- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Generation of Insulin-Producing Alpha TC1-6 Cells Using EpiCRISPR System for Targeted DNA Methylation
Published: Vol 15, Iss 20, Oct 20, 2025 DOI: 10.21769/BioProtoc.5481 Views: 1230
Reviewed by: Laxmi Narayan MishraAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Genetic Knock-Ins of Endogenous Fluorescent Tags in RAW 264.7 Murine Macrophages Using CRISPR/Cas9 Genome Editing
Beverly Naigles [...] Nan Hao
Mar 20, 2024 4244 Views
Multiplex Genome Editing of Human Pluripotent Stem Cells Using Cpf1
Haiting Ma
Nov 20, 2024 2642 Views
Reprogramming White Fat Cells for Adipose Manipulation Transplantation (AMT) Therapy
Kelly An [...] Nadav Ahituv
Aug 5, 2025 2238 Views
Abstract
Diabetes lacks concrete curative strategies due to diverse aetiologies and, therefore, represents the perfect candidate for cell replacement therapy, since it is caused by either an absolute (type 1 diabetes) or relative (type 2 diabetes) defect in the insulin-producing beta cells of the pancreas. Pancreatic alpha cells are a promising source for transdifferentiation into insulin-producing cells as they share a common developmental origin with beta cells and exhibit a certain degree of cellular plasticity. Furthermore, impairment of glucagon signaling in diabetes leads to a marked increase in alpha cell mass, raising the possibility that such alpha cell hyperplasia provides an increased supply of alpha cells for their transdifferentiation into new beta cells.
In this protocol, we used the modular epigenetic CRISPR/dCas9 toolbox for targeted DNA methylation (EpiCRISPR) and silencing of the Arx gene (Aristaless Related Homeobox, Arx), which is essential for the maintenance of alpha cell identity. Methylation-based silencing of Arx initiates the reprogramming of pancreatic alpha cells into insulin-producing cells. As a key novelty, this protocol provides a direct route for epigenetically induced transdifferentiation of mouse pancreatic alpha TC1-6 cells into insulin-producing cells and thereby confirms a proof of concept of reversible cellular epigenetic reprogramming in vitro. In addition, this streamlined workflow addresses the inherent challenges of transfecting clustered alpha TC1-6 cells by optimizing their dissociation into single-cell suspensions, thereby improving uptake and reproducibility.
In summary, this approach for cell transdifferentiation involves precise epigenetic editing of a lineage-specific marker gene, thereby enabling direct lineage conversion in a safe and versatile strategy to generate insulin-producing cells by epigenetic reprogramming. In contrast to approaches that rely on viral vectors or permanent genome editing, this method reduces the risk of off-target effects and immunogenic responses while ensuring reproducibility. The combination of efficiency and precision makes it a valuable tool to advance regenerative approaches for diabetes therapy and to explore the epigenetic regulation of cell identity.
Key features
• This protocol describes a reprogramming of alpha TC1-6 cells into insulin-producing cells by inducing transdifferentiation through targeted epigenetic modulation of a single gene.
• Targeted repression of Arx reduces off-target effects while maintaining genomic integrity.
• This protocol requires at least five days to complete and includes cell preparation, nucleofection, and cell sorting.
• This approach avoids agents like viral vectors or nonspecific demethylating compounds that could limit its potential for therapeutic translation.
Keywords: Alpha cellsGraphical overview
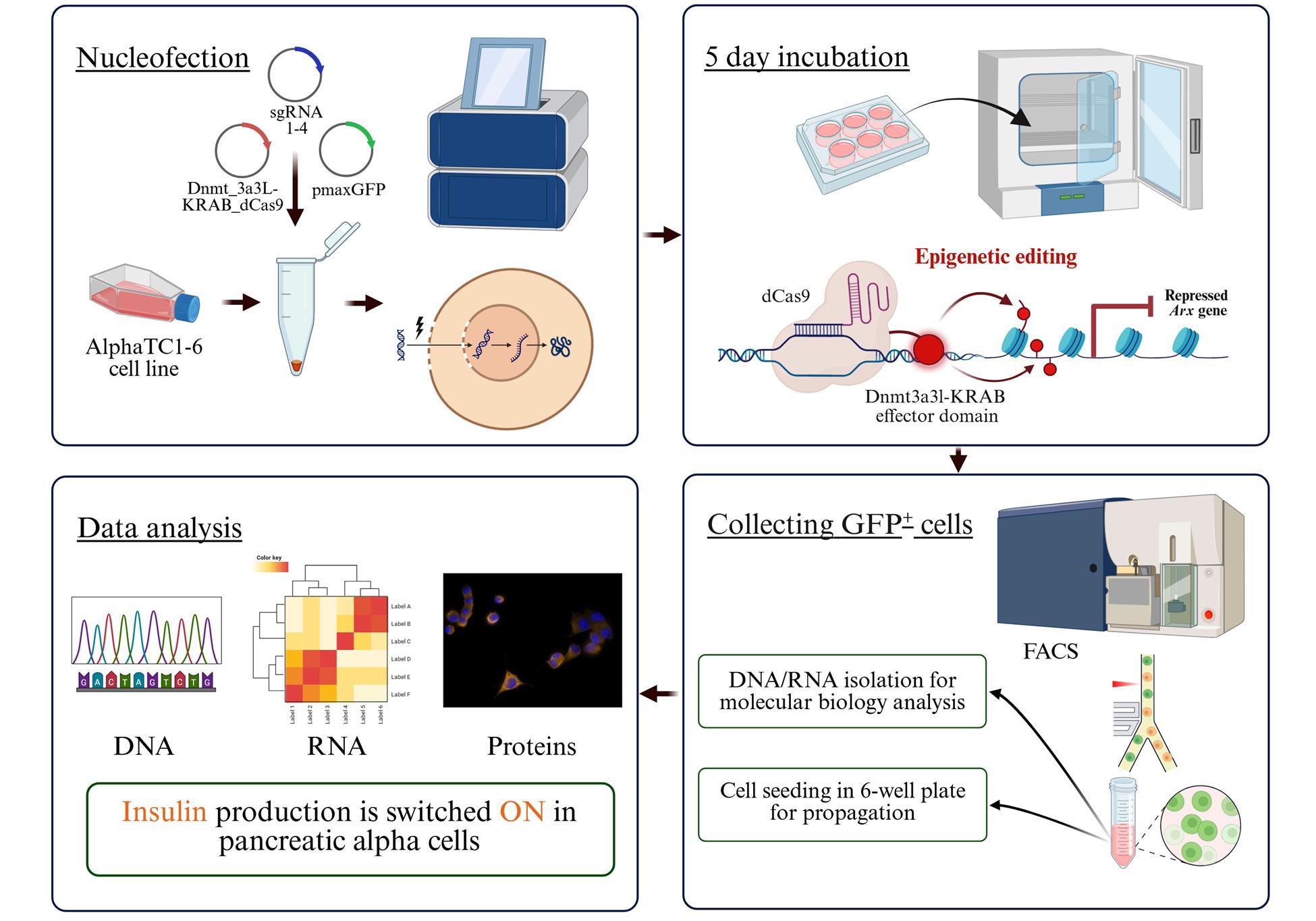
Workflow for the generation of insulin-producing pancreatic alpha TC1-6 cells using the EpiCRISPR system
Background
Cell transdifferentiation, which refers to the direct conversion of one mature cell type into another without passing through a pluripotent state, has emerged as a powerful strategy in regenerative biology [1]. Epigenetic modifications, such as DNA methylation, histone modifications, higher-order chromatin structures, or noncoding RNAs, play a regulatory role in gene expression, determining the developmental trajectories and the maintenance of cell identity and function [2].
