Advanced Search
Fluorescence-based ion transport assays using Proteoliposomes
Last updated date: Sep 20, 2025 Views: 242 Forks: 0
Abstract
Divalent metal ion transporters are conserved across all domains of life and play essential roles in diverse processes such as manganese acquisition during nutritional immunity in bacteria [1] and iron homeostasis in higher eukaryotes [2], [3]. Traditional techniques, such as electrophysiological assays, are often unsuitable due to the slow kinetics of many membrane transporters. To overcome these limitations and to investigate both the activity and ion selectivity of transporters, also including those normally expressed intracellularly, we have developed a fluorescence-based transport assay using purified proteins. This in vitro assay uses encapsulated fluorophores to monitor the movement of divalent metal ions (e.g., Mn²⁺, Ca²⁺, Mg²⁺) or protons across liposomal membranes reconstituted with purified transporter proteins. This approach provides detailed functional insight that complements structural and cellular data.
Key features
- Enables detection of real-time transport activity through precise timing of reagent addition and controlled generation of membrane potential.
- Compatible with a wide range of divalent metal ions and ionophores, allowing adaptation to various transporter types.
- Applicable to transporters that are naturally expressed in intracellular compartments
- Allows detailed analysis of transporter function in a defined lipid environment.
- Allows for testing effects of binders and compounds.
Keywords
Fluorescence-based in vitro transport assay, metal ion transporters, reconstituted proteoliposomes, Calcein, Fura-2, Magnesium Green, ACMA
This protocol is used in [4], [5], [6], [7], [8], [9]:
Nat Commun. (2017), DOI: 10.1038/ncomms14033
Elife (2019), DOI: 10.7554/eLife.51913
Elife (2022), DOI: 10.7554/eLife.74589
Elife (2023), DOI: 10.7554/eLife.83053
Elife (2023), DOI: 10.7554/eLife.85641
Nat Commun. (2025), DOI: 10.1038/s41467-024-54705-0
Graphical overview
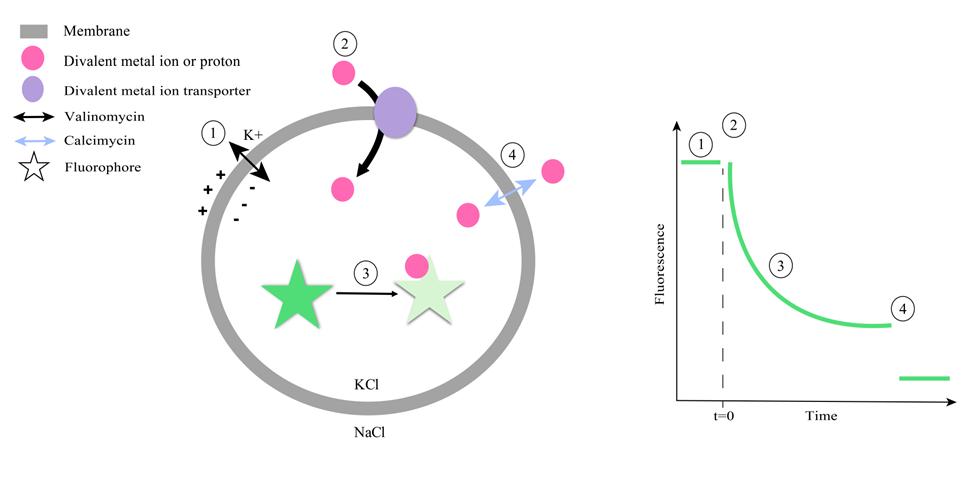
Setup and time flow of the fluorescence-based ion transport assay.
