Advanced Search
Measurement of vaccine-induced IgA antibody mucosal samples
Last updated date: Sep 8, 2022 Views: 547 Forks: 0
human iga measurements via msd
1.0 PRINCIPLE
This document describes detection of SARS-CoV-2 IgA in nasal samples using an MSD immunoassay for the specific IgA and an ELISA for the detection of total IgA in human nasal samples to normalize samples.
2.0 REAGENTS – SPECIAL SUPPLIES AND EQUIPMENT
2.1 EQUIPMENT
2.1.1 Biological safety cabinet (BSC).
2.1.2 Pipette-Aid.
2.1.3 Vortex.
2.1.4 Refrigerator capable of the following parameters: 2 - 8°C.
2.1.5 Single-channel pipettes: 20 µl, 200 µl, 1000 µl.
2.1.6 Multi-channel pipettes (12): 20 µl, 200 µl, 1000 µl
2.1.7 Plate washer.
2.1.8 Plate shaker.
2.1.9 Microplate reader. Spectra Max M2.
2.2 SUPPLIES
2.2.1 Coating plate. Nunc™ 96-Well MaxiSorp™ Polystyrene Flat Bottom Non-Sterile Plates. Thermo Fisher Scientific, catalog # 439454.
2.2.2 Dilution plate. Corning 96 Well Clear Round Bottom Polypropylene Not Treated Microplate, Nonsterile. Corning, catalog # 3365.
2.2.3 Plate Sealers. VWR, catalog # 60941-120.
2.2.4 15 and 50 mL sterile centrifuge tubes.
2.2.5 Sterile bottles.
2.2.6 5, 10, and 25 mL sterile, disposable serological pipettes.
2.2.7 Sterile reservoirs.
2.2.8 20, 200, and 1000 µL sterile, filtered, disposable tips for pipettes.
2.2.9 Microcentrifuge Tubes, Polypropylene 1.5 mL.
2.3 REAGENTS
Storage and expiration per manufacturer’s instructions unless otherwise noted.
2.3.1 Capture Ab. Purified Anti-Human IgA mAb MT57. Mabtech, catalog #3860-3-1000.
2.3.2 IgA From Human Serum. Sigma-Aldrich, catalog #I4036.
2.3.3 Detection Ab. Anti-human IgA mAb MT20; ALP conjugated. Mabtech, catalog # 3860-9A-1000.
2.3.4 P-Nitrophenyl Phosphate (pNPP) substrate. Mabtech, catalog #3652-P10.
2.3.5 Dulbecco’s Phosphate-Buffered Saline (DPBS), 10X, without calcium and magnesium. Corning, catalog # 20-031-CV (or equivalent).
2.3.6 Phosphate-Buffered Saline (PBS), 1X, without calcium and magnesium. Corning, catalog # 21-040-CV (or equivalent).
2.3.7 Albumin, Bovine. Amresco, catalog #0332-100G.
2.3.8 Tween-20 (reagent grade). Amresco, catalog # 0777.
2.3.9 BupH™ Carbonate-Bicarbonate Buffer Packs. Thermo Fisher Scientific, catalog # 28382.
2.4 KITS
2.4.1 MSD V-PLEX Serology IgA Kit for COVID-19. MSD, catalog #K15361U, K15385U, or equivalent.
2.5 PREPARED BUFFERS
2.5.1 MSD - Blocker A Buffer
2.5.1.1 Add 1.25 g of MSD Blocker A to 20 ml of dH2O and 5 ml of MSD 5X Phosphate Buffer. Quantity for 1 plate.
2.5.2 MSD and ELISA - Wash Buffer and Dilution Buffer (PBST)
2.5.2.1 Add 200 mL 10X DPBS and 1 ml Tween-20 to 1800 mL H2O (final concentration 1X PBS + 0.1% Tween-20).
2.5.2.2 MSD’s provided Diluent-100 can substitute PBST as a dilution buffer in an MSD assay.
2.5.3 ELISA - Blocking Buffer (PBST + 1% BSA)
2.5.3.1 Add 0.22 g BSA to 22 mL PBST. Quantity for 1 plate.
2.6 Determination of Spike-specific IgA – MSD Assay
*This protocol was tailored for MSD V-PLEX Serology Kit SARS-CoV-2 Panel 2 IgA specificity (catalog # K15385U) but can be modified to fit other MSD COVID-19 serology kits.