Platforms for epigenetic editing, such as CRISPR/dCas9, which utilize catalytically inactive dCas9 fused to epigenetic effectors (e.g., DNMT3A, TET1, or KRAB domains), possess wide applicability across diverse biological systems and pathological conditions [3]. The EpiCRISPR toolbox that we used utilizes an engineered dCas9 that can specifically recognize a unique sequence at a target site, coupled with an effector domain capable of altering the methylation status of that site (DNA methyltransferases) and also repressing it due to the presence of the KRAB repressor (Figure 1C) [4]. Retargeting of the dCas9 effector complex to another locus is achieved by introducing a new single-guide RNA (sgRNA) sequence that is complementary to the desired target site. Regenerative medicine, which focuses on the repair and restoration of tissues and organs damaged by disease or injury, could significantly benefit from rapid progress in epigenetic reprogramming technologies. The initial steps for manipulating gene expression levels include target gene(s) identification, effector domain selection, and sgRNA design. The precision and efficiency of targeted reprogramming approaches, particularly those based on single-gene induced transdifferentiation, make them especially attractive for therapeutic applications, e.g., in monogenic disorders, reactivation of tumor suppressor genes, or oncogene silencing [5].
The strategy to use pancreatic alpha cells from the same organ (pancreas) for transdifferentiation was based on the fact that alpha-to-beta cell transdifferentiation can lead to restoration of beta cell mass while simultaneously reducing alpha cell mass, thus restoring the balance between pancreatic hormones (insulin and glucagon), which is perturbed in diabetes (bihormonal hypothesis of diabetes) [6]. Increasing the number of insulin-producing beta cells while decreasing the number of glucagon-producing alpha cells turns out to be a promising therapeutic avenue. Furthermore, impairment of glucagon signaling in diabetes leads to a marked increase in alpha cell mass, raising the possibility that such alpha cell hyperplasia provides an increased supply of alpha cells for their transdifferentiation into new beta cells [7]. In addition, the advantages of using pancreatic alpha cells for transdifferentiation into insulin-producing cells with this particular method for gene repression are numerous. Targeted epigenetic silencing of the lineage-specific marker gene for pancreatic alpha cells, Arx, combines cellular plasticity with precision control in targeting, offering a promising strategy to restore insulin production from the patient’s own pancreas (endogenously). Since other pancreatic endocrine and exocrine cell types already display silenced Arx expression through promoter methylation [8], this approach minimizes off-target effects and ensures specificity to alpha cells, which are resilient, numerous, and remain intact in most diabetic patients. The strength of the protocol described here lies in its distinction from previous approaches to alpha cell transdifferentiation, such as direct Arx knockout [9], which causes a permanent DNA alteration, or drug-induced Arx inhibition, which represents a non-invasive and scalable, but less specific, alternative, potentially affecting off-target GABA signaling pathways [10]. Directed epigenome editing avoids double-stranded DNA breaks and offers reversible, adjustable effects with a profile that is safer than permanent gene knockouts.
Despite the promise of reprogramming pancreatic alpha cells into insulin-producing beta cells, some challenges remain. Achieving high reprogramming efficiency without compromising cell viability still remains an important limitation. A major impediment of this approach is the low reprogramming efficiency, emphasizing the tremendous need for further optimization of EpiCRISPR systems to achieve robust and durable Arx promoter methylation and sustained insulin expression (Figure 2) [4]. In addition, the immunogenicity of reprogrammed alpha cells remains undefined. It is yet to be determined whether these cells are susceptible to autoimmune attack in the context of type 1 diabetes or if parallel immunomodulatory strategies are required. Besides, this approach is confronted by in vivo delivery challenges, as achieving specific and efficient targeting of pancreatic alpha cells is critical for experimental reproducibility and remains technically demanding. Therefore, the development of advanced delivery platforms, such as cell type–selective lipid nanoparticles, will be essential to ensure targeted, long-term, and non-toxic expression of epigenetic editing components [11]. Overcoming these challenges is crucial to developing safe and effective epigenome-based reprogramming strategies for diabetes therapy.
