Advanced Search
Baseline Separation of Six Hexose Phosphate Isomers by Liquid Chromatography-Mass Spectrometry from Tissues
Last updated date: Feb 11, 2022 Views: 673 Forks: 0
Baseline Separation of Six Hexose Phosphate Isomers by Liquid Chromatography-Mass Spectrometry from Tissues
Yusong Wang1, Xueying Wang1, Lina Xu1, Yupei Jiao1 and Xiaohui Liu1 *
1School of Life Sciences, Tsinghua University, China; National Protein Science Facility (Beijing), Tsinghua University, China.
*For correspondence: Xiaohui2013@mail.tsinghua.edu.cn
[Abstract] Hexose phosphates are important intracellular metabolites and involved in many biosynthesis pathways. Quantitative analysis is a prerequisite for understanding the role of sugar phosphates in regulating energetic metabolism. Although various methods have been proposed, such as enzymatic assay, GC-MS and CE-MS, analysis of sugar phosphates is still challenging because of the structural similarity of various isomers. Our work describes a method based on liquid chromatography-mass spectrometry (LC-MS) for separation of hexose phosphates with tributylamine as an ion pairing reagent. In this protocol, six structural isomeric hexose phosphates were successfully resolved.
Keywords: Hexose phosphates, Isomers, LC-MS, Glucose-6-phosphate, Fructose-6-phosphate, Glucose-1-phosphate, Fructose-1-phosphate, Galactose-1-phosphate, Mannose-6-phosphate
[Background] Hexose phosphates are key intermediates in many biosynthesis pathways including glycolysis, glycogenolysis, oxidative pentose phosphate pathway and so on. The isomeric hexose phosphates are interconvertible through enzymatic reaction, but each of them plays a unqiue role in organism. For example, the interconverstion of glucose-6-phosphate and glucose-1-phsophate is mediated by phosphoglucomutase in glycogenolysis (Adeva-Andany et al., 2016). The dysregulation of hexose-phosphate metabolism is strongly correlated with various diseases. Galactose-1-phosphate uridyltransferase (GALT) deficiency, which leads to elevated galactose-1-phosphate (Gal1P) in patients, is the most common cause of galactosemia. The concentration of Gal1P in erythrocytes is also a sensitive index of dietary control (Timson., 2019). Modification of mannose 6-phosphate is a well-characterized sorting signal for lysosomal enzymes (Kornfeld., 1986; Von Figura et al., 1986). Therefore, establishing a reliable approach to quantitively analyze isomeric hexose phosphates is of key importance to further investigate these metabolic disorders.
Traditionally, sugar phosphates in vivo have been determined using enzymatic assays with high sensivitity and specificity (Gibon et al.,2002; Zhu et al.,2009). However, simultaneous analysis of multiple metabolites could not be achieved. Mass spectrometry is a technique that allows analysis of thousands of molecues at one time, especially when couples with chromatographic separation. Previous research has determined th levels of sugar phosphates without discriminating isomeric forms (Bajad et al., 2006; Preinerstorfer et al., 2010; Wamelink et al., 2005; Vizán et al., 2007; Kiefer et al., 2008 ). Incorporation of chromatographic techniques benefits efficient separation of isomeric molecules. Therefore, GC-MS, CE-MS, and LC-MS has been extensively applied for metabolite analysis. GC-MS provides predominant separation efficiency via derivatization of nonvolatile compounds (Gullberg et al., 2004; Okahashi et al., 2019). CE-MS has been reported to analyze glucose-1-phosphate, glucose-6-phosphate and fructose-1-phosphate. However, wide application of CE-MS analysis is challenging due to poor reproducibility (Soga et al., 2009). By far, LC-MS is more popular to study polar metabolites including hexose phosphates.
However, hexose phosphates separate by conventional reversed-phase HPLC with some problems due to their highly hydrophilic nature(Hinterwirth et al., 2010). Herein, we propose a methodology that provides sufficient chromatographic selectivity for the separation of six isomeric hexose phosphate isomers including glucose-1-phosphate (G1P), mannose-6-phosphate (M6P), fructose-6-phosphate (F6P), fructose-1-phosphate (F6P), glucose-6-phosphate (G6P) and galactose-6-phosphate (Gal6P).
