- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Establishing and Maintaining 3D Organoid Cultures From Human Fallopian Tube Epithelium
Published: Vol 15, Iss 16, Aug 20, 2025 DOI: 10.21769/BioProtoc.5412 Views: 2195
Reviewed by: Anu ThomasAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3751 Views
Examining the Roles of m6A Sites in mRNA Using the Luciferase Gene Fused With Mutated RRACH Motifs
Nobuko Katoku-Kikyo and Nobuaki Kikyo
Nov 5, 2025 1934 Views
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1249 Views
Abstract
The female reproductive tract is comprised of different regions, each with distinctive physiological characteristics. One of them is the fallopian tubes, which are vital for human reproductive health and success. The ability to model their function and physiology is of utmost importance. So far, in vitro models have been based on a few immortalized or cancer cell lines derived from fallopian tube cells that lacked differentiated, specialized cell types and did not allow for the study of cancer initiation due to their implicit biases. Organoids, in contrast, overcome these limitations and provide an advanced, three-dimensional system for the study of healthy fallopian tube physiology and pathology. Fallopian tube organoids are comprised of epithelial progenitors that can be enriched using chemical or hormonal treatment into the different cell types that are found in the in vivo tissue, namely detyrosinated-tubulin-positive ciliated cells or paired-box protein 8 (PAX8)-positive secretory cells. This protocol provides a step-by-step guide for the establishment and maintenance of a long-term culture of organoids from healthy human fallopian tube tissue. The organoid model described here closely mimics the in vivo physiology and anatomy of human fallopian tube epithelium and provides a comprehensive basis for future studies on its underlying molecular characteristics and possible pathology.
Key features
• Provides a step-by-step guide for the establishment of long-term fallopian tube organoid cultures.
• Allows for rapid extension of fallopian tube epithelial progenitor cells with a yield of up to 1 × 108 cells within 3 weeks of isolation.
• Fallopian tube organoids closely mimic healthy physiology, being comprised of multiple different cell types, like detyrosinated-tubulin-positive ciliated cells or paired-box protein 8 (PAX8)-positive secretory cells.
• Further enrichment of secretory cells by hormonal treatment and ciliated cells by chemical treatment is possible.
Keywords: OrganoidsGraphical overview
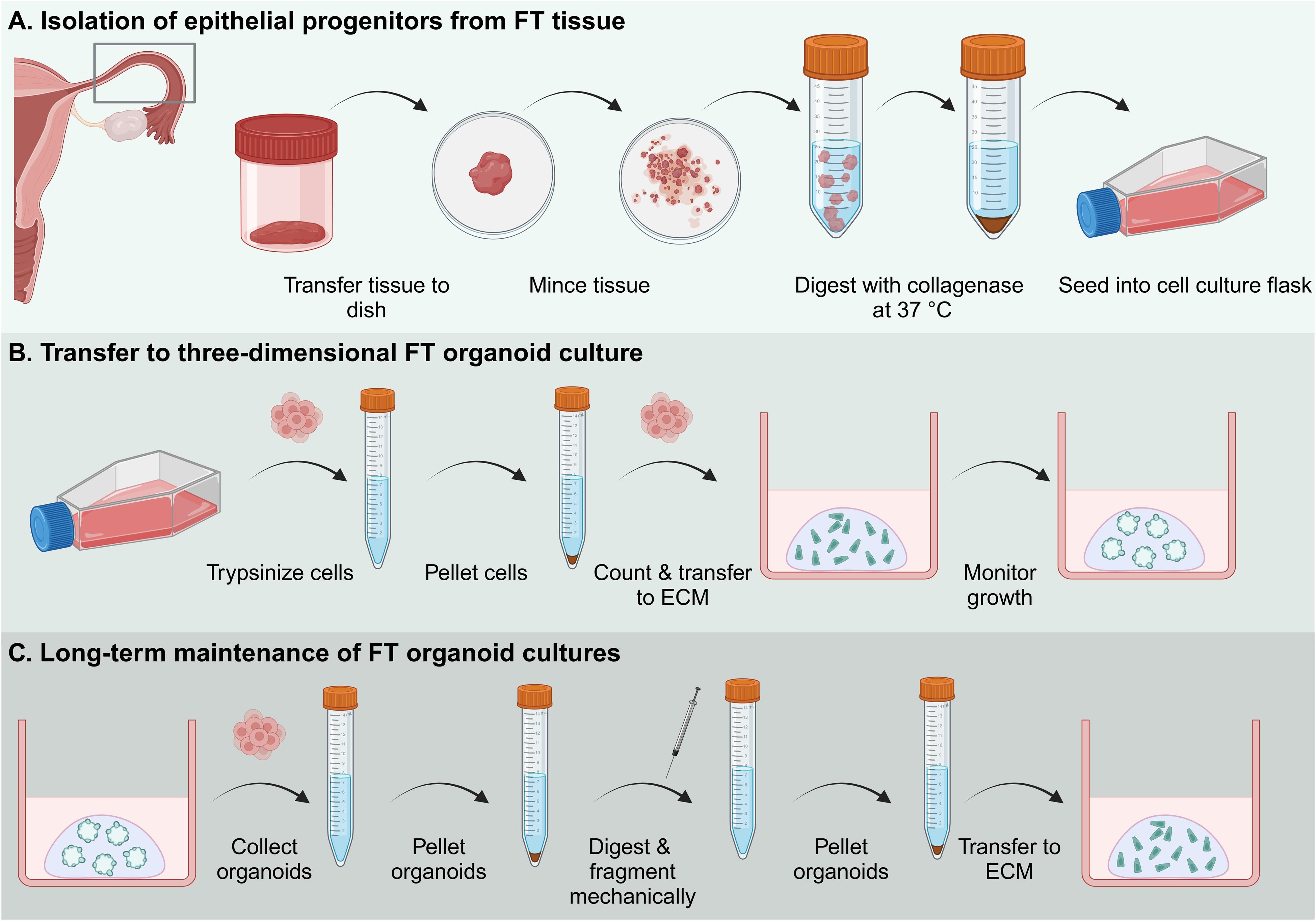