2.6.1 Bring all materials and reagents provided in the MSD kit to room temperature.
2.6.2 Block the MSD plate by adding 150 µl/well of Blocker A buffer. Seal plate with an adhesive plate sealer.
2.6.3 Incubate for at least 30 minutes at room temperature on the plate shaker (low-setting). During this time, prepare the calibrators, controls, and samples in a dilution plate.
2.6.4 Using the Reference Standard-1 provided in the MSD V-plex IgA kit and dilution buffer, make a 7-point calibration curve with 4-fold serial dilution steps and a blank. Vortex the Reference Standard-1 prior to dilution. Discard any excess diluted calibrator.
2.6.5 Each control provided by the MSD V-Plex IgA kit is ready for use. Wait to add each control until ready to load the calibrator and the samples to the MSD plate (step 3.2.9).
2.6.6 Use the dilution buffer to prepare a 1:10 and 1:100 dilution for every nasal sample.
2.6.7 After blocking, wash the MSD plate 3x with PBST (300 µl/well) using a plate washer.
2.6.8 Transfer 50 µl /well of prepared calibrators, controls, and samples from the dilution plate onto the MSD plate. Seal the MSD plate.
2.6.9 Incubate for 2 hours at room temperature on a plate shaker (low-setting. During this time, prepare the detection antibody dilution.
2.6.10 Use the dilution buffer to prepare a 1X working solution of MSD IgA Detection Antibody (200X stock) in a Falcon conical tube.
2.6.11 After sample incubation, wash the MSD plate 3x with PBST (300 µl/well) using a plate washer.
2.6.12 Load 50 µl/well of 1X Detection Antibody to the MSD plate. Seal the plate.
2.6.13 Incubate for 1 hour at room temperature on the plate shaker (low-setting).
2.6.14 Wash plate 3x with PBST (300 µl/well) using a plate washer. Pat dry.
2.6.15 Add 150 µl/well of MSD Gold Reader Buffer B (ready for use).
2.6.16 Read the plate immediately on the MSD QuickPlex instrument. Do not shake the plate.
2.7 Determination of Total IgA – ELISA
2.7.1 Prepare 2 µg/mL MT57 capture Ab in 1X PBS.
2.7.2 Add 100 µl/well of 2 µg/mL MT57 capture antibody to a Nunc Maxisorb coating plate. Seal plate.
2.7.3 Incubate overnight at 2-8°C.
2.7.4 Wash plate 3x with PBST (300 µl/well) using a plate washer.
2.7.5 Block plate by adding 100 µl/well of blocking buffer. Seal plate.
2.7.6 Incubate for 1 hour at room temperature on a plate shaker (low-setting).
2.7.7 Prepare dilutions of human IgA standard with PBST in a dilution plate. Create an 8-step serial dilution with the 8th dilution as a blank. Begin the first dilution at 200 ng/ml and serially dilute down the plate 2-fold. Standards are run as a single or duplicate. The total volume per well is 0.24 mL.
2.7.8 Prepare dilutions of human nasal samples with PBST in a dilution plate. Create an 8-step serial dilution. Begin the first dilution at 1:100 and serially dilute 1:3 down the plate. Samples are run in duplicate or triplicate. The total volume per well is 0.24 mL.
2.7.9 After 1 hour of blocking, wash the plate 3x with PBST.
2.7.10 Transfer 100 µl/well of samples and standards from the dilution plate to the coated plate following the plate layout.
2.7.11 Incubate for 2 hours at room temperature on the plate shaker (low-setting).
2.7.12 10 min prior to the end of incubation, prepare a 1:1000 dilution of MT20-ALP detection Ab in blocking buffer.