Background
This protocol allows characterization of transport kinetics and substrate specificity of purified transporters. These types of assays are also well suited for structure-function analyses of wild type and mutant proteins as well as for testing inhibitors and other regulatory factors [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Traditionally, electrophysiological approaches such as voltage-clamp recordings [16], [17], [18], [19] or radioactive tracer [20] uptake assays have been used to study metal ion transport. While valuable, these methods can be limited by technical complexity, low temporal resolution, or incompatibility with certain transporter types. Fluorescent metal ion indicators have also been applied in live-cell studies [21], but controlling the ionic environment and membrane potential in such systems can be difficult. The fluorescence-based in vitro transport assay presented here overcomes many of these limitations by reconstituting purified transporters into artificial liposomes loaded with fluorophores, which allows monitoring ion transport under controlled conditions, including defined ionic composition, membrane potential, and lipid environment. One important limitation of the protocol is that transporter orientation within liposomes cannot be precisely controlled, leading to a mix of outside-out and inside-out populations. Although some membrane proteins may insert preferentially in one direction under certain conditions [22], [23], [24], [25], this was not the case for divalent metal ion transporters investigated in our studies [5]. Transporter orientation needs to be taken into account when interpreting kinetic data with respect to the used membrane potential and when exploring the effects of inhibitors or regulatory factors, as access to the relevant binding site depends on the transporter’s orientation.
Materials and reagents
Biological materials
1. Membrane transporter reconstituted into liposomes
2. Liposomes devoid of proteins (negative control)
The reconstitution protocol is described in the articles listed above (“This protocol is used in”) and in a Nature protocols article [23].
Reagents
1. Calcein (Thermo Fisher Scientific, catalog number: C481)
2. Fura-2 (ThermoFischer Scientific, catalog number: F1200)
3. Magnesium Green (ThermoFischer Scientific, catalog number: M3733)
4. Valinomycin (ThermoFischer Scientific, catalog number: V1644)
5. Cacimycin (ThermoFischer Scientific, catalog number: V1644)
6. Ionomycin (ThermoFischer Scientific, catalog number: I24222)
7. ACMA (ThermoFischer Scientific, catalog number: A1324)
8. CCCP (ThermoFischer Scientific, catalog number: C2759)
9. Sodium chloride (Sigma, catalog number: 71380)
10. Potassium chloride (Sigma, catalog number: 746436)
11. HEPES (Sigma, catalog number: H3375)
12. Manganese chloride (Fluka, catalog number: 31422)
13. Magnesium chloride (Fluka, catalog number: 63065)
14. Calcium chloride (Fluka, catalog number: 223506)
Solutions
1. Buffer IN (see Recipes)
2. Buffer IN with Fluorophore (see Recipes)
3. Buffer OUT (see Recipes)
4. Valinomycin, Calcimycin, Ionomycin and CCCP Stock Solutions
5. ACMA Stock Solution
6. Metal Ion Stock solutions
Recipes
1. Buffer IN
Reagent | Final concentration | Quantity or Volume |
HEPES pH 7.0, 1M | 20 mM | 1 mL |
KCl, 2M | 100 mM | 2.5 mL |
Total | n/a | 50 mL with deionized water |
2. Buffer IN with Fluorophore
Reagent | Final concentration | Quantity or Volume |
HEPES pH 7.0, 1M | 20 mM | 0.1 mL |
KCl, 2M | 100 mM | 0.25 mL |
Fluorophore |
| Respective quantity to reach 250 µM Calcein, 100 µM Fura-2 or 400 µM Magnesium Green |
Total | n/a | 5 mL with deionized water |
For Calcein it is possible to prepare a stock solution of higher concentration that can be stored for several weeks at 4 °C. Due to reduced stability and high costs for Fura-2 and Magnesium Green, it is advisable to use freshly prepared solutions and smaller volumes.
3. Buffer OUT
Reagent | Final concentration | Quantity or Volume |
HEPES pH 7.0, 1M | 20 mM | 1 mL |
NaCl, 5M | 100 mM | 1 mL |
Total | n/a | 50 mL with deionized water |
4. Valinomycin, Calcimycin, Ionomycin and CCCP Stock Solutions
Reagent | Final concentration | Quantity or Volume |
Ionophore solution 500 µM (see below) | 10 µM | 200 µL |
100% EtOH |
| 9.8 mL |
Total |
| 10 mL |
A 5 mM stock solution in 100% EtOH can be stored at -80 °C for long term (several months).
The 10 µM stock solution in 100% EtOH used in the experiment can be stored at -20 °C for several weeks.
Preparation of the 500 µM stock solution: dilute the 5 mM stock 1:10 with 100% EtOH to reach a concentration of 500 µM.
5. ACMA Stock Solution
Reagent | Final concentration | Quantity or Volume |
ACMA solution 10 mM in 80% EtOH | 1 mM | 10 µL |
20% EtOH |
| 90 µL |
Total |
| 100 µL |
A 10 mM stock solution in 80% EtOH can be stored at 4 °C for long term (several months). ACMA is not completely soluble in this mixture, therefore mix shortly before use.