Graphical overview of the establishment and maintenance of human fallopian tube organoid cultures. Abbreviations: FT: fallopian tube; ECM: extracellular matrix.
Background
Since the initial description by Sato et al. [1], organoid technology has been expanded across entities and species barriers. In the past, researchers have predominantly relied on immortalized cancer cell lines or mouse models to study cancer biology, and both have their distinct advantages and shortcomings [2,3]. In comparison to these models, organoids have the advantage of primary origin and multiple differentiated cell types in combination with comparable low costs, scalability, and three-dimensional architecture [2,4]. At the same time, they retain patient characteristics, making them a powerful tool for disease modeling and cancer research as so-called patient avatars [4–6], personalized models derived from patient material representing individual patient characteristics and genotype, allowing for targeted therapeutic recommendations [4]. Previous work has focused on the establishment of organoids derived from healthy tissue from human intestines [7], liver [8], pancreas [9], stomach [10], and other organs. Here, we present a protocol for the establishment of organoids from a vital organoid for reproductive success, the fallopian tubes, based on our earlier descriptions [11]. The fallopian tubes are tube-like organs of the female reproductive system. Their primary function is to transport oocytes from the adjacent ovaries for fertilization and subsequently the embryo to the uterus for implantation [12]. For this, the tubes are equipped with an epithelium composed of secretory and ciliated cells that undergo dynamic change within the reproductive and hormonal cycle [13].
We previously showed that the establishment of proliferative cultures of fallopian tube epithelial progenitors is dependent on active Notch and WNT-signaling and requires constant supplementation of adequate ligands in the growth media [11]. These organoids closely mimic the in vivo differentiation [11] by differentiation of epithelial progenitors into detyrosinated-tubulin-positive ciliated cells and PAX8-positive secretory cells that can be further enriched by supplementing with chemical or hormonal treatments, such as dibenzazepine or β-estradiol and progesterone, respectively. The model proposed here has been successfully applied in the study of fallopian tube physiology [11], infection [14], and cancer initiation [15,16] by us and others.
Materials and reagents
Note: Examples for providers/manufacturers are given; similar cells, reagents, supplies, and devices might perform equivalently but have not been tested for these procedures.