2.7.13 After the 2 hour incubation, wash 3x with PBST.
2.7.14 Add 100 µl/well of 1:1000 detection Ab.
2.7.15 Incubate for 1 hour at room temperature on the plate shaker (low-setting).
2.7.16 Wash 3x with PBST.
2.7.17 Add 100 µl/well of pNPP substrate.
2.7.18 Incubate for 1 hour at room temperature in the dark on the plate shaker (low-setting).
2.7.19 At the 1 hour mark, measure the optical density (405 nm) in an microplate reader.
Hamster IgA U-PLEX MSD Protocol
(1) SulfoTag the Detection Antibody
Materials
- Rabbit anti-hamster IgA unconjugated, stock 100 ug/mL, Brookwood Biomedical cat#sab3001a, lot#002
- Sulfotag kit - MSD cat#R91AO-1
- Amicon Ultra 0.5 mL Centrifugal Filters -Millipore Sigma, cat#UFC500396, 0.5 mL sample volume, 3000 MWCO https://www.emdmillipore.com/US/en/product/Amicon-Ultra-0.5-Centrifugal-Filter-Unit,MM_NF-UFC500396#anchor_BRO
Protocol
https://www.mesoscale.com/~/media/files/product%20inserts/msd%20sulfo%20tag%20nhs%20ester.pdf
*instructions for a 150 nmol kit to conjugate 1 mg of Ig.
*Using a 20:1 challenge ratio (ratio can be altered. See notes section of MSD’s provided material)
Pre-Conjugation Procedure:
- Equilibrate the protein to be conjugated to RT.
- Prepare a 1-2 mg/mL solution of the protein to be conjugated using the Conjugation Buffer.
- Use the Amicon 0.5 mL centrifugal units. Protocol: https://www.emdmillipore.com/US/en/product/Amicon-Ultra-0.5-Centrifugal-Filter-Unit,MM_NF-UFC500396#anchor_BRO
- Protein in preservatives (sodium azide or EDTA) or in buffers with primary amines (Tris, glycine) and glycerol must be removed by buffer-exchange using the supplied Zeba Spin Desalting Columns or the Amicon device.
- Filter the protein using the 0.2 µm filter.
- Measure the concentration of the protein solution from an OD280 absorbance or with a colorimetric protein concentration assay.
- Calculate the amount of Sulfo-Tag NHS-Ester stock solution required using the formula below:
*use a challenge ratio of 20
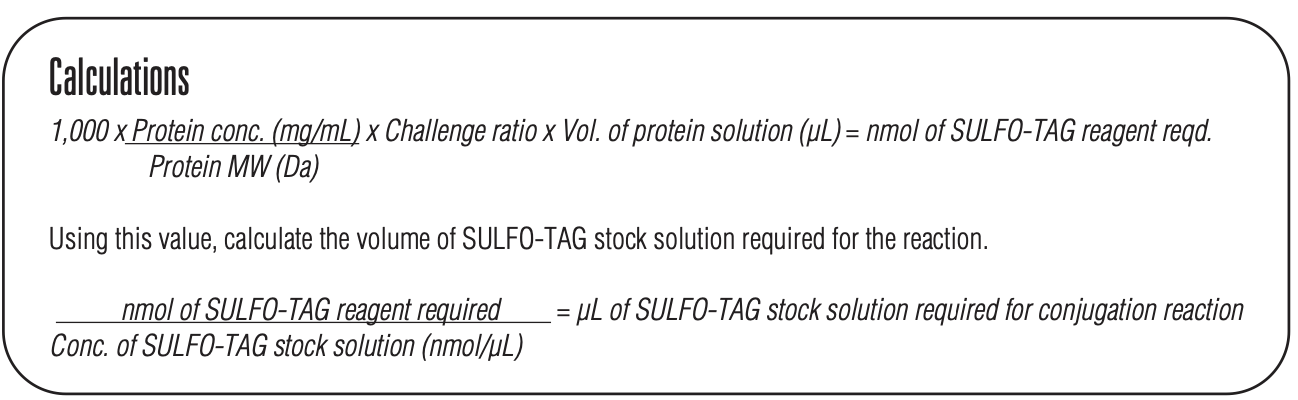

Conjugation Procedure:
- Gently tap the Sulfo-Tag NHS-Ester vial or spin for 1 min at 1,000 x g to bring lyophilized material to the bottom.