The 1 mM stock in 20% EtOH used in the experiment is soluble and can be stored at 4 °C for several months.
6. Metal Ion Stock solutions
Reagent | Final concentration | Quantity or Volume |
MnCl₂·4H₂O (MW = 197.91 g/ml) | 0.5M | 0.49g |
Total | n/a | 5 mL with deionized water |
Reagent | Final concentration | Quantity or Volume |
MgCl₂·6H₂O (MW = 203.30 g/mol) | 0.5M | 0.51g |
Total | n/a | 5 mL with deionized water |
Reagent | Final concentration | Quantity or Volume |
CaCl₂·2H₂O (MW = 147.01 g/mol) | 0.5M | 0.37g |
Total | n/a | 5 mL with deionized water |
These stock solutions are diluted to reach the desired concentrations needed in the experiments. For each experiment, freshly prepare metal ion stock solutions.
Laboratory supplies
1. Microfuge Tube Polypropylene (Beckman Coulter, catalog number: 357448)
2. 96-well black walled microplates (Thermofischer Scientific, catalog number: M33089)
3. FIOLAX test glass tubes with beaded rim 12 x 100mm (Duran Group, catalog number: 261101105)
Equipment
1. Optima MAX-XP Ultracentrifuge or related (Beckman Coulter, product number: 393315)
2. Rotor TLA 100.3 (Beckman Coulter, catalog number: 349490)
3. Diameter Delrin Tube Adapter 11mm (Beckman Coulter, catalog number: 355919)
4. Avestin Extruder kit (Sigma, catalog number: Z373400)
5. Polycarbonate filters 400 nm (Sigma, catalog number: Z373435)
6. Tecan Infinite or Spark or related (Tecan)
7. Bath sonicator model G112SPIT with power supply G112SPIG with transformer 2422-530-05415K (Laboratory Supplies Co. Inc, Hicksville N.Y. and Filec, Netherlands)
Procedure
General note: Unilamellar vesicles can be prepared using different techniques depending on the experimental objective. For metal ion transport assays, extrusion has proven to be the most suitable method, as it produces liposomes with a uniform and defined size, which is important for reproducibility and quantitative analysis (Section A). In contrast, for proton transport measurements, sensitivity is a limiting factor. The sensitivity of pH-sensitive fluorescence signals depends on the internal volume of the liposomes. Smaller vesicles enable more rapid and detectable pH changes upon proton flux. Therefore, sonication was found to be the preferred method for generating small unilamellar vesicles in this context (Section B).
A. Preparation of liposomes for metal ion transport assay by extrusion
1. Add respective amount of proteoliposomes to 400 µl Buffer IN with Fluorophore in a 2 mL Eppendorf tube.
Note: An advisable amount of proteoliposomes to use for one series of experiments for one day is 1 mg of liposomes.
2. Freeze the mixture in liquid nitrogen and thaw it again at room temperature. Repeat 3 times. Make sure that liposomes are warmed up to room temperature after thawing, especially before extrusion. We advise wiping off the condensate outside the tube and assessing that it is at room temperature by finger-touch at each freeze-thaw cycle. If the tube is tilted in a slight horizontal direction for freezing, the thawing process will be faster and easier to evaluate.
Note: In case of light sensitive fluorophores, protect from light using an aluminum foil.
3. Assemble the extruder and wash the extruder first with Buffer IN, then with Buffer IN with Fluorophore. Add the proteoliposomes to one side and extrude 9 to 11 times. Rinse the extruder with 500 µl Buffer IN with Fluorophore by extruding a couple of times. Add the rinsing solution to the liposomes.
Note: Extrude an uneven number of times to collect extruded liposomes from the side which was NOT used to load the extruder. Make sure that the extruder is properly washed, dried and at room temperature before each use.
Critical: After the extrusion step, always keep the liposomes above the phase transition temperature for the used lipid mixture.
4. Pellet the liposomes by centrifugation at 170’000 g and 22 °C for 25 minutes using a TLA 100.3 rotor and polypropylene tubes.
Critical: Make sure that the rotor and the tube adaptors are equilibrated to room temperature beforehand. Often rotors are kept at 4 °C and need several hours to equilibrate to room temperature. It is therefore advisable to place the rotor at room temperature already the evening before the planned experiment.