Materials and reagents
Biological materials
1. Alpha TC1 clone 6 cell line, αTC1-6 (American Type Culture Collection, Manassas, VA, USA, CRL-2934; origin: pancreas of a mouse with adenoma)
2. TOP10 Escherichia coli cells [Thermo Fisher Scientific, Invitrogen, C404003; origin: E. coli (K12)]
Reagents
1. Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F9665) (-20 °C)
2. Bovine serum albumin (BSA) (SERVA Electrophoresis GmbH, catalog number: 11930.03) (4 °C)
3. DMEM, high glucose, GlutaMAXTM Supplement, HEPES (Gibco, Thermo Fisher Scientific, catalog number: 32430027) (4 °C)
4. DMEM, low glucose (Sigma-Aldrich, catalog number: D6046) (4 °C)
5. Cell dissociation buffer, enzyme-free, PBS (Gibco, Thermo Fisher Scientific, catalog number: 13151014) (4 °C)
6. RPMI 1640 medium (Gibco, Thermo Fisher Scientific, catalog number: 52400025) (4 °C)
7. Penicillin/streptomycin solution (Sigma-Aldrich, catalog number: P4333) (-20 °C)
8. Kanamycin sulfate from Streptomyces kanamyceticuc (Sigma-Aldrich, catalog number: K1377-1G) (4 °C)
9. Trypan blue solution, 0.4% (Gibco, Thermo Fisher Scientific, catalog number: 15250-061) [room temperature (RT)]
10. SF Cell Line 4D-Nucleofector X kit L (Lonza Solution and Supplement, catalog number: V4XC-2024); solution box (catalog number: PBC2-02250), supplement 1 (catalog number: S-08983), pmaxGFP 1.0 (lot. no. D-00068) (4 °C)
11. Plasmid Plus Midi kit (25) (Qiagen, catalog number: 12943); components: buffer P1(4 °C), buffer P2 (RT), buffer P3 (RT), buffer BB (RT), buffer ETR (RT), and buffer EB (RT)
12. Ethanol 100% (ZORKA Pharma, catalog number: 56/238) (RT)
13. HBSS w/o calcium, w/o magnesium, w/o phenol red (Biowest, catalog number: L0605) (4 °C)
14. Dimethyl sulfoxide (DMSO) (Santa Cruz Biotechnology, catalog number: sc-358801) (RT)
15. Tryptone (Difco, catalog number: 0123-01) (RT)
16. Yeast extract (Difco, catalog number: 0127-01) (RT)
17. KCl (Alkaloid Skopje, catalog number: 06743) (RT)
18. NaH2PO4 × 12 H2O (Merck, catalog number: 6579) (RT)
19. KH2PO4 (Moss Hemos, catalog number: 369K2) (RT)
20. NaCl (ZORKA Pharma, catalog number: 7647-14-5) (RT)
21. T4 DNA ligase (Thermo Fisher Scientific, catalog number: EL0011) (-20 °C)
22. 10× T4 ligase buffer (Thermo Fisher Scientific, catalog number: B69) (-20 °C)
23. BbsI enzyme (New England Biolabs, U.S.A, catalog number: R0539S) (-20 °C)
Solutions
1. DMEM high glucose full medium (see Recipes)
2. DMEM low glucose full medium (see Recipes)
3. PBS (see Recipes)
4. α final medium (see Recipes)
5. Buffer for cell transfection (see Recipes)
6. Buffer for cell sorting (see Recipes)
7. Lysogeny broth (see Recipes)
Recipes
1. DMEM high glucose full medium
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM high glucose | 89% | 445 mL |
| Penicillin/streptomycin | 1× | 5 mL |
| FBS | 10% | 50 mL |
| Total | 100% | 500 mL |
a. Perform all the steps in sterile conditions.
b. Pour out the 55 mL of clean medium from the 500 mL bottle; then, add FBS and penicillin/streptomycin solution to the bottle and mix by inverting several times.
c. Store at 4 °C until use.
2. DMEM low glucose full medium
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM low glucose | 89% | 445 mL |
| Penicillin/streptomycin | 1× | 5 mL |
| FBS | 10% | 50 mL |
| Total | 100% | 500 mL |
a. Perform all the steps in sterile conditions.
b. Pour out the 55 mL of clean medium from the bottle; then, add FBS and penicillin/streptomycin solution and mix by inverting several times.
c. Store at 4 °C until use.
3. PBS (1×, pH 7.4)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | 137 mM | 8 g |
| Na2HPO4·12H2O | 10 mM | 3.6 g |
| KCl | 2.7 mM | 0.2 g |
| KH2PO4 | 1.8 mM | 0.24 g |
| H2O | 1 L | |
| Total | 1× | 1 L |
a. Prepare 800 mL of distilled water in a 1 L glass cup.
b. Add 8 g of NaCl, 0.2 g of KCl, 3.6 g of NaH2PO4·12H2O, and 0.24 g of KH2PO4 to the solution.
c. Adjust the solution to pH ≈ 7.4.
d. Add distilled water to a final volume of 1 L.
e. After autoclaving, store the buffer at 4 °C (maximum 12 months).
f. Warm to 37 °C before use.
4. α final medium
| Reagent | Volume |
|---|---|
| DMEM high glucose full medium | 24.5 mL |
| DMEM low glucose full medium | 24.5 mL |
| BSA (1% in PBS) | 1 mL |
| Total | 50 mL |
a. Dissolve 0.5 g of BSA in 50 mL of PBS (1% BSA) and then filter the solution through a syringe filter (pore size 0.2 μm).
b. Mix DMEM high and low glucose full media and 1% BSA solution in a 50 mL conical tube by inverting several times.
c. Store at 4 °C until use.
d. Warm to 37 °C before use.
5. Buffer for cell transfection
| Reagent | Final concentration | Quantity or volume | ||||
| SF NucleofectorTM X solution | 77.52% | 82 μL | ||||
| Supplement 1 | 17.02% | 18 μL | ||||
| Plasmids | pMax eGFP | 0.47% | 0.375 μg | |||
| dCas9-Dnmt3a3L-KRAB | 0.99% | 1.5 μg | ||||
| Arx sgRNA | 1 | 0.99% | 3.99% | 1.406 μg | 5.625 μg | |
| 2 | 0.99% | 1.406 μg | ||||
| 3 | 0.99% | 1.406 μg | ||||
| 4 | 0.99% | 1.406 μg | ||||
| Total | n/a | 100 μL | ||||
a. First, warm the SF solution and supplement to RT.
b. Mix all the components of the buffer for cell transfection freshly prior to use.
c. The volume of added plasmids should not exceed 10 μL.
6. Buffer for cell sorting
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HBSS | 97.6% | 48.8 mL |
| EDTA | 2 mM | 0.2 mL (0.5 M) |
| FBS | 2% | 1 mL |
| Total | n/a | 50 mL |
a. Mix the HBSS and EDTA solution with FBS freshly prior to use.
b. Warm to 37 °C before use.
c. Store the HBSS at 4 °C until use.
7. Lysogeny broth (LB)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tryptone | 1% | 10 g |
| Yeast extract | 0.5% | 5 g |
| NaCl | 1% | 10 g |
| Distilled water | 1 L |
a. Dissolve all the components in 0.8 L of water on a mixer.
b. After component dissolving, adjust the pH to 7.2 and add water up to 1 L.
c. Sterilize by autoclaving and store at 4 °C for up to 6 months.