- Reconstitute the vial containing 150 nmol Sulfo-Tag NHS-Ester with 50 µL of cold distilled water to generate a 3 nmol/µL stock solution. Vortex. Keep on ice for up to 10 min prior to use.
- For conjugating <100 µg protein – add 100 µL of Conjugation Buffer to the 3 nmol/µL stock solution prepared above. This will make a 1 nmol/µL solution of Sulfo-Tag NHS-Ester.
- Add the calculated volume of Sulfo-Tag stock solution to the protein solution. Vortex. Incubate for 2 hours in the dark at RT. Discard any remaining Sulfo-Tag stock solution. Note the final volume of your tagged antibody.
Post-Conjugation Procedure:
1. Based on the volume of the tagged antibody, determine the size of Zeba column you need.
2. Remove the Zeba Spin Desalting Column’s bottom closure & loosen the cap. Place the column in a Eppendorf tube.
3. Centrifuge & discard the storage solution buffer it came in (See table for g and minutes).
4. Wash the column 3x with MSD Conjugation Storage Buffer. Centrifuge & remove wash (see table).
5. Add the Tagged Antibody to the spin column in a drop-wise manner. Centrifuge the column in a new Eppendorf tube. The purified Sulfo-Tag conjugated protein will be present in the eluate. Keep the collection tube and discard the column.
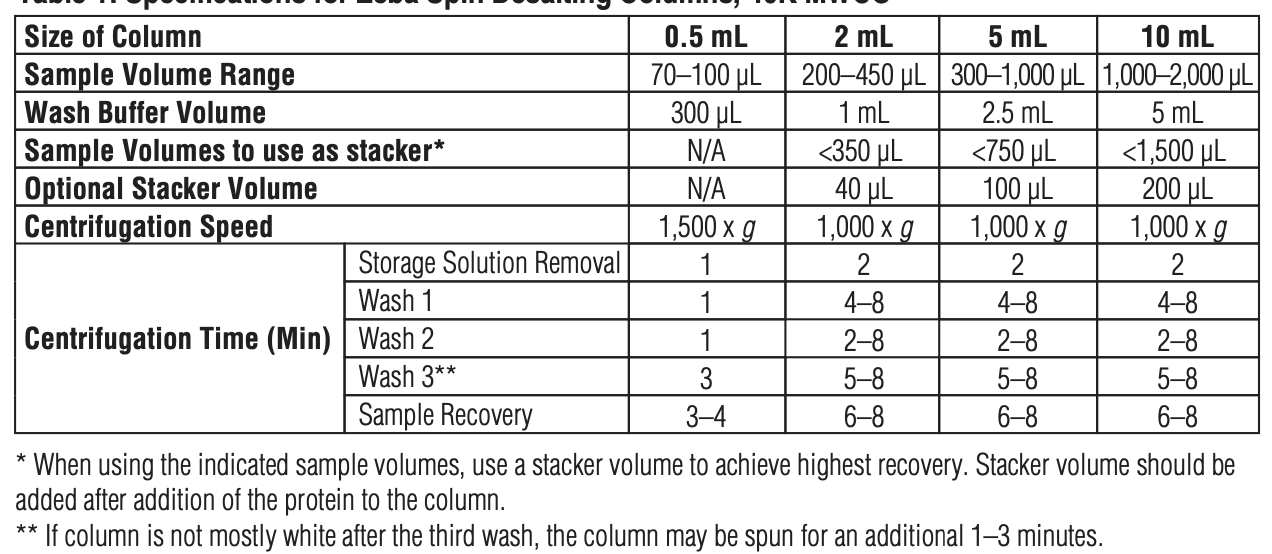
6. Filter the conjugated protein using the 0.2 µm filter.
7. Determine the molar protein concentration of the purified conjugated protein using a colorimetric protein assay (BCA, Bradford, or Lowry). SEE protocol below. Do NOT use an OD280 absorbance reading.
