffer then spin down cells at 1,300 rpm for 5 min at 4 °C. Remove supernatant.
- Resuspend cells in 100 µl FACS buffer.
- Acquire FACS data using a flow cytometer.
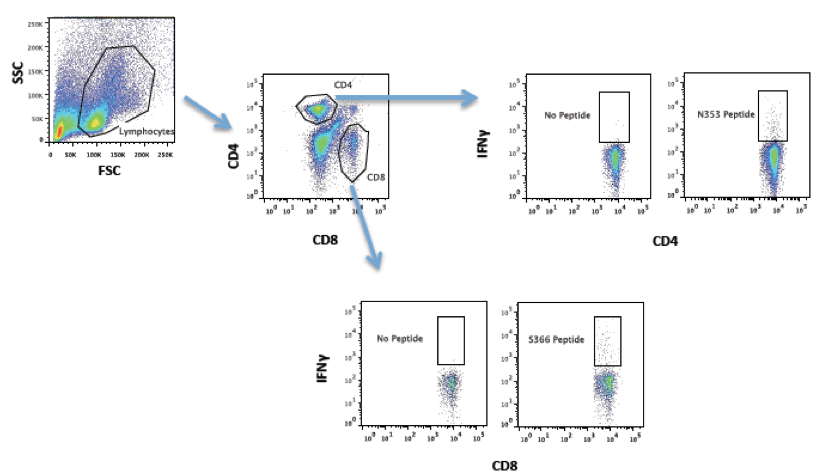
Figure 1. Strategy for identifying SARS-CoV specific T cell responses. Mice were infected with mouse-adapted SARS-CoV. After 6-8 days, mice were euthanized, lungs removed and single cell suspensions prepared for flow cytometry. Left hand panel shows mononuclear cell gate. CD4 and CD8 T cells were identified and assessed for IFN-γ expression after stimulation with peptides.
- Resuspend cells in 100 µl of intracellular staining mixture per sample. Mix thoroughly by lightly vortexing tubes.
Recipes
- Ketamine solution (10 ml)
1.75 ml ketamine
0.25 ml xylazine
8 ml 0.9% sodium chloride irrigation
Stored at room temperature
- Digestion buffer (100 ml)
100 mg collagenase D
10 mg DNase I
2 ml FBS
1 ml glutamine
2.5 ml Hepes
5 ml penicillin streptomycin
89.5 ml HBSS
Stored at -20 °C
- RP10 (500 ml)
50 ml FBS
500 µl 2-Mercaptoethanol (from 50 mM stock made in H2O)
450 ml RPMI medium 1640
Stored at 4 °C
- FACS buffer (500 ml)
1.7 ml sodium azide (from 30% stock made in H2O)
20 ml FBS
480 ml PBS
Stored at 4 °C
- Cell surface staining mixture (100 µl = 1 sample)
1 µl rat serum
0.25 µl CD4 PerCP-Cyanine5.5
0.5 µl CD8a FITC
100 µl FACS buffer
- Intracellular staining mixture (100 µl = 1 sample)
0.3 µl IFNγ APC
100 µl BD Perm/WashTM buffer
Acknowledgments
This work was supported by grants from the National Institutes of Health (PO1AI060699, RO1AI091322). The protocol described herein was based on the following manuscript: Zhao et al. (2010).
References
- Fett, C., DeDiego, M. L., Regla-Nava, J. A., Enjuanes, L. and Perlman, S. (2013). Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein. J Virol 87(12): 6551-6559.
- Fett, C., Zhao, J. C. and Perlman, S. (2014). Virus infection and titration of SARS-CoV in mouse lung. Bio-protocol 4(6): e1084.
- Netland, J., DeDiego, M. L., Zhao, J., Fett, C., Alvarez, E., Nieto-Torres, J. L., Enjuanes, L. and Perlman, S. (2010). Immunization with an attenuated severe acute respiratory syndrome coronavirus deleted in E protein protects against lethal respiratory disease. Virology 399(1): 120-128.
- Zhao, J., Zhao, J. and Perlman, S. (2010). T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol 84(18): 9318-9325.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Fett, C., Zhao, J. and Perlman, S. (2014). Measurement of CD8 and CD4 T Cell Responses in Mouse Lungs. Bio-protocol 4(6): e1083. DOI: 10.21769/BioProtoc.1083.
Category
Immunology > Animal model > Mouse
Immunology > Immune cell function > Lymphocyte
Microbiology > Microbe-host interactions > Virus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