Laboratory supplies
1. Cell culture flasks, 25 cm2 (T-25 adherent flask) (Thermo Fisher Scientific, catalog number: 130189)
2. 6-well sterile culture plates (Thermo Fisher Scientific, catalog number: 130184)
3. 10 mL conical tubes (Sarstedt, catalog number: 62.9924.284)
4. 50 mL conical tube (Sarstedt, catalog number: 62.547.254)
5. Cell counting chamber slide, Neubauer Pattern (Blaubrand, catalog number: BR718605-1EA)
6. Sterile syringes 50 mL (Weigao, catalog number: SP503PLS)
7. Syringe filter, pore size: 0.2 µm (Sarstedt, catalog number: 83.1826.102)
8. Reaction tube, 1.5 mL, PP (Sarstedt, catalog number: 72.690.001)
9. Reaction tube, 2 mL, PP (Sarstedt, catalog number: 72.691)
10. 10 μL pipette tips (Eppendorf, catalog number: 0030 000.811)
11. 200 μL pipette tips (Sarstedt, catalog number: 70.3030)
12. 1,000 μL pipette tips (Sarstedt, catalog number: 70.3050)
13. FACS tube, 3.5 mL, L×Ø: 55 × 12 mm, PS (Sarstedt, catalog number: 55.484)
14. Glass cup, 1 L (LLG, Pirex catalog number: 9014005*LLG)
Equipment
1. Cell culture incubator (37 °C, 5% CO2) (Thermo Fisher Scientific, BB15, catalog number: 51023121)
2. Water bath (Thermo Fisher Scientific, Precision GP 20, catalog number: TSGP20)
3. Light microscope (Nikon, model: ECLIPSE Ts2)
4. Axio Observer Z1 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany)
5. High-speed centrifuge (Eppendorf, model: 5804)
6. Swing bucket rotor (Eppendorf, model: A-4-44)
7. Mini centrifuge with standard rotor (Carl Roth, model: SD 220 VC)
8. AmaxaTM 4D-NucleofectorTM Core Unit (Lonza, 100-240, catalog number: 817B1102)
9. AmaxaTM 4D-NucleofectorTM X Unit (Lonza, catalog number: 817X0291)
10. Flow cytometer and cell sorter (FACS) (BD Biosciences, San Diego, USA, BD FACSAria III)
11. Vortex mixer (Neuation I Swix, model: TA1707AA0301)
12. Vertical laminar flow bench (Technology. Science. Life, Telstar, model: AV-100)
13. Ultracentrifuge (Beckman Coulter, model: Optima L-100 KSP)
14. Titanium rotor (Beckman Coulter, Type 70 Ti Fixed-Angle, catalog number: 06E 2591)
15. Incubated/refrigerated stackable shaker [Thermo Scientific MaxQ, model: SHKE6000-1CE (4352)]
16. NanoPhotometer MicroVolume spectrophotometer (Implen, Germany, catalog number: N60 UV/Vis)
17. Thermomixer (Eppendorf, Thermomixer comfort with 2 mL Thermoblock, catalog number: 5355)
18. Laboratory vacuum pump (Sartorius, Microstart Mini-Vac, catalog number: 025250)
19. Refrigerator (2–8 °C)
20. Freezer (-20 °C)
21. Deep freezer (-80 °C)
Software and datasets
1. FACS Diva software v6.1.3, 2011
2. BioRender (https://biorender.com)
3. SnapGene Viewer Version 7.0.1
4. GraphPad’s Prism8.0.1 (263) software for statistical analyses
Procedure
A. Preparation of sgRNA expression vectors
See General note 1.
1. Ligation cloning
a. Preparation of sgRNA oligos
i. Design and order complementary oligonucleotides encoding the desired sgRNA sequence.
ii. Resuspend oligos in nuclease-free water to a concentration of 100 μM.
b. Oligo annealing
i. Mix 1 μL each of forward and reverse sgRNA oligos (100 μM), 1 μL of 10× T4 ligase buffer, and 7 μL of water.
ii. Heat to 95 °C for 5 min and allow to cool slowly to RT (~30 min) to anneal the oligos.
c. Vector preparation
i. Digest 2 μg of sgRNA plasmid (with dual BbsI sites) using BbsI at 37 °C for 1 h.
ii. Purify the digested vector using a gel extraction cleanup kit.
d. Ligation reaction
i. Combine 50 ng of BbsI-digested plasmid, annealed oligos (3:1 molar ratio), 1 μL of 10× T4 ligase buffer, 1 μL of T4 DNA ligase, and water to 10 μL.
ii. Incubate at 16 °C overnight.
2. Heat shock transformation of bacteria
a. Add 10 ng of ligation reaction to competent E. coli TOP10 cells (50 μL) that have just begun to thaw and mix by tapping gently.
b. Incubate the tubes with cells on ice (4 °C) for 30 min.
c. Transfer the tubes to 42 °C for 45 s.
d. Pour 900 μL of prewarmed LB medium on the cells and incubate for 1 h at 37 °C in a shaking incubator (250 rpm).
e. Centrifuge the tubes for 3 min at 0,8× g (RT).
f. Discard half of the supernatant (SN). Resuspend the cells in the rest of the SN and sow on a prepared solid medium with kanamycin as a selection antibiotic.
g. Incubate the plates at 37 °C overnight.
h. Store plates with bacteria at 4 °C for 1–2 weeks.
3. Plasmid isolation
a. Transfer a single bacterial colony from a solid substrate to a liquid LB medium with a selective antibiotic and incubate overnight (12–16 h) at 37 °C on a shaker.
b. Centrifuge the overnight culture at 7,000× g for 15 min at 4 °C.
c. Resuspend the bacterial pellets in 4 mL of buffer P1.
d. Add 4 mL of P2 lysis buffer to the cell suspensions. Mix by gently inverting the 50 mL tube until the lysate becomes viscous.
e. Incubate the lysates for 3 min at RT.
f. Add 4 mL of P3 neutralization buffer to the lysate and mix immediately by vigorously inverting the tube 4–6 times.
g. Transfer the lysate to the QIAfilter and incubate for 10 min at RT.
h. Filter the lysate through the QIAfilter, add 2 mL of BB buffer to the filtrate, and mix by inverting 4–6 times.
i. Transfer the lysate to QIAGEN Plasmid Plus Midi spin columns and apply a vacuum of ~300 mbar until all the liquid has passed through the column.
j. Wash the column 2× with 700 μL of ETR buffer each time using a vacuum.
k. Centrifuge the columns at 10,000× g for 1 min to eliminate residual wash buffer.
l. Transfer the columns to 1.5 mL tubes.
m. Add 200 μL of EB buffer and incubate for ≥1 min at room temperature.
n. Centrifuge at 10,000× g for 1 min to obtain dissolved plasmid DNA in the eluate.