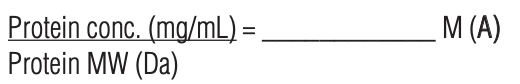
8. Measure the absorbance of the conjugated protein at 455 nm using a spectrophotometer. Divide the measured value by the pathlength in cm. Then divide by the extinction coefficient of the label (15,400 M-1cm-1) to obtain the MSD Sulfo-Tag label concentration in moles/per liter.
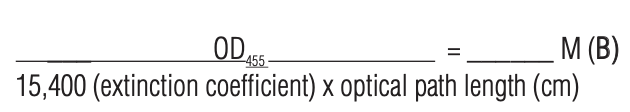
9. Determine the Sulfo-tag label to protein ratio.
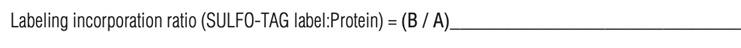
10. Store Sulfo-tag conjugated proteins in dark amber vials. Antibodies are stable for 2 years at 2-8˚C in conjugate storage buffer.
(2) BCA Assay – determine concentration of sulfo-tagged detection Ab
Materials
- Pierce BCA Protein Assay Kit, Thermo Scientific ref#23227
Protocol:
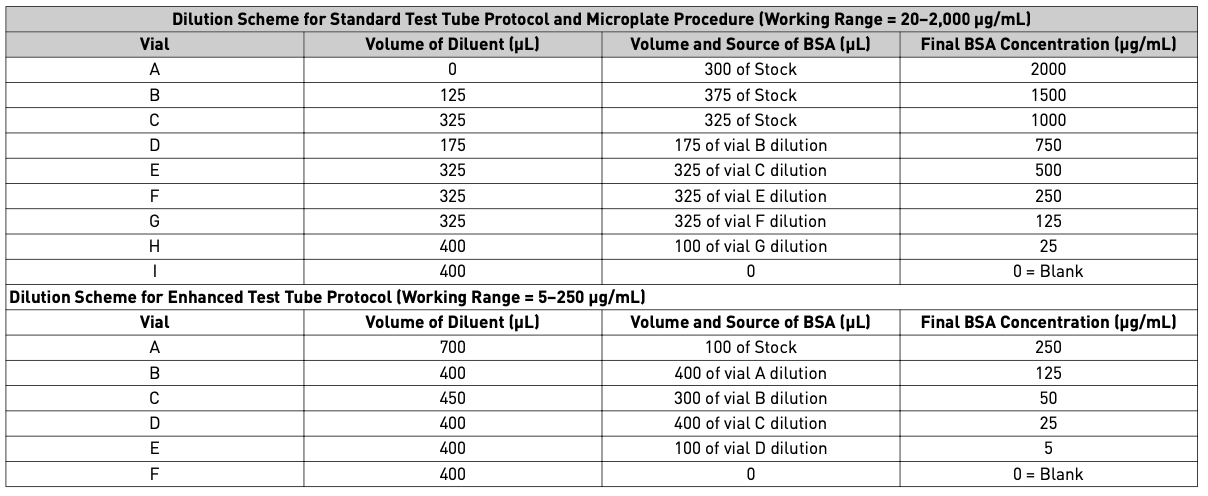
Prepare diluted BSA standards
- Dilute the contents of 1 BSA ampule (starting 2 mg/mL) to prepare protein standards. Use the table below based on the working range. There will be sufficient volume for 3x replications at each dilution level.
Prepare the BCA working reagent (WR)
- Determine the total volume of WR required:
(# standards + # unknowns) × (# replicates) × (volume of WR per sample*) = total volume WR required
*200ul of WR required for each sample in a microplate - Prepare WR = Reagent A to Reagent B ratio 50:1
WR is stable for several days at RT
Microplate procedure (sample to WR ratio = 1:8)
- Pipette 25 µL of each standard or unknown sample replicate into a microplate well (working range = 20-2000 µg/mL)
- If sample size is limited, 10 µL of each unknown sample and standard can be used (sample to WR ratio = 1:20). However, the working range of the assay in this case is be limited to 125-2000 µg/mL.