5. Carefully remove the supernatant and gently resuspend the pellet containing the liposomes in 800 µl Buffer IN. Pellet the liposomes by centrifugation as described above. We advise to cut first millimeters of the P1000 tip extremity, thus allowing for gentle resuspension of the pellet without disrupting liposomes.
6. Repeat the wash step to reach a total of two wash steps.
7. Carefully resuspend the pellet in a total volume of 40 µl Buffer IN. This is the liposome stock solution.
B. Preparation of liposomes for proton transport assay by sonication
1. Prepare a proteoliposome stock of 15 mg/ml in 5 mM HEPES pH 7.0, 100 mM KCl, 50 µM ACMA. Depending on the composition of the reconstituted proteoliposomes this can be reached in different ways. If the proteoliposome stock has sufficiently high concentration and the buffer composition is sufficiently low, this final composition can be reached by simple dilution. Alternatively, it can be reached by diluting into larger volumes of 5 mM HEPES pH 7.0, 100 mM KCl and subsequent wash steps as described in protocol A (steps 5-7). In this later case, ACMA should be added in the last resuspension step.
Note: The required buffer capacity may vary depending on the experimental goal and should be optimized accordingly. For the study of metal ion transporters in the Dutzler laboratory, HEPES buffer concentrations of 5 to 10 mM have proven to be adequate.
2. To generate small unilamellar vesicles, sonicate the proteoliposome solution until it appears optically clear.
Note: Optical clarity is easier to assess when the solution is placed in a glass tube with sufficient volume. It is therefore recommended to use a total volume of 50 µL and the glass tubes listed in the laboratory supply section. The sonication intensity can be adjusted by changing the position of the tube within the sonication bath, as energy distribution is not uniform and depends on bath geometry and water level. These parameters should be taken into account for optimization of the sonication setup. For our setup, the sonication bath was filled to about 2 cm below the rim, set to 80 V, and liposomes were sonicated for 30–60 seconds with short breaks.
Critical: After the sonication step, always keep the liposomes above the phase transition temperature for the used lipid mixture.
3. Collect all liquid to the bottom by brief centrifugation at room temperature, as after sonication, droplets may adhere to the inner wall of the tube. This is the liposome stock solution.
C. Recording of transport activity by change of fluorescence signal
1. Dilute the liposome stock solution about 100 times by adding 8 µl to 830 µl Buffer OUT. Use 100 µl for one experiment. Note: It is advisable to do series of 8 experiments in a 96-black walled microplate.
2. Use a plate reader such as the Tecan Infinite or Spark to record fluorescence. Set the device to measure as continuously as possible. For Tecan Infinite M100 we recorded fluorescence every 4 seconds for a total of up to 360 cycles (24 minutes). Fluorescence settings vary depending on the indicator:
a. Mn²⁺ detection with Calcein, use λex=492 nm; λem=518 nm
b. Ca²⁺ detection with Fura-2, use the ratio between Ca2+ bound and unbound state.
- Ca²⁺-bound Fura-2: λex=340 nm; λem=510 nm
- Ca²⁺-unbound Fura-2: (λex=380 nm; λem=510 nm
c. Mg²⁺ detection with Magnesium Green: λex=506 nm; λem=531 nm
d. Detection of pH change with ACMA: λex=412 nm; λem=482 nm
3. Perform the following steps during the measurement:
a. Baseline recording: Begin by recording 60–90 cycles (about 4 to 6 minutes) to establish a stable baseline fluorescence.
b. Initiate membrane potential: Add 1 µL of Valinomycin stock solution to generate a negative membrane potential. Continue measuring for another 20–30 cycles (about 1.5 to 2 minutes).
c. Start transport: Add 1 µL of the respective metal ion stock solution (e.g., Mn²⁺, Ca²⁺, or Mg²⁺) to initiate transport activity. Monitor for 75–270 additional cycles (about 5 to 18 minutes) depending on your experimental goal.
d. Apply positive control: Add 1 µL of the respective ionophore to validate the maximum possible fluorescence signal (Calcimycin for Mn²⁺ and Mg²⁺, Ionomycin for Ca²⁺, and CCCP for protons). Record fluorescence for another 10–20 cycles (about 1 to 1.5 minutes) after ionophore addition.