B. Cultivation and propagation of αTC1-6 cells
Note: Warm all solutions to 37 °C prior to use.
1. Incubate a mouse pancreatic αTC1 clone 6 cell line at 37 °C in humidified air containing 5% CO2 in α final medium, which contains 15.25 mM glucose. Change the medium every second day, without PBS washing. When cells reach 85% confluency (T-25 flask), transfer half of the cells to a new T-25 flask for further cell propagation:
a. Wash the cells with 5 mL of PBS.
b. Add 1 mL of cell dissociation buffer and gently rock the flask to immerse the cells at RT for 2 min.
c. Leave the flask for an additional 3 min at RT in the laminar flow hood.
d. Detach the cells with 4 mL of α final medium and transfer the cell suspension to the 10 mL centrifuge tube.
e. Centrifuge the tube with the cell suspension at 120× g for 5 min. Remove medium and resuspend the cell pellet in fresh α final medium.
f. Add half an aliquot of cell suspension to a new T-25 flask.
g. Incubate cells at 37 °C.
C. Nucleofection of αTC1-6 cells
Note: All steps for nucleofection include gently handling the cells.
1. Prewarm the 6-well plate with 1,800 μL/well of α final medium at 37 °C.
2. After reaching 70% cell confluency (see General note 2), aspirate the medium from the flask and rinse the cells with 5 mL of PBS. Detach the cells using cell dissociation buffer (as described in step B1a–d).
3. Mix the cell suspension with Trypan Blue solution (1:1), load onto a hemocytometer, and count viable cells to determine the concentration per unit volume.
4. Centrifuge 5 × 106 cells per one 100 μL single NucleocuvetteTM at 90× g for 10 min at RT.
5. Pour out the medium and rest the centrifuge tube on filter paper to absorb residual liquid to complete removal of media without drying the pellet.
6. Add 100 μL of transfection buffer (4D-NucleofectorTM SF Solution with Supplement and 7.5 μg of plasmid DNA) to the dry cell pellet. Mix gently by pipetting 3–4×.
7. Transfer the cell suspension into a 100 µL single NucleocuvetteTM.
8. Insert the NucleocuvetteTM into the retainer of the 4D-NucleofectorTM X Unit (Figure 1A). Initiate the CM-156 nucleofection program.
9. Upon completion of the program for nucleofection, add 400 μL of prewarmed RPMI medium to each NucleocuvetteTM slowly down the cuvette wall and incubate for 10 min at 37 °C.
10. (Critical) Gently resuspend the cells by pipetting up and down 2–3 times. The application of the electrical pulse creates temporary pores in the cell membrane, making the cells highly sensitive to mechanical stress.
11. Transfer the 200 μL of resuspended cells from one NucleocuvetteTM into one well of a pre-incubated 6-well plate containing α final medium (two wells per sample in total).
12. Cultivate the transfected cells under standard conditions until further analysis (Figure 2).
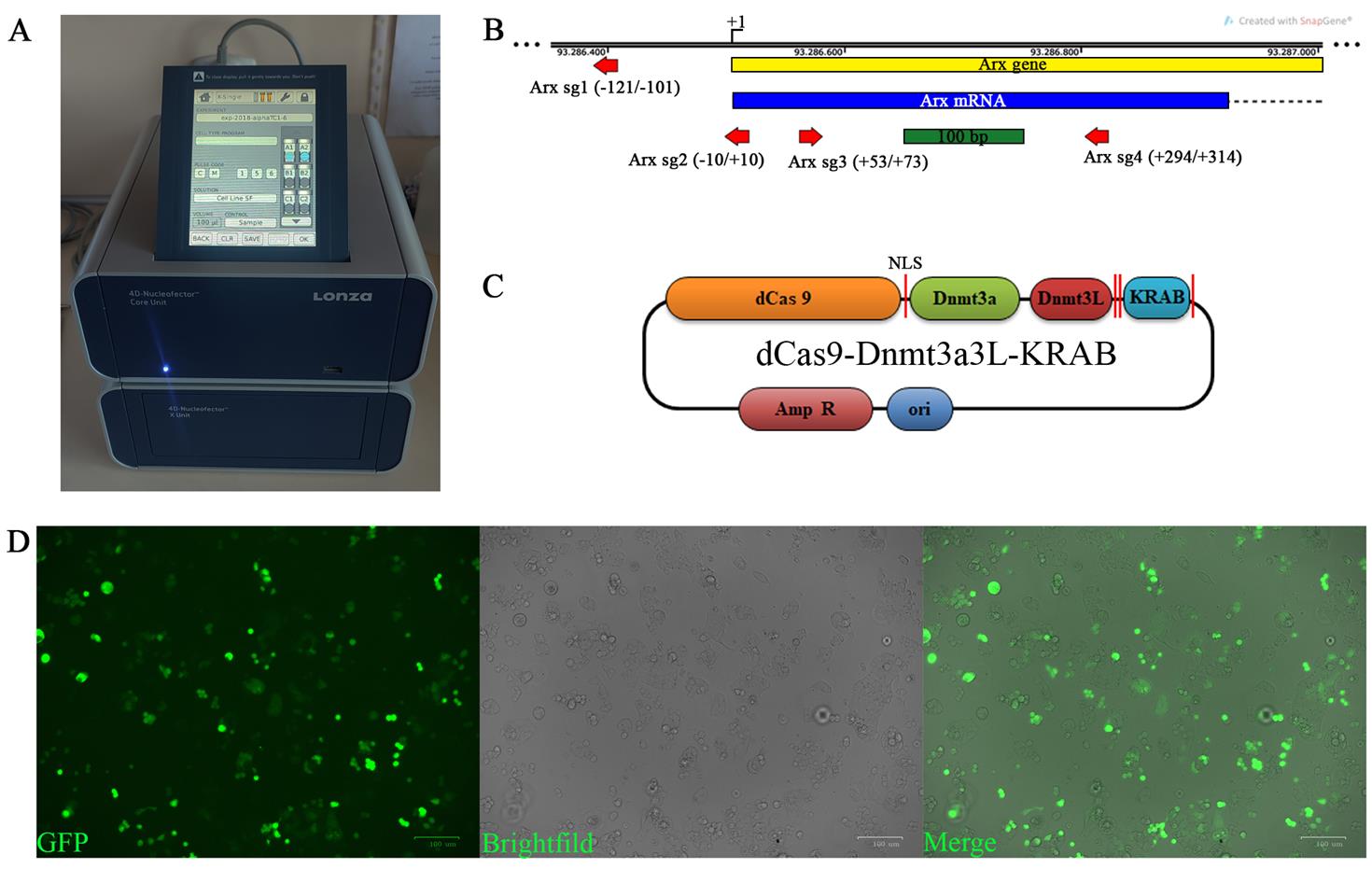
Figure 1. Alpha TC1-6 transfection. (A) 4D-Nucleofector X unit with set parameters for transfection of alpha TC1-6 cells in the Nucleocuvette. (B) The map illustrates the positions of the four sgRNAs (indicated by red arrows; the sequences are presented in Table 1) used for transfection and targeting of the EpiCRISPR fusion construct. The Arx coding region is represented as a blue box, while the intron is depicted as a dashed line. NLS, nuclear localization signal. (C) Schema of the dCas9-Dnmt3a3L-KRAB plasmid that was used to transfect the alpha TC1-6 cells for silencing the expression of the Arx gene. (D) Alpha TC1-6 cells at day 5 post-transfection. Images were taken with an Axiocam digital camera attached to the Axio Observer Z1 microscope. Scale bars, 100 µm.