- Add 200 µL of the WR to each well and mix plate thoroughly on a plate shaker for 30 seconds.
- Cover plate and incubate at 37 ˚C for 30 minutes.
- Cool plate to RT. Measure the absorbance at or near 562 nm on a plate reader.
Read
- Set spectrophotometer to 562 nm and measure the absorbance of all samples within 10 minutes.
- Subtract the reading of the Blank from the readouts of the standard and unknown samples.
- Prepare a standard curve by plotting the average Blank-corrected 562 nm measurement for each standard vs its concentration in µg/mL.
- Use the standard curve to determine the protein concentration of each unknown sample.
(3) Biotinylate Coating Protein
Materials:
- EZ-Link Sulfo-NHS-LC-Biotin, Thermo Fisher cat#A39257 https://www.thermofisher.com/order/catalog/product/A39257?SID=srch-hj-A39257
- 1xPBS
- Zeba Dye and Biotin Removal Spin Columns 2 mL, Thermo Fisher cat#A44299 https://www.thermofisher.com/order/catalog/product/A44299
- Ultrapure water, Teknova cat#W3335
Protocol:
Procedure for Biotinylating Proteins in Solution
A. Calculations
Note – labeling reactions with dilute solutions require a greater fold molar excess of biotin. Using a 20-fold molar excess of biotin to label 1-10 mg antibody (in 0.5-2 mL) resulted in 4-6 biotin groups per antibody molecule. Using a 50-fold molar excess of biotin to label 50-200 µg antibody (in 200-700 µL) resulted in 1-3 biotin groups per antibody molecule. Adjust if necessary.
1. Calculate millimoles of biotin reagent to add to the reaction for a 50-fold molar excess:
(mL protein) ( (mg protein)/(mL protein) ) ( (mmol protein)/(mg protein) ) ( (50 mmol biotin)/(mmol protein) ) = mmol biotin
2. Calculate microliters of 10 mM biotin reagent solution (step B.3) to add to the reaction:
(mmol biotin)( (1,000,000 µL)/L ) ( L/(10 mmol) ) = µL biotin
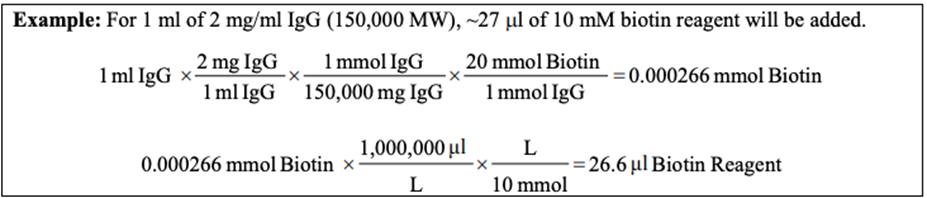
*Note: IgG is 150 kDa = 150,000 Da = 150,000 g/mol
B. Biotin Labeling Reaction
- Remove vial of Sulfo-NHS-LC-Biotin from freezer and equilibrate to RT before opening in step 3.
- Prepare protein in PBS. Proteins in Tris or other amine-containing buffers must be buffer exchanged into a suitable buffer.
- Immediately before use, prepare a 10 mM solution of biotin reagent:
- Add 180 µL of ultrapure water to the 1 mg vial. 0.321
- Add the appropriate volume of 10 mM biotin reagent to the protein solution. Discard any unused biotin reagent as it hydrolyzes over time.
- Incubate reaction on ice for 2 hours or at RT for 30 minutes. (reaction can go over)
- Purify the labeled protein to remove non-reacted biotin using desalting or dialysis.