Biological materials
1. L-Wnt3a-producing cell line (ATCC, catalog number: CRL-2647 [17], RRID: CVCL_0635)
2. R-Spondin-1-producing cell line (i.a. Cultrex HA-R-Spondin1-Fc 293T Cells, catalog number: 3710-001-01 or Kim, et al. [18])
Critical: Gene technological approval from the local authority must be obtained when working with GMOs.
Note: The conditioned media from the L-Wnt3a, R-Spondin-1, and Noggin can be replaced by the addition of 25% conditioned media of the L-WRN cell line (ATCC, catalog number: CRL-3276 [19], RRID: CVCL_DA06). A protocol for the generation of the conditioned media can be found at ATCC or in Warschkau et al. [20].
Reagents
1. L-Wnt3a conditioned medium [17] (made in-house; aliquot and store at -80 °C)
2. R-Spondin-1-conditioned medium [18] (made in-house; aliquot and store at -80 °C)
3. Extracellular matrix; growth factor reduced, i.e., Matrigel (Corning, catalog number: 354230; aliquot and store at -80 °C) or Cultrex BME type 2 (R&D Systems, catalog number: 3533-010-02; aliquot and store at -80 °C)
Note: The protocols listed here were established using Matrigel and Cultrex. The use of another substitute may affect organoid growth.
4. Fetal calf serum (FCS) (Corning, catalog number: A5256702; aliquot and store at -20 °C)
5. Advanced DMEM/F12 (Gibco, catalog number: 12634010; store at 4 °C)
6. B27 (Thermo, catalog number: 17504044; store at -80 °C) or alternatively NCS21 (Capricorn, catalog number: C21-H; store at -80 °C)
7. N2 for organoid medium (Capricorn, catalog number: N2-K; store at -80 °C)
8. Recombinant human epithelial growth factor (Thermo, catalog number: AF-100-15; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
9. Human recombinant Noggin (Thermo, catalog number: 120-10C; dissolve in DPBS containing 0.1% bovine serum albumin, aliquot, and store at -80 °C)
Note: The recombinant Noggin can be replaced by Noggin-secreting cell lines when the conditioned medium is added in a concentration of 10% (v/v).
10. Human recombinant fibroblast growth factor 10 (FGF10) (Thermo, catalog number: 100-26; dissolve in DPBS containing 0.1% bovine serum albumin, aliquot, and store at -80 °C)
11. Nicotinamide (Sigma, catalog number: N0636; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
12. N-acetyl-L-Cysteine (Sigma, catalog number: A9165; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
13. A83-01 (Biomol, catalog number: Cay9001799; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
14. Y-27632 (Biomol, catalog number: Cay9001799; dissolve in Advanced DMEM/F12, aliquot, and store at -80 °C)
15. HEPES (PanBioTech, catalog number: P05-01100; store at 4 °C)
16. GlutaMax (Gibco, catalog number: 35050038; store at 4 °C)
17. CryoSFM (PromoCell, catalog number: C-29922; store at 4 °C)
18. DPBS (PanBioTech, catalog number: P04-36500; store at 4 °C)
19. TrypLE Express (Gibco, catalog number: 12605010; store at 4 °C)
20. Penicillin/Streptomycin (Gibco, catalog number: 15140122; aliquot and store at -20 °C)
21. Fungin (InvivoGen, catalog number: ant-fn-1; store at -20 °C)