Critical: It is important to be fast in order to record as much as possible of the initial transport activity. Therefore, it is advisable to use a programmable multi-channel pipette. Aliquot the ionophore stock solutions and the metal ion stock solutions in PCR tubes (strips of 8 tubes). After pipetting 1 µl of the stock solution wash the tip once by pipetting up and down with 1.5 µl. Change the pipette tips and mix by pipetting up and down three to five times with a volume of 50 µl. Avoid air bubbles while mixing. For technical reasons, plate readers may be localized in climatized rooms with temperatures set close to 20 °C. Since these temperatures might be close to the phase transition of the used lipid mixture, it is possible that the measurements will be compromised if the sample is not measured fast enough. Although the integrity of the proteoliposomes and the quality of the measurement is controlled by the final addition of calcimycin or CCCP, we advise the use of heating block set at 22 °C to store the stock of proteoliposomes to be measured.
Note: The timing of the sequence may vary depending on the purpose of the experiment and transport properties. The sequence outlined above are suitable for the investigation of metal ion transporters studied in the Dutzler laboratory. To monitor proton transport, steps 3b and 3c were performed in reverse order, as adding valinomycin before the substrate led to a significant decrease in fluorescence due to the uncoupled proton leak.
4. Perform a linear regression in the linear range of signal change to obtain initial transport velocities. Analyse these values with respect to the substrate concentration using a Michaelis Menten equation to obtain KM and Vmax values.
5. Normalize the data to the fluorescence signal at the timepoint of substrate addition and plot the data. When using Fura-2, normalization is done by calculating the ratio F340/F380.
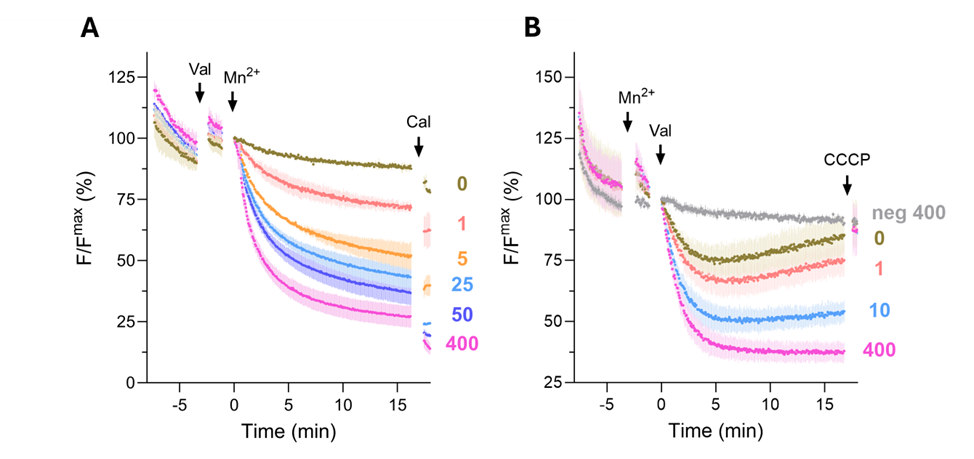
Figure 1. Transport properties of the prokaryotic divalent metal transporter EcoDMT. A) Mn2+ transport into EcoDMT proteoliposomes containing EcoDMT assayed by the quenching of the fluorophore calcein trapped inside the vesicles. B) Mn2+ coupled H+ transport into EcoDMT proteoliposomes assayed by the quenching of the fluorophore ACMA. After equilibration of the fluorescent signal, the K+ Ionophore Valinomycin (Val) and the Substrate Mn2+ were added at the indicated timepoints to establish a negative membrane potential and to start the transport. At the end, the Mn2+ Ionophore Calcimycin (A) or H+ Ionophore CCCP (B) was added as an internal positive control. In both cases, the mean of three experiments from three independent experiments is displayed. The data is normalized to the value after addition of substrate or Valinomycin (t = 0). Applied micromolar ion concentrations of Mn2+ to the outside are indicated on the right. A subset of these data is published in Ramanadane et al, 2022, in Figures 2A and 4H.