Materials and Reagents
1. D-Glucose-6-phosphate sodium salt (Sigma-Aldrich, catalog number: G7879)
2. D-Fructose-6-phosphate disodium salt (Sigma-Aldrich, catalog number: F3627)
3. D-Fructose-1-phosphate disodium salt (Santa Cruz, catalog number: sc-285345)
4. Glucose-1-phosphate dipotassium salt dihydrate(Santa Cruz, catalog number: sc-487156)
5. D-Galactose-1-phosphate dipotassium salt Pentahydrate(Santa Cruz, catalog number: sc-203795)
6. D-Mannose-6-phosphate, Disodium Salt(Santa Cruz, catalog number: sc-203019)
7. Water, optima(LC-MS grade) (Thermo Fisher Scientific, catalog number: 51140 )
8. Methonal, optima (LC-MS grade) (Thermo Fisher Scientific, catalog number: A456-4)
9. Tributylamine(TBA) (Sigma-Aldrich, catalog number: 90781-10ML)
10. Acetic Acid, Glacial (Fisher Scientific, catalog number: A113-50)
11. NaCl (Sigma-Aldrich, catalog number: S5886-500G)
12. Mobile Phase Eluent A (see Recipes)
13. Mobile Phase Eluent B (see Recipes)
14. Saline(see Recipes)
Equipment
1. Homogenizer (Fluko, model: F6)
2. Vacuum Concentrator Systems (Labconco, model: CentriVap)
3. Column: ACQUITY UPLC BEH C18 Column, 130Å, 1.7 µm, 2.1 mm X 100 mm(Waters, catalog number: 186002352)
4. LC: Dionex Ultimate 3000 UPLC system (Thermo Fisher Scientific)
5. MS: TSQ Quantiva Ultra triple-quadrupole mass spectrometer (Thermo Fisher Scientific)
Software
1.Xcalibur software (Thermo Fisher Scientific,web address:https://www.thermofisher.cn/order/catalog/product/OPTON-30965)
Procedure
A.Tissue collection
Note: Mouse liver is taken as an example here.
1. At the designated period of time, sacrifice the mouse by cervical dislocation.
2. Immediately, open the abdomen of mouse, avoid damaging the internal organs, find and then isolate the liver. The dissection can be performed on tissue papers spread on ice.
3. Transfer liver to a clean dish, rinse by saline three times. Cut the liver to pieces at about 50mg and put them in a Freezing Tube, flash-freeze in liquid nitrogen, and store at -80 °C before use.
B.Metabolites extraction
1. Add 500uL of 80%(v/v) LC-MS grade methanol (pre‐chilled at ‐80°C) to 50mg tissue in a 1.5mL/2mL tube.
Note: Add of 80% (v/v) methanol proportionally according to the weight of tissue to make the same concentration.
2. Grind for 1‐2min with Homogenizer on dry ice in the tube, vortex for 1min (<=1min) at 4‐8°C and incubate at ‐80°C for 2h or overnight.
3. Centrifuge at 14,000g (or the highest speed) for 20min at 4°C and transfer the same volume of supernatant to a new tube.
Note: Please AVOID touching the bottom pellet when transferring supernatant.
4. Lyophilize to pellet using Vacuum Concentrator Systems (room temperature).
5. Store the dried samples in -80°C freezer.
C.Sample preparation for liquid chromatography-mass spectrometry (LC-MS)
1.Resuspend dried samples in 100 µl 100% water before LC-MS analysis.
2.Incubate at 4°C for 10 min.
3.Centrifuge samples at 14,000 × g at 4°C for 20 min.
4.Transfer supernatant into LC-MS sample vials and load into the autosampler.
D.Hexose phosphates seperation by LC-MS/MS
1.Liquid chromatography
a.Column: ACQUITY UPLC BEH C18 Column, (2.1 mm X 100 mm, 130Å, 1.7 µm, Waters, catalog number: 186002352)
b. Mobile phase: .