22. Gentamicin (Thermo, catalog number: 15750060; aliquot and store at -20 °C)
23. Collagenase type I (Sigma-Aldrich, catalog number: SCR103, dissolve in ethanol, aliquot, and store at -80 °C)
24. Dibenzazepine (DBZ, YO-01027) (MedChemExpress, catalog number: HY-13526; dissolve in DMSO, aliquot, and store at -80 °C)
25. β-estradiol (Sigma-Aldrich, catalog number: E2257; dissolve in ethanol, aliquot, and store at -80 °C)
26. Progesterone (Sigma-Aldrich, catalog number: P8783; dissolve in ethanol, aliquot, and store at -80 °C)
27. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650; store at room temperature)
28. Bovine serum albumin (BSA) (Carl Roth, catalog number: 3854; store at 4 °C)
Solution
1. ADF+++ (see Recipes)
2. FT isolation medium (see Recipes)
3. FT expansion medium (see Recipes)
Recipes
1. ADF+++
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Advanced DMEM/F12 | - | 500 mL |
| HEPES | 10 mM | 5 mL |
| GlutaMax | 1× | 5 mL |
| Penicillin/Streptomycin | 1% (v/v) | 5 mL |
2. FT isolation medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Human recombinant epithelial growth factor (EGF) | 10 ng/mL | 100 μL of a 10 µg/mL stock |
| Fetal calf serum | 5% (v/v) | 5 mL |
| Y-27632 (ROCK inhibitor) | 10 μm | 100 μL of a 10 mM stock |
| HEPES | 10 mM | 1,000 μL of a 1 M stock |
| GlutaMax | 1× | 1,000 μL |
| Penicillin/Streptomycin | 1% (v/v) | 1,000 μL |
| Fungin (only for isolation) | 0.1% (v/v) | 100 μL |
| Gentamicin (only for isolation) | 10 µg/mL | 100 μL of a 10 mg/mL stock |
| Advanced DMEM/F12 | - | Top up to 100 mL |
Note: Filter the freshly made medium through a 0.22 μm filter to avoid contamination and keep at 4 °C for a maximum of two weeks.
3. FT expansion medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Wnt3a conditioned medium [17] | 25% (v/v) | 25 mL |
| Rspondin-1 conditioned medium [18] | 25% (v/v) | 25 mL |
| B27 or NCS21 supplement | 2% (v/v) | 2 mL |
| N2 supplement | 1% (v/v) | 1 mL |
| Human recombinant epithelial growth factor (EGF) | 10 ng/mL | 100 μL of a 10 µg/mL stock |
| Human recombinant Noggin | 100 ng/mL | 100 μL of a 100 µg/mL stock |
| Human recombinant fibroblast growth factor 10 (FGF10) | 100 ng/mL | 100 μL of a 100 µg/mL stock |
| Nicotinamide | 1 mM | 100 μL of a 1 M stock |
| A83-01 (TGF-β inhibitor) | 1 μm | 100 μL of a 1 mM stock |
| Y-27632 (ROCK inhibitor) | 10 μm | 100 μL of a 10 mM stock |
| HEPES | 10 mM | 1,000 μL of a 1 M stock |
| GlutaMax | 1× | 1,000 μL |
| Penicillin/Streptomycin | 1% (v/v) | 1,000 μL |
| Advanced DMEM/F12 | - | Top up to 100 mL |
Note: Filter the freshly made medium through a 0.22 μm filter to avoid contamination and keep at 4 °C for a maximum of one week. The conditioned media from the L-Wnt3a, R-Spondin-1, and the recombinant Noggin can be replaced by the addition of 25% conditioned media of the L-WRN cell line (ATCC, CRL-3276 [19]). The recombinant Noggin can be replaced by Noggin-secreting cell lines when the conditioned medium is added in a concentration of 10% (v/v).