Validation of protocol
For each experimental condition, at least two independent proteoliposome reconstitutions have been analyzed, and at least three independent biological replicates were performed. The respective amount of replicates are stated in the figure legends of the research articles listed below. Positive controls included known metal ion transporters with well characterized transport properties (EcoDMT in most cases) and the addition of ionophores such as Calcimycin, Ionomycin, or CCCP to assess maximum fluorescence signals. Negative controls consisted of protein-free liposomes.
This protocol (or parts of it) has been used and validated in the following research articles:
Ehrnstorfer et al., DOI: 10.1038/nsmb.2904, Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport, Nat Struct Mol Biol., 2014, (Figure 1 panel b and c, Figure 2 panel k and I, supplementary Figure 6)
Ehrnstorfer et al., DOI: 10.1038/ncomms14033, Structural and mechanistic basis of proton-coupled metal ion transport in the SLC11/NRAMP family, Nat Commun., 2017, (Figure 1, Figure 4 panel c and c, Figure 6, supplementary Figure 1 panels b to e)
Manatschal et al., DOI: 10.7554/eLife.51913, Mechanistic basis of the inhibition of SLC11/NRAMP-mediated metal ion transport by bis-isothiourea substituted compounds, eLife, 2019, (Figure 2 panel a, Figure 2 – figure supplement 1, Figure 4 – figure supplement 2)
Ramanadane et al., DOI: 10.7554/eLife.74589, Structural and functional properties of a magnesium transporter of the SLC11/NRAMP family, eLife, 2022, (Figure 2, Figure 2 – figure supplement 1, Figure 2 panels a to f, Figure 4, Figure 8 panels b to i)
Lehmann et al., DOI: 10.7554/eLife.83053, Structures of ferroportin in complex with its specific inhibitor vamifeport, eLife, 2023, (Figure 1 panel a, Figure 1 – figure supplement 1 panel c)
Ramanadane et al., DOI: 10.7554/eLife.85641, Structural and functional properties of a plant NRAMP-related aluminum transporter, eLife, 2023, (Figure 1 panel a to g and j to l, Figure 1 – figure supplement 2 panel c to k, Figure 5 panel b to e and g to k)
Liziczai et al., DOI: 10.1038/s41467-024-54705-0, Structural basis for metal ion transport by the human SLC11 proteins DMT1 and NRAMP1, Nat Commun., 2025, (Figure 1, Figure 2, Figure 6 panel b to e, supplementary Figure 1, supplementary Figure 2, supplementary Figure 11, supplementary Table 1, supplementary Table 2)
General notes and troubleshooting
General notes
In general, the assay is highly sensitive to numerous variables (e.g., temperature, timing between extrusion and measurement, and other small procedural differences). Therefore, it is essential to always include appropriate positive and negative controls, such as an established transporter or wild-type protein, and protein-free liposomes, to ensure reliable interpretation of results.
In our assay, the negative control was prepared in parallel to the reconstitution of the protein of interest using the exact same protocol with the difference that size exclusion chromatography buffer devoid of protein was used. This approach allows monitoring of complete detergent removal, which is critical for ensuring liposome tightness. Incomplete detergent removal during liposome preparation can lead to leaky vesicles, even in the absence of protein. Thus, verifying the integrity of protein-free liposomes is essential to confirm that any observed transport activity is indeed protein-mediated.
It is advisable to perform all experimental steps on the same day, as unilamellar proteoliposomes are only stable for a limited time before multilamellar structures begin to form. As the fluorescent lamp varies between instruments, we advise to adjust the Tecan gain for each fluorophore at the aforementioned concentration and keep it the same for all measurements.
Troubleshooting
Problem 1: Fluorescence level remains rather high even after addition of the positive control (Calcimycin, Ionomycin or CCCP) .
Possible cause: Presence of multilamellar liposomes.
Solution: Perform a longer extrusion to ensure unilamellarity (generally 17-25 cylces), minimize the time between extrusion and the start of the measurement and keep the temperature above the phase transition temperature of the lipid mixture. In case of liposome preparation by sonication, repeat the sonication step.
Problem 2: Noisy baseline or increased noise after addition of ionophore or metal ion solution.
Possible cause: Presence of an air bubble in the measurement well.
Solution: Gently tap the plate on the table to dislodge bubbles before starting the measurement, or carefully remove bubbles using a fine needle.