Eluent A Aqueous phase: 10mM Tributylamine(TBA), 15mM Acetic Acid in 100% Water
Eluent B Organic phase: Methanol
c. Gradient:
Time | Flow (ml/min) | Percent Eluent A | Percent Eluent B |
0min | 0.300 | 99.0 | 1.0 |
1.5min | 0.300 | 99.0 | 1.0 |
7min | 0.300 | 95.0 | 5.0 |
11min | 0.300 | 1.0 | 99.0 |
13min | 0.300 | 1.0 | 99.0 |
13.1min | 0.300 | 99.0 | 1.0 |
15min | 0.300 | 99.0 | 1.0 |
d. Column temperature: 35°C
e. Flow rate: 0.3 ml/min
d. Injection volume: 1 µl
2.Mass spectrometry
a. Ion source parameter
Polarity | Negative |
Ion spray voltage (V) | 4500 |
Sheath Gas(Arb) | 30 |
Aux Gas(Arb) | 10 |
Ion Transfer Tube Temp(°C) | 350 |
Vaporizer Temp(°C) | 300 |
b. For data acquisition, multiple reaction monitoring (MRM) mode and the Xcalibur software is used. Hexose phoshates are analyzed with the mass transition 259.1 m/z -> 79 m/z. Collision Energy(V) is 37, RF Lens(V) is 50.
Data analysis
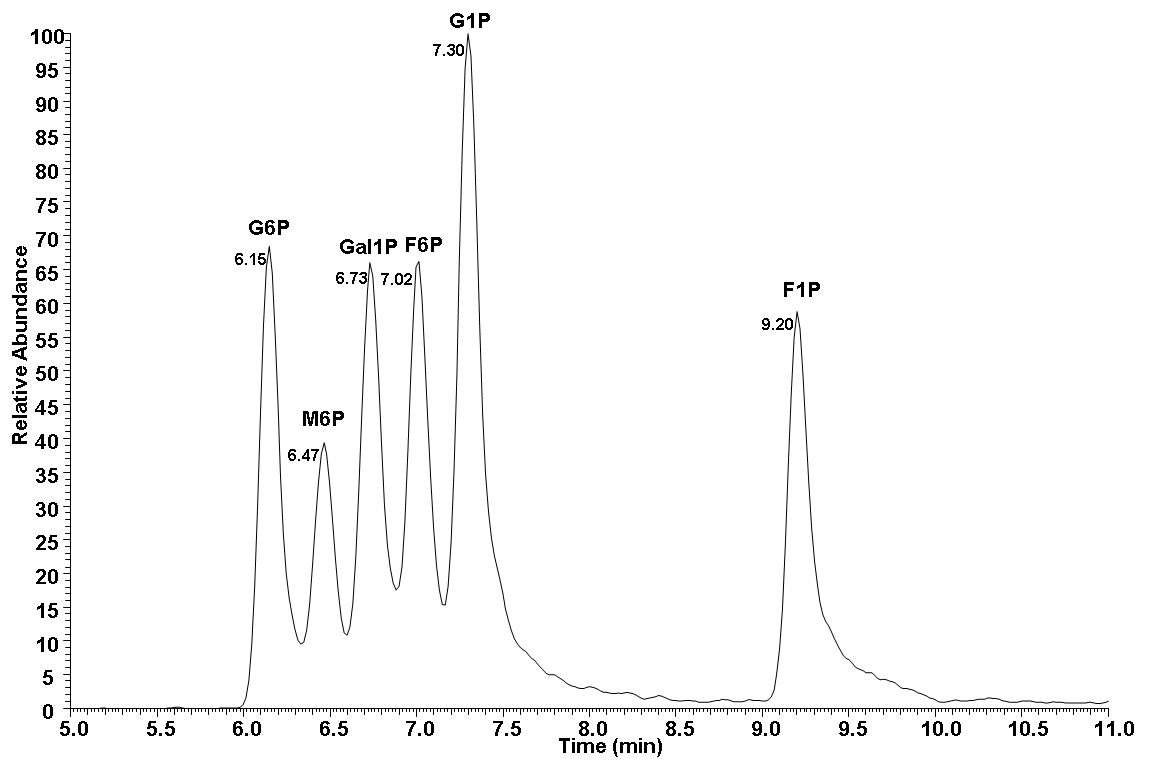
1.STD result
Chromatographic peak areas for G6P, F6P, M6P, G1P, F1P and Gal1P are integrated using Xcalibur
software.