Laboratory supplies
1. 24-well tissue culture plates (F-base; TPP, catalog number: 920 12/24; or comparable product)
2. T25/T75 flasks (Sarstedt, catalog numbers: 83.3910.302/83.3911.302; or comparable product)
3. 15/50 mL conical centrifuge tubes (TPP, catalog number: 910 15/50; or comparable product)
4. 10/200/1,000 μL ART barrier pipette tips (Thermo Scientific, catalog numbers: 10098960/10029040/10313272; or comparable product)
5. Vacuum filtration system "rapid"-Filtermax (0.22 μm) (TPP, catalog number: 99505; or comparable product)
6. Sartorius MinisartR sterile filters (0.22 μm) (Thermo Scientific, catalog number: 10730792; or comparable product)
7. Duran glass beakers (25/600 mL) (Schott, catalog numbers: 2110614, 2110648; or comparable product)
8. Duran glass bottles (500/1,000 mL) (Schott, catalog numbers: 4459/5455; or comparable product)
9. B Braun solo cone Luer syringes (1 mL/20 mL) (Braun, catalog number: 12752637; or comparable product)
10. Single-use 18 G syringe needles (blunt, 40 mm) (VWR, BD Medical, catalog number: BDAM303129)
11. Disposable, sterile scalpels (Type 11 or 22; Feather; or comparable product)
12. Disposable, sterile forceps (Faust, MediSet, catalog number: 7661698; or comparable product)
13. Cell strainer (100 μm pore size) (PluriSelect, catalog number: 43-50100-51; or comparable product)
14. CoolCell LX cell freezing vial containers (Fisher Scientific, Corning, catalog number: 15572771; or comparable product)
15. Cell culture dishes (100 × 22 mm) (Th. Geyer, LabSolute, catalog number: 7696774; or comparable product)
Equipment
1. Safety cabinet for BSL-2 work, Safe 2020 Class II biological safety cabinet (Thermo Scientific, catalog number: 51026640; or comparable device)
2. Shaking water bath (Lauda, catalog number/model: Hydro H 20 S; or comparable device)
3. Centrifuge for 50/15 mL tubes (Hettich, catalog number/model: Rotina 420R; or comparable device)
4. Incubator (Binder, catalog number/model: CB-UL; or comparable device)
5. Rocker (IKA, KS 4000 i Control; or comparable device)
6. Automated cell counter (DeNovix, CellDrop BF; or comparable device)
7. Disposable hemocytometer (Kisker Biotech, catalog number: M-NZ; or comparable product) as an alternative for manual cell counting
8. Standard benchtop brightfield microscope for cell monitoring
Procedure
A. Isolation of epithelial progenitors from fallopian tube tissue
Caution: Primary human samples might contain pathogens. Use appropriate protective equipment and barrier tips.
Note: It is recommended to source fallopian tubes from standard surgical procedures for benign gynecological disease to minimize the chances of contamination with cancer cells. It is recommended that each sample be evaluated by a pathologist. Samples are best collected in ADF+++.