Table 1. sgRNA targeting sequences
| Arx sgRNA | sgRNA targeting sequence |
|---|---|
| sgRNA 1 | GCAAAGCGCAAAGCGCGAAC |
| sgRNA 2 | TAACAAGTGTAGTGAGCCGC |
| sgRNA 3 | AGGGTGGGAGCCCGCAACCG |
| sgRNA 4 | GATGCTGTCGATGCAGTAGG |
D. Sorting of transfected αTC1-6 cells
Note: All steps for cell sorting should be done at RT.
1. At day 5 post-transfection, aspirate medium and wash the cells with 1 mL of PBS and detach with 0.7 mL of cell dissociation buffer per well (as described in step B1a–d).
2. Centrifuge the cells at 90× g for 10 min at RT in a FACS tube.
3. Pour out the medium from the tubes.
4. Resuspend the cell pellet in 1 mL of buffer for cell sorting.
5. Collect the GFP-positive αTC1-6 cells using the FACS Aria III flow cytometer and cell sorter (cell sorting parameters are listed in Table 2).
6. Collect the cells into the 10 mL conical tubes containing 2 mL of α final medium. Coat the inner walls of the tubes with α final medium immediately before placing them in the holder.
7. After sorting and collection, centrifuge the cells at 90× g for 10 min at RT to pellet them.
8. The cell pellet can be used immediately for molecular biology analyses or further propagated in cell culture.
Table 2. Cell sorting parameters
| Parameter | Value |
|---|---|
| Pressure | 45 psi |
| Nozzle | 85 µm |
| Frequency | 47 Hz |
| Gap | 8 |
| FSC voltage | 20 |
| SSC voltage | 315 |
| FITC/GFP | 330 |
| Purity mode | 4-way purity |
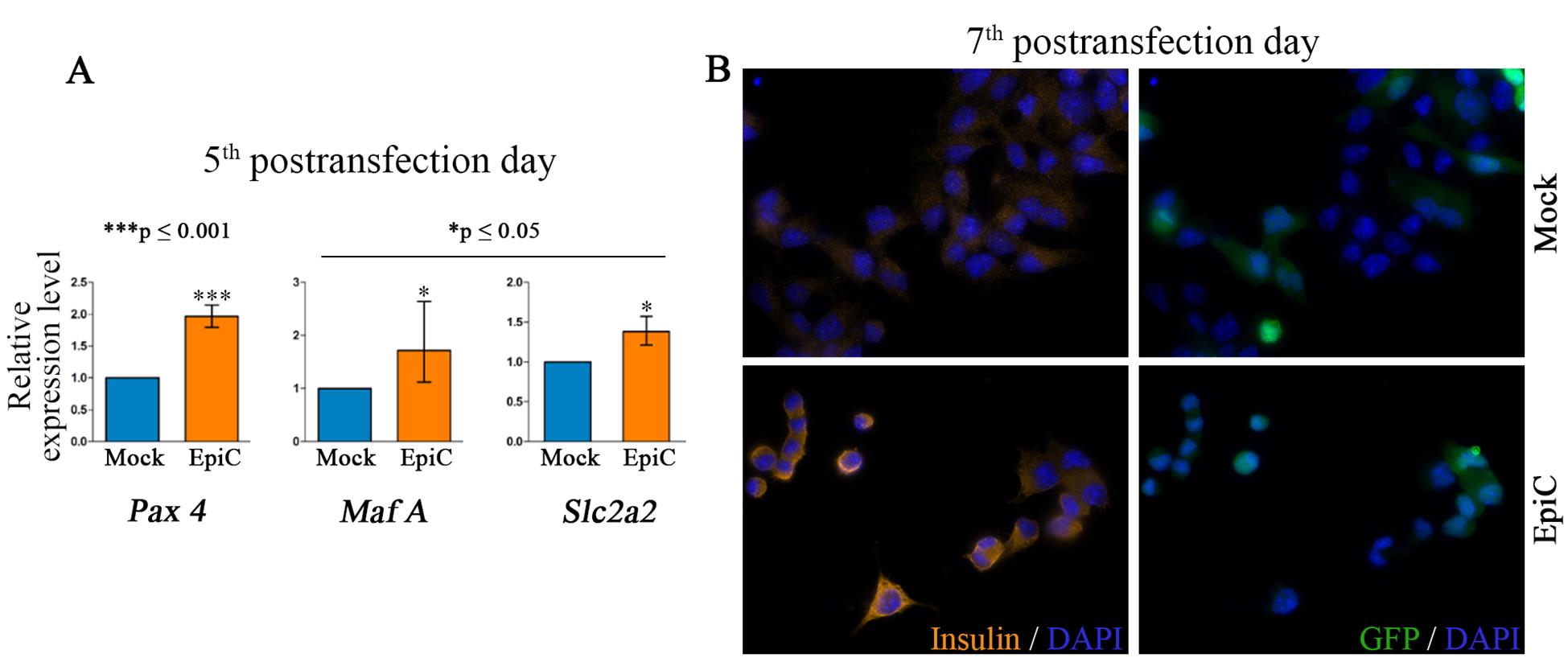
Figure 2. Alpha TC1-6 cells exhibit a beta-cell-like phenotype. (A) Relative expression at the mRNA level of pancreatic beta cell key marker genes (Pax 4, Maf A, and Slc2a2) in Mock (cells transfected with a mix of plasmid with empty sgRNA instead of the four listed sgRNAs for Arx) and EpiC transfected and sorted αTC1-6 at day 5 post-transfection. The error bars denote SD from three biological replicates. A one-sample t-test was used for determining statistical significance, *p ≤ 0.05, ***p ≤ 0.001. The figure is modified from Đorđević et al [4]. (B) Immunocytochemistry (ICC) analysis of Mock and EpiC-transfected αTC1-6 at day 7 post-transfection shows that insulin is present in the EpiC cells at the protein level, while it was not detected in Mock-transfected cells.