Acknowledgments
The described transport assays were initialized by Ines A. Ehrnstorfer and Eric R. Geertsma using the procaryotic transporter ScaDMT [26] and further optimized for different metal ion transporters and applications by Cristina Manatschal, Karthik Ramanadane, Márton Liziczai and Elena F. Lehmann. Raimund Dutzler contributed to the conceptualization and troubleshooting of the assays and acquired the funding. The manuscript was conceptualized, written, reviewed and edited by Karthik Ramanadane and Cristina Manatschal. The work was funded by the Swiss National Science Foundation (NCCR TransCure) granted to Raimund Dutzler.
Competing interests
The authors declare no conflicts of interest.
References
[1] L. J. Juttukonda and E. P. Skaar, “Manganese homeostasis and utilization in pathogenic bacteria,” Mol. Microbiol., vol. 97, no. 2, pp. 216–228, July 2015, doi: 10.1111/mmi.13034.
[2] N. Montalbetti, A. Simonin, G. Kovacs, and M. A. Hediger, “Mammalian iron transporters: families SLC11 and SLC40,” Mol. Aspects Med., vol. 34, no. 2–3, pp. 270–287, 2013, doi: 10.1016/j.mam.2013.01.002.
[3] N. C. Andrews, “Metal transporters and disease,” Curr. Opin. Chem. Biol., vol. 6, no. 2, pp. 181–186, Apr. 2002, doi: 10.1016/S1367-5931(02)00307-1.
[4] C. Manatschal, J. Pujol-Giménez, M. Poirier, J.-L. Reymond, M. A. Hediger, and R. Dutzler, “Mechanistic basis of the inhibition of SLC11/NRAMP-mediated metal ion transport by bis-isothiourea substituted compounds,” eLife, vol. 8, p. e51913, Dec. 2019, doi: 10.7554/eLife.51913.
[5] I. A. Ehrnstorfer, C. Manatschal, F. M. Arnold, J. Laederach, and R. Dutzler, “Structural and mechanistic basis of proton-coupled metal ion transport in the SLC11/NRAMP family,” Nat. Commun., vol. 8, p. 14033, Jan. 2017, doi: 10.1038/ncomms14033.
[6] K. Ramanadane, M. S. Straub, R. Dutzler, and C. Manatschal, “Structural and functional properties of a magnesium transporter of the SLC11/NRAMP family,” eLife, vol. 11, p. e74589, Jan. 2022, doi: 10.7554/eLife.74589.
[7] K. Ramanadane et al., “Structural and functional properties of a plant NRAMP-related aluminum transporter,” eLife, vol. 12, p. e85641, Apr. 2023, doi: 10.7554/eLife.85641.
[8] E. F. Lehmann et al., “Structures of ferroportin in complex with its specific inhibitor vamifeport,” eLife, vol. 12, p. e83053, Mar. 2023, doi: 10.7554/eLife.83053.
[9] M. Liziczai, A. Fuchs, C. Manatschal, and R. Dutzler, “Structural basis for metal ion transport by the human SLC11 proteins DMT1 and NRAMP1,” Nat. Commun., vol. 16, no. 1, p. 761, Jan. 2025, doi: 10.1038/s41467-024-54705-0.
[10] “Conserved methionine dictates substrate preference in Nramp-family divalent metal transporters | PNAS.” Accessed: Aug. 13, 2025. [Online]. Available: https://www.pnas.org/doi/10.1073/pnas.1607734113
[11] A. T. Bozzi et al., “Crystal Structure and Conformational Change Mechanism of a Bacterial Nramp-Family Divalent Metal Transporter,” Struct. Lond. Engl. 1993, vol. 24, no. 12, pp. 2102–2114, Dec. 2016, doi: 10.1016/j.str.2016.09.017.
[12] “Structures in multiple conformations reveal distinct transition metal and proton pathways in an Nramp transporter | eLife.” Accessed: Aug. 13, 2025. [Online]. Available: https://elifesciences.org/articles/41124
[13] B. At, M. Al, B. Bc, and G. R, “Transmembrane helix 6b links proton and metal release pathways and drives conformational change in an Nramp-family transition metal transporter,” J. Biol. Chem., vol. 295, no. 5, Jan. 2020, doi: 10.1074/jbc.RA119.011336.