- Count how many Zeba Spin Desalting columns needed to achieve recovery (0.5 mL column size: 70-100 µL sample, 2 mL column size: 200-450 µL sample, 5 mL column size: 300-1000 µL sample)
- Twist off bottom plug of the Zeba column and loosen cap. Place column in a collection tube.
- Spin at 1,500 x g for 1 minute to remove storage buffer. Discard the flow -through.
- Wash the column 3 times by adding 1 mL of buffer solution similar to what the protein solution is in. Spin at 1,500 x g for 1-3 minutes, discarding the flow-through each time.
- Add the sample 1 drop at a time to the center of the resin. Centrifuge at 1,500 x g for 4 minutes. Collect the flow-through and store.
- If the Thermo Scientific™ Pierce™ Biotin Quantitation Kit (HABA assay; Product No. 28005) will be performed to determine the level of biotin incorporation, the protein first must be desalted or dialyzed to remove non-reacted biotin.
(4) MSD U-PLEX protocol
Materials
- Dilution Buffer: MSD Diluent-100
- PBST (0.05% Tween-20, 1X PBS)
- 3% MSD Blocker B in MSD Diluent-100
- MSD U-PLEX kit
- Sulfo-Tagged Detection Ab
- Biotinylated proteins
- Samples
Protocol
https://www.mesoscale.com/~/media/files/product%20inserts/u-plex%20development%20pack.pdf
- U-Plex unique linker to each biotinylated proteins. Record the identity of each linker number.
Dilute each biotinylated protein in dilution buffer for a final concentration of 66 nM in a total volume 200 ul.
Add 200ul of the 66 nM biotinylated protein to 300 µL of its assigned linker (use a different linker for each biotinylated protein). Vortex. Incubate at room temperature for 30 minutes.
Add 200 µL of Stop Solution (provided by MSD). Vortex at room temperature for 30 minutes.
Each individual U-PLEX coupled protein can be stored up to 7 days at 2-8 ˚C. - Prepare Multiplex Coating Solution
Combine all linked proteins - Pool 600 µL of each U-PLEX coupled proteins into a 15 mL conical tube.
Bring the solution up to 6 mL (volume for 1 plate) with Stop Solution to result in a final 1X concentration per linked protein. Vortex.
The multiplex coating solution can be stored for up to 7 days at 4 ˚C. - Coat U-Plex Plate
Add 50 µL of the multiplex coating solution to each well. Seal plate with adhesive plate and incubate with shaking at room temperature for 1 hour or coat the day before & leave 2-8˚C. - Block the plate
Wash plate 3x with > 150 µL/well of PBST.
Add 150 µL of blocking solution to each well, seal, and incubate for 1 hour at room temperature with shaking. - Prepare sample dilutions
Dilute sample in PBST in a low-protein binding polypropylene plate. - Add the ferret serum or nasal wash sample
Wash blocked plate 3x with > 150 µL/well of PBST.
Dispense 50 µL of each sample into the MSD plate as per the plate layout. Seal the plate with an adhesive plate seal and incubate for 1 hour with shaking at room temperature. - Prepare Detection Antibody
Dilute the Sulfo-Tagged Ab in dilution buffer (recommend 1 µg/mL). - Add of Detection Antibody
Wash plate 3x with > 150 µL/well of PBST.
Add 50 µL of detection antibody into each well of the MSD plate as per plate layout.
Seal the plate and incubate for 1 hour with shaking at room temperature. - Read Plate
Wash plate 3x with > 150 µL/well of PBST.
Add 150 µL/well of 1X Read Buffer B.
Read plate immediately using MSD QuickPlex instrument.
- Langel, S and Tucker, S(2022). Measurement of vaccine-induced IgA antibody mucosal samples. Bio-protocol Preprint. bio-protocol.org/prep1914.
- Langel, S. N., Johnson, S., Martinez, C. I., Tedjakusuma, S. N., Peinovich, N., Dora, E. G., Kuehl, P. J., Irshad, H., Barrett, E. G., Werts, A. D. and Tucker, S. N.(2022). Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Science Translational Medicine 14(658). DOI: 10.1126/scitranslmed.abn6868
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link