Validation of protocol
This protocol has been used and validated in the following research articles:
Đorđević et al. [14]. Nucleofection as an Efficient Method for Alpha TC1-6 Cell Line Transfection. Applied Sciences (Figure 9).
Đorđević, M. B. [15]. Transdiferencijacija alfa ćelija pankreasa miša u ćelije koje proizvode insulin ciljanom metilacijom DNK primenom Epi-CRISPR sistema [Doctoral dissertation, University of Belgrade (Serbia)].
Đorđević et al. [16]. Changes in up and down regulated gene expression after transient suppression of Arx gene in pancreatic alpha TC1-6 cell line. CoMBoS2-the Second Congress of Molecular Biologists of Serbia; 2023 Oct 6-8; Belgrade, Serbia (pp. 151–151). Belgrade: Institute of Molecular Genetics and Genetic Engineering, University of Belgrade.
Đorđević et al. [17]. Uloga ciljanih (epi) genetičkih modifikacija u potencijalnoj terapiji dijabetesa. Trendovi u molekularnoj Biologiji.
General notes and troubleshooting
General notes
1. The Arx gene DNA sequences with NCBI ref. s. NC_000086.7 [assembly: GRCm38.p6 (GCF_000001635.26)] were obtained using the UCSC Genome Browser [12]. Potential binding sites for sgRNAs targeting the dCas9-3a3L-KRAB fusion protein to the Arx promoter were designed by the online E-CRISP platform [13]. The sgRNAs were chosen to target the Arx promoter region and CpG island, with a focus on high efficiency and low off-target effects (Figure 1B).
2. Transfection efficiency is strongly influenced by cell density. For optimal results, cells should reach 70%–75% confluency at the time of transfection, as this ensures they are actively dividing and most receptive to DNA uptake. Higher confluency levels can lead to reduced efficiency due to contact inhibition and changes in cellular physiology.
3. High plasmid concentration, good purity, and the absence of endotoxin are necessary for high transfection efficiency.
Troubleshooting
Problem: Low transfection efficiency.
Possible causes:
1. The transfection protocol is not well optimized for cells other than alphaTC1-6.
2. Cells are of high density/confluency.
3. Low cell viability after nucleofection.
4. Low sorting recovery.
5. Low plasmid purity.
Solutions:
1. Optimize the nucleofection program (pulse settings optimization), the amount of the DNA construct, and the number and viability of input cells. Expected efficiency ranges should be 70%–80% GFP-positive cells (Figure 1D).
2. Consider using a transfection method other than nucleofection (e.g., chemical transfection: lipid- or polymer-based reagents).
3. Adjust post-transfection recovery conditions, including medium composition, supplements, and handling during the first 24 h.
4. Adjust the buffers for collecting cells by adding FBS or BSA and HEPES (Ca/Mg-free).
5. Check endotoxin levels after plasmid isolation.
Acknowledgments
1) Specific contributions of each author: Conceptualization, M.V. and J.A.J.; Investigation, M.Đ., S.M.H., A.S., and T.M.; Formal analysis, M.Đ., J.R., S.D., and A.U.; Writing—Original Draft, M.Đ.; Writing—Review & Editing, N.G., M.M. and M.V.; Funding acquisition, M.V.; Supervision, J.A.J.
2) Funding sources that supported the work: Ministry of Science, Technological Development and Innovation (grant no.: 451-03-68/2020-14/ 200007, 451-03-9/2021-14/ 200007, 451-03-68/2022-14/ 200007, 451-03-47/2023-01/ 200007, 451-03-66/2024-03/ 200007, 451-03-136/2025-03/ 200007); European Foundation for the Study of Diabetes (EFSD), European Diabetes Research Programme in Cellular Plasticity Underlying the Pathophysiology of Type 2 Diabetes, research grant from AstraZeneca. AstraZeneca was not involved in the study design, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. Funding source is partially based upon work from COST Actions CM1406 and CA16119, supported by COST (European Cooperation in Science and Technology). The results presented in this manuscript are in line with Sustainable Development Goal 3 (Good Health and Well-being) of the United Nations 2030 Agenda.
3) Citation of the original research paper in which the protocol was described and validated: Đorđević, M., Stepper, P., Feuerstein-Akgoz, C., Gerhauser, C., Paunović, V., Tolić, A., Rajić, J., Dinić, S., Uskoković, A., Grdović, N., Mihailović, M., Jurkowska, R. Z., Jurkowski, T. P., Jovanović, J. A., & Vidaković, M. (2023). EpiCRISPR targeted methylation of Arx gene initiates transient switch of mouse pancreatic alpha to insulin-producing cells. Frontiers in endocrinology, 14, 1134478. https://doi.org/10.3389/fendo.2023.1134478.
4) We would like to express our sincere thanks to Prof. Tomasz P. Jurkowski for providing the Epi-CRISPR epigenetic editing tool used in this study. Special thanks to Dr. Peter Stepper for his assistance in designing and cloning the gRNAs. We are also grateful to Dr. Verica Paunović for her valuable assistance with FACS sorting.
5) The Graphical abstract was created using BioRender by Vidakovic, M. (2025) https://BioRender.com/1jrpv6n.
Competing interests
The authors declare no conflicts of interest.