[14] A. T. Bozzi, L. B. Bane, C. M. Zimanyi, and R. Gaudet, “Unique structural features in an Nramp metal transporter impart substrate-specific proton cotransport and a kinetic bias to favor import,” J. Gen. Physiol., vol. 151, no. 12, pp. 1413–1429, Dec. 2019, doi: 10.1085/jgp.201912428.
[15] “High-resolution structures with bound Mn2+ and Cd2+ map the metal import pathway in an Nramp transporter | eLife.” Accessed: Aug. 13, 2025. [Online]. Available: https://elifesciences.org/articles/84006
[16] H. Gunshin et al., “Cloning and characterization of a mammalian proton-coupled metal-ion transporter,” Nature, vol. 388, no. 6641, pp. 482–488, July 1997, doi: 10.1038/41343.
[17] B. Mackenzie, M. L. Ujwal, M. H. Chang, M. F. Romero, and M. A. Hediger, “Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes,” Pflugers Arch., vol. 451, no. 4, pp. 544–558, Jan. 2006, doi: 10.1007/s00424-005-1494-3.
[18] B. Mackenzie, H. Takanaga, N. Hubert, A. Rolfs, and M. A. Hediger, “Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1),” Biochem. J., vol. 403, no. 1, pp. 59–69, Apr. 2007, doi: 10.1042/BJ20061290.
[19] J. Pujol-Giménez, M. A. Hediger, and G. Gyimesi, “A novel proton transfer mechanism in the SLC11 family of divalent metal ion transporters,” Sci. Rep., vol. 7, no. 1, p. 6194, July 2017, doi: 10.1038/s41598-017-06446-y.
[20] C. J. Mitchell, A. Shawki, T. Ganz, E. Nemeth, and B. Mackenzie, “Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc,” Am. J. Physiol.-Cell Physiol., vol. 306, no. 5, pp. C450–C459, Mar. 2014, doi: 10.1152/ajpcell.00348.2013.
[21] N. Montalbetti, A. Simonin, M. G. Dalghi, G. Kovacs, and M. A. Hediger, “Development and Validation of a Fast and Homogeneous Cell-Based Fluorescence Screening Assay for Divalent Metal Transporter 1 (DMT1/SLC11A2) Using the FLIPR Tetra,” SLAS Discov., vol. 19, no. 6, pp. 900–908, July 2014, doi: 10.1177/1087057114521663.
[22] J. Garcia-Celma, A. Szydelko, and R. Dutzler, “Functional characterization of a ClC transporter by solid-supported membrane electrophysiology,” J. Gen. Physiol., vol. 141, no. 4, pp. 479–491, Apr. 2013, doi: 10.1085/jgp.201210927.
[23] E. R. Geertsma, N. a. B. Nik Mahmood, G. K. Schuurman-Wolters, and B. Poolman, “Membrane reconstitution of ABC transporters and assays of translocator function,” Nat. Protoc., vol. 3, no. 2, pp. 256–266, Feb. 2008, doi: 10.1038/nprot.2007.519.
[24] J. L. Rigaud, M. T. Paternostre, and A. Bluzat, “Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin,” Biochemistry, vol. 27, no. 8, pp. 2677–2688, Apr. 1988, doi: 10.1021/bi00408a007.
[25] G. D. Eytan, “Use of liposomes for reconstitution of biological functions,” Biochim. Biophys. Acta BBA - Rev. Biomembr., vol. 694, no. 2, pp. 185–202, Oct. 1982, doi: 10.1016/0304-4157(82)90024-7.
[26] I. A. Ehrnstorfer, E. R. Geertsma, E. Pardon, J. Steyaert, and R. Dutzler, “Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport,” Nat. Struct. Mol. Biol., vol. 21, no. 11, pp. 990–996, Nov. 2014, doi: 10.1038/nsmb.2904.
Related files
 250920_Transport-Assay_Bioprotocols_Ramanadane_Manatschal.docx
250920_Transport-Assay_Bioprotocols_Ramanadane_Manatschal.docx - Ramanadane, K and Manatschal, C(2025). Fluorescence-based ion transport assays using Proteoliposomes. Bio-protocol Preprint. bio-protocol.org/prep2859.
- Ramanadane, K., Straub, M. S., Dutzler, R. and Manatschal, C.(2022). Structural and functional properties of a magnesium transporter of the SLC11/NRAMP family. eLife. DOI: 10.7554/eLife.74589
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