A
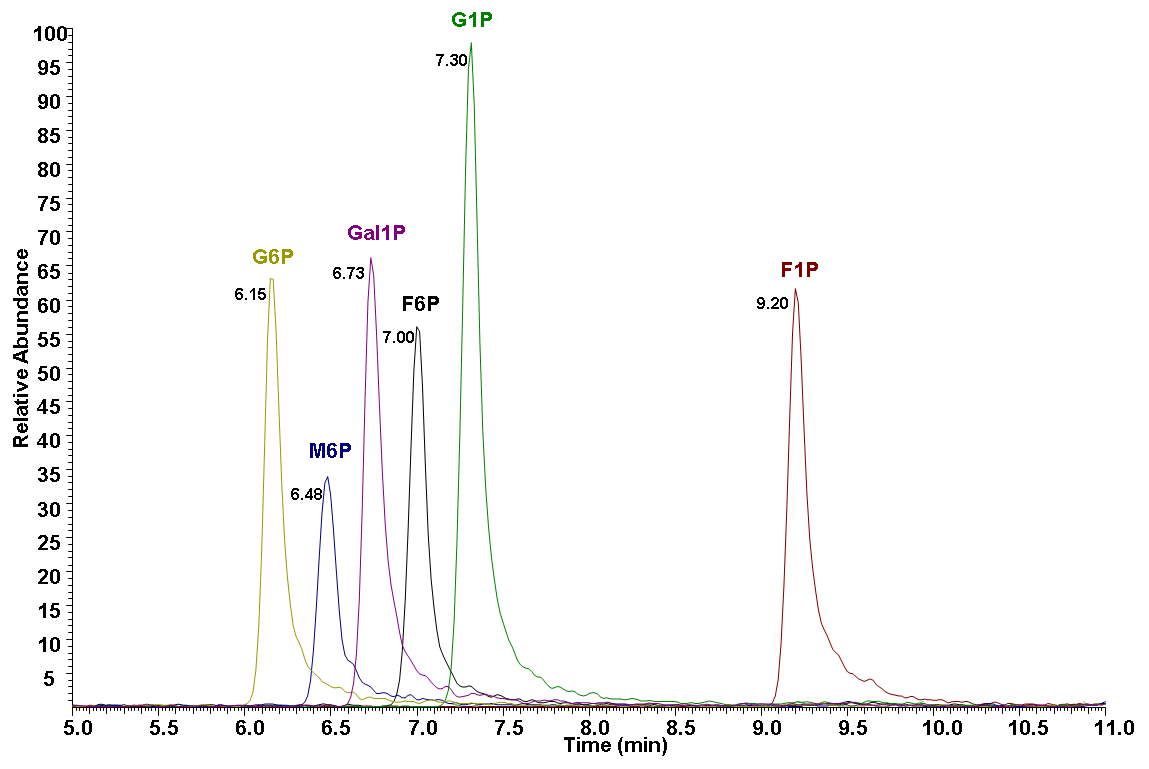
B
2.Representative data
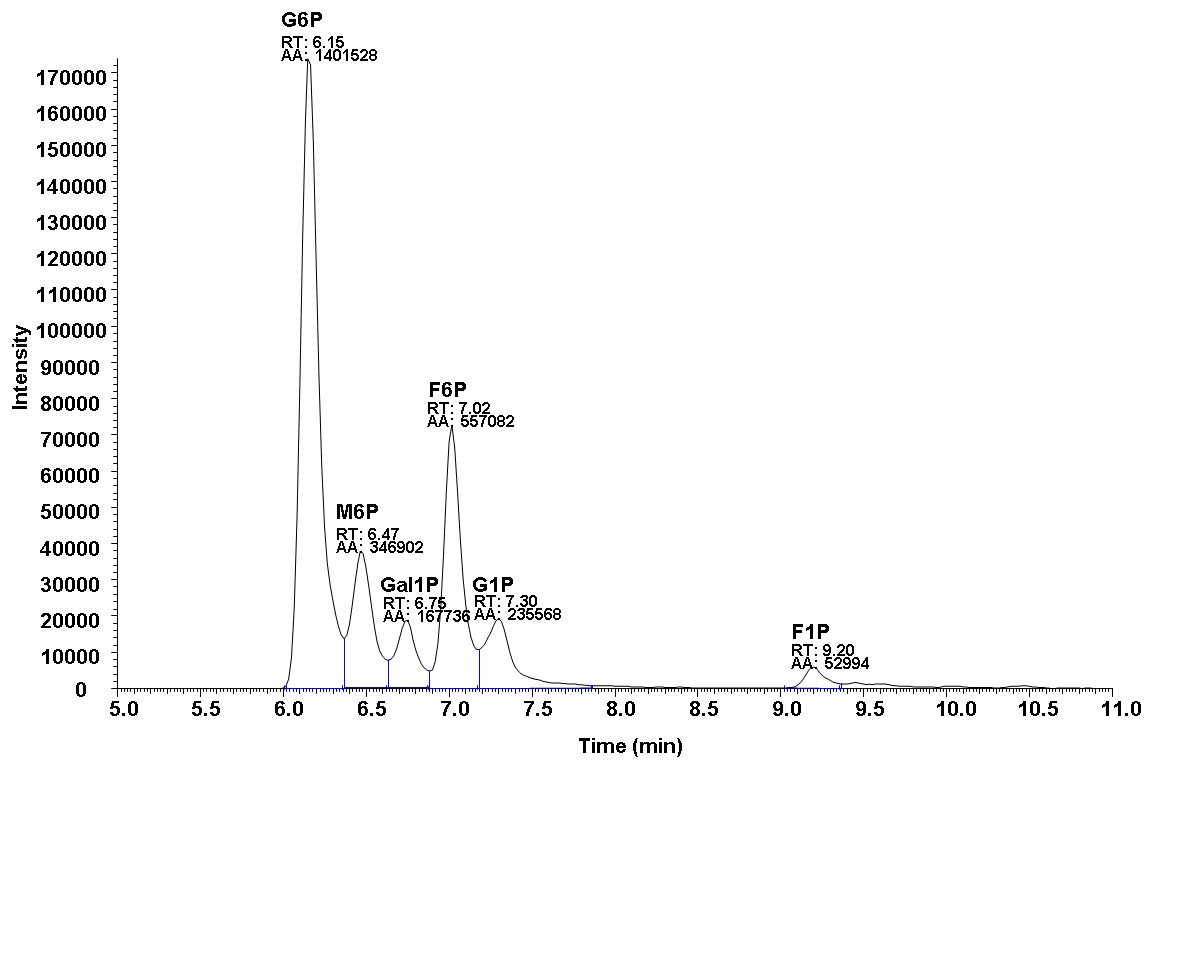
Notes (optional)
1.This protocol also can be used for analysis of the six hexose phosphate isomers from other organ.
2.Please shack strongly the Eluent A to make sure TBA dissolved completely.
Recipes
1. Saline
0.9% NaCl in water (LC-MS grade)
2. Mobile Phase Eluent A
100% water (LC-MS grade)
10 mM Tributylamine (LC-MS grade)
15 mM acetic acid (LC-MS grade)
3.Mobile Phase Eluent B
100% MeOH (LC-MS grade)
Acknowledgments
This research was supported by National Protein Science Facility (Beijing), Tsinghua University, China.
Competing interests
The authors declare no competing interests.
Ethics
Mouse experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Laboratory Animal Resources Center, Tsinghua University.