References
- Sepyani, S., Momenzadeh, S., Safabakhsh, S., Nedaeinia, R. and Salehi, R. (2024). Therapeutic approaches for Type 1 Diabetes: Promising cell-based approaches to achieve ultimate success. SLAS Discovery. 29(1): 23–33. https://doi.org/10.1016/j.slasd.2023.11.002
- Wu, Y. L., Lin, Z. J., Li, C. C., Lin, X., Shan, S. K., Guo, B., Zheng, M. H., Li, F., Yuan, L. Q., Li, Z. h., et al. (2023). Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study. Signal Transduction Targeted Ther. 8(1): e1038/s41392–023–01333–7. https://doi.org/10.1038/s41392-023-01333-7
- Gilbert, L. A., Larson, M. H., Morsut, L., Liu, Z., Brar, G. A., Torres, S. E., Stern-Ginossar, N., Brandman, O., Whitehead, E. H., Doudna, J. A., et al. (2013). CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell. 154(2): 442–451. https://doi.org/10.1016/j.cell.2013.06.044
- Đorđević, M., Stepper, P., Feuerstein-Akgoz, C., Gerhauser, C., Paunović, V., Tolić, A., Rajić, J., Dinić, S., Uskoković, A., Grdović, N., et al. (2023). EpiCRISPR targeted methylation of Arx gene initiates transient switch of mouse pancreatic alpha to insulin-producing cells. Front Endocrinol (Lausanne). 14: e1134478. https://doi.org/10.3389/fendo.2023.1134478
- Saunderson, E. A., Stepper, P., Gomm, J. J., Hoa, L., Morgan, A., Allen, M. D., Jones, J. L., Gribben, J. G., Jurkowski, T. P., Ficz, G., et al. (2017). Hit-and-run epigenetic editing prevents senescence entry in primary breast cells from healthy donors. Nat Commun. 8(1): 1450. https://doi.org/10.1038/s41467-017-01078-2
- Unger, R. H. and Cherrington, A. D. (2012). Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 122(1): 4–12. https://doi.org/10.1172/jci60016
- Stanojevic, V. and Habener, J. F. (2015). Evolving function and potential of pancreatic alpha cells. Best Pract Res Clin Endocrinol Metab. 29(6): 859–871. https://doi.org/10.1016/j.beem.2015.10.002
- Arda, H. E., Tsai, J., Rosli, Y. R., Giresi, P., Bottino, R., Greenleaf, W. J., Chang, H. Y. and Kim, S. K. (2018). A Chromatin Basis for Cell Lineage and Disease Risk in the Human Pancreas. Cell Syst. 7(3): 310–322.e4. https://doi.org/10.1016/j.cels.2018.07.007
- Chakravarthy, H., Gu, X., Enge, M., Dai, X., Wang, Y., Damond, N., Downie, C., Liu, K., Wang, J., Xing, Y., et al. (2017). Converting Adult Pancreatic Islet α Cells into β Cells by Targeting Both Dnmt1 and Arx. Cell Metab. 25(3): 622–634. https://doi.org/10.1016/j.cmet.2017.01.009
- Zhang, Y., Lin, X. and Li, J. (2023). The controversy about the effects of artemisinins on pancreatic α cell reprogramming and diabetes. Trends Endocrinol Metab. 34(3): 131–134. https://doi.org/10.1016/j.tem.2022.12.005
- Zhang, T., Yin, H., Li, Y., Yang, H., Ge, K., Zhang, J., Yuan, Q., Dai, X., Naeem, A., Weng, Y., et al. (2024). Optimized lipid nanoparticles (LNPs) for organ-selective nucleic acids delivery in vivo. iScience. 27(6): 109804. https://doi.org/10.1016/j.isci.2024.109804
- Kent, W. J., Sugnet, C. W., Furey, T. S., Roskin, K. M., Pringle, T. H., Zahler, A. M. and Haussler, D. (2002). The human genome browser at UCSC. Genome Res. 12(6): 996–1006. https://doi.org/10.1101/gr.229102
- Heigwer, F., Kerr, G. and Boutros, M. (2014). E-CRISP: fast CRISPR target site identification. Nat Methods. 11(2): 122–123. https://doi.org/10.1038/nmeth.2812
- Đorđević, M., Paunović, V., Jovanović Tucović, M., Tolić, A., Rajić, J., Dinić, S., Uskoković, A., Grdović, N., Mihailović, M., Marković, I., et al. (2022). Nucleofection as an Efficient Method for Alpha TC1-6 Cell Line Transfection. Appl Sci. 12(15): 7938. https://doi.org/10.3390/app12157938
- Đorđević, M. B. (2023). Transdiferencijacija alfa ćelija pankreasa miša u ćelije koje proizvode insulin ciljanom metilacijom DNK primenom Epi-CRISPR Sistema. University of Belgrade. https://phaidrabg.bg.ac.rs/o:31809
- Đorđević, M., et al. (2023). Changes in up and down regulated gene expression after transient suppression of Arx gene in pancreatic alpha TC1-6 cell line. CoMBoS2-the Second Congress of Molecular Biologists of Serbia. Belgrade: Institute of Molecular Genetics and Genetic Engineering, University of Belgrade.
- Đorđević, M., Dinić, S., Mihailović, M., Uskoković, A., Grdović, N., Arambašić Jovanović, J. and Vidaković, M. (2023). Uloga ciljanih (epi) genetičkih modifikacija u potencijalnoj terapiji dijabetesa. Trendovi u molekularnoj Biologiji. 3: 138–150. https://hdl.handle.net/21.15107/rcub_imagine_2252
Article Information
Publication history
Received: Aug 14, 2025
Accepted: Sep 17, 2025
Available online: Oct 10, 2025
Published: Oct 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Đorđević, M. B., Hadžić, S. �. M., Rajić, J. J., Sarić, A. D., Marković, T. R., Dinić, S. S., Uskoković, A. S., Grdović, N. M., Mihailović, M. V., Jovanović, J. A. D. and Vidaković, M. S. (2025). Generation of Insulin-Producing Alpha TC1-6 Cells Using EpiCRISPR System for Targeted DNA Methylation. Bio-protocol 15(20): e5481. DOI: 10.21769/BioProtoc.5481.
Category
Cell Biology > Cell engineering > CRISPR-cas9
Biological Engineering > Synthetic biology > Genetic modification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