References
1.Adeva-Andany, M. M., González-Lucán, M., Donapetry-García, C., Fernández-Fernández, C., and Ameneiros-Rodríguez, E. (2016). Glycogen metabolism in humans. BBA clinical, 5, 85-100.
2.Timson, D. J. (2019). Type IV galactosemia. Genetics in Medicine, 21(6), 1283-1285.
3.Kornfeld, S. (1986). Trafficking of lysosomal enzymes in normal and disease states. The Journal of clinical investigation, 77(1), 1-6.
4.Von Figura, K. (1985). Lysosomal enzymes and their receptors. J Cell Biol, 101, 2253-2262.
5.Gibon, Y., Vigeolas, H., Tiessen, A., Geigenberger, P., & Stitt, M. (2002). Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. The Plant Journal, 30(2), 221-235.
6.Zhu, A., Romero, R., & Petty, H. R. (2009). An enzymatic fluorimetric assay for glucose-6-phosphate: application in an in vitro Warburg-like effect. Analytical biochemistry, 388(1), 97-101.
7.Bajad, S. U., Lu, W., Kimball, E. H., Yuan, J., Peterson, C., & Rabinowitz, J. D. (2006). Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. Journal of chromatography A, 1125(1), 76-88.
8.Preinerstorfer, B., Schiesel, S., Lämmerhofer, M., & Lindner, W. (2010). Metabolic profiling of intracellular metabolites in fermentation broths from β-lactam antibiotics production by liquid chromatography–tandem mass spectrometry methods. Journal of chromatography A, 1217(3), 312-328.
9.Wamelink, M. M., Struys, E. A., Huck, J. H., Roos, B., van der Knaap, M. S., Jakobs, C., & Verhoeven, N. M. (2005). Quantification of sugar phosphate intermediates of the pentose phosphate pathway by LC–MS/MS: application to two new inherited defects of metabolism. Journal of Chromatography B, 823(1), 18-25.
10.Vizán, P., Alcarraz-Vizán, G., Díaz-Moralli, S., Rodríguez-Prados, J. C., Zanuy, M., Centelles, J. J., ... & Cascante, M. (2007). Quantification of Intracellular Phosphorylated Carbohydrates in HT29 Human Colon Adenocarcinoma Cell Line Using Liquid Chromatography− Electrospray Ionization Tandem Mass Spectrometry. Analytical chemistry, 79(13), 5000-5005.
11.Kiefer, P., Portais, J. C., & Vorholt, J. A. (2008). Quantitative metabolome analysis using liquid chromatography–high-resolution mass spectrometry. Analytical biochemistry, 382(2), 94-100.
12.Gullberg, J., Jonsson, P., Nordström, A., Sjöström, M., & Moritz, T. (2004). Design of experiments: an efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Analytical biochemistry, 331(2), 283-295.
13.Okahashi, N., Maeda, K., Kawana, S., Iida, J., Shimizu, H., & Matsuda, F. (2019). Sugar phosphate analysis with baseline separation and soft ionization by gas chromatography-negative chemical ionization-mass spectrometry improves flux estimation of bidirectional reactions in cancer cells. Metabolic engineering, 51, 43-49.
14.Soga, T., Igarashi, K., Ito, C., Mizobuchi, K., Zimmermann, H. P., & Tomita, M. (2009). Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Analytical chemistry, 81(15), 6165-6174.
15.Hinterwirth, H., Lämmerhofer, M., Preinerstorfer, B., Gargano, A., Reischl, R., Bicker, W., ... & Lindner, W. (2010). Selectivity issues in targeted metabolomics: Separation of phosphorylated carbohydrate isomers by mixed‐mode hydrophilic interaction/weak anion exchange chromatography. Journal of separation science, 33(21), 3273-3282.
- Wang, Y and Wang, X(2022). Baseline Separation of Six Hexose Phosphate Isomers by Liquid Chromatography-Mass Spectrometry from Tissues. Bio-protocol Preprint. bio-protocol.org/prep1535.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