Critical: Dissect samples within 2–3 hours after surgery. Ethical and genetic technological approval from the local authority must be obtained.
Note: Coat all tubes with sterile 0.1% BSA in DPBS. Apply the solution to all surfaces that come into contact with cells by up-and-down-pipetting or decanting.
1. Place the fallopian tubes in an appropriate container, like a Petri dish, for further dissection. Cut the tissue into several pieces if it is too large to handle whole (Figure 1).
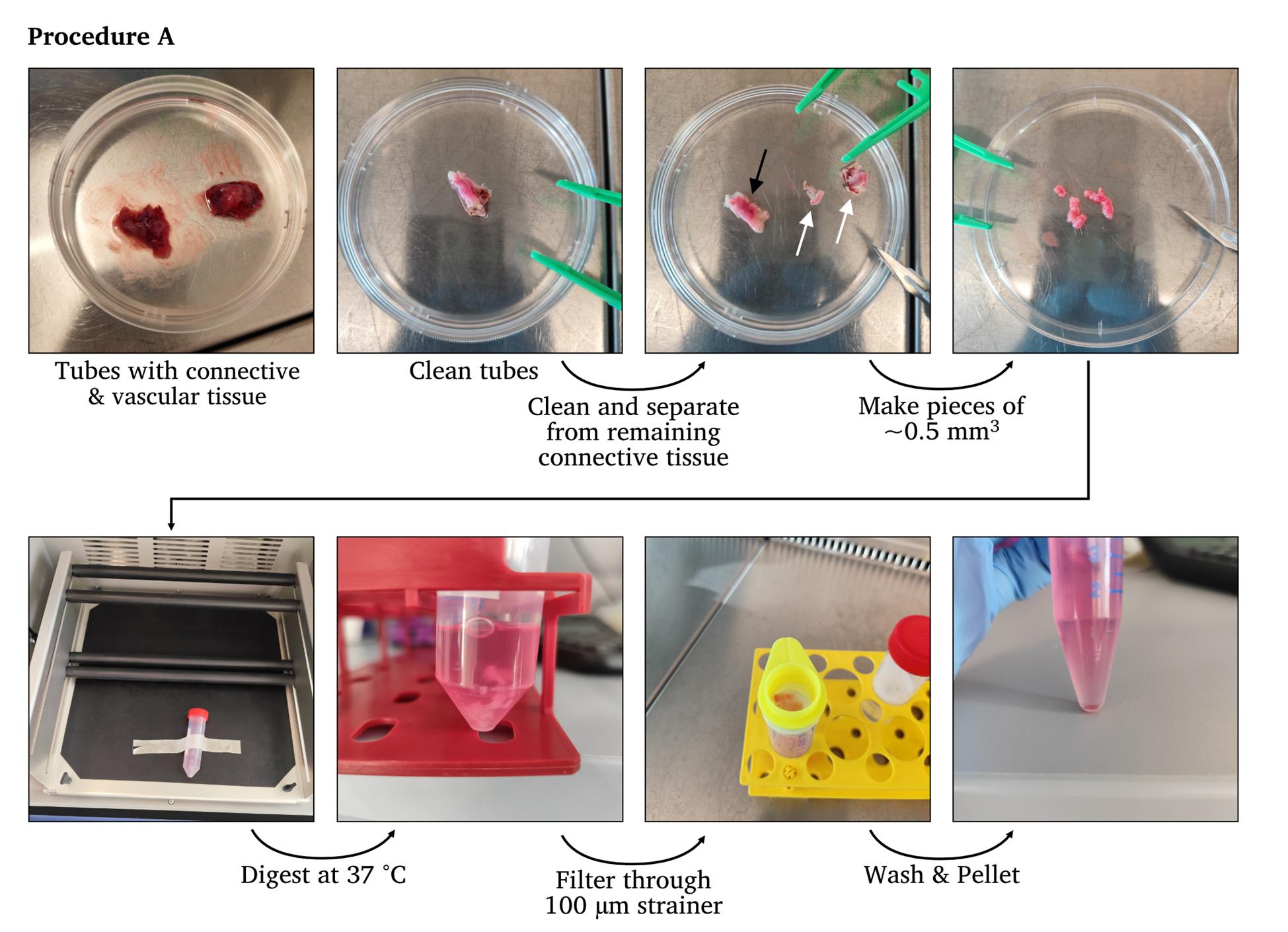
Figure 1. Representative images of the workflow of Procedure A. White arrows indicate connective tissues, and black arrows indicate cleaned fallopian tubes.
2. Wash the tissue with DPBS to remove remnants of body fluids.
3. Aspirate the DPBS.
4. Remove the connective and vascular tissues with a scalpel or surgical scissors.
5. Open the fallopian tubes longitudinally to expose the mucosal folds with surgical scissors.
6. Wash the inside with DPBS.
7. Aspirate the DPBS.
8. Scrape off epithelial cells from the submucosa and collect them in a Falcon tube. For this, the cells can be scraped three to five times vigorously along the lumen of the tube, at an approximately 45–60º angle to minimize vertical pressure using a disposable scalpel. Alternatively, cut into pieces of approximately 0.5 mm3 using a scalpel and/or surgical scissors.
Note: Scrapping of epithelial cells from the submucosa greatly improves the purity of the organoid cultures, but this is not always possible as some samples tend to be softer than others. Especially if connective tissue has been removed before, the scraping tends to be tedious or even impossible as the tissue falls apart.
9. Incubate the pieces in 0.5 mg/mL collagenase type I in ADF for 45 min at 37 °C to dissociate the epithelial cells from the extracellular matrix. We recommend a total of 10 mL of digestion volume.
Note: Incubation on a rocking plate at 37 °C and 100–200 rpm gives the best results. Digestion is sufficient when tissue pieces disintegrate and stretch out during rocking.
10. Filter through a 100 μm strainer into a 0.1% BSA-coated 50 mL tube to retain leftover tissue fragments.
11. Transfer to a coated 15 mL tube.
12. Pellet the collected cells by centrifugation at 500× g for 5 min at 25 °C.
13. Remove supernatant.
14. Wash with Advanced DMEM/F12 (ADF).
15. Pellet the collected cells by centrifugation at 500× g for 5 min at 25 °C.
16. Remove supernatant.
17. Resuspend the pellet in FT isolation medium (advanced DMEM/F12 containing 10 mM HEPES, 1% GlutaMax, 5% FCS, 10 ng/mL human EGF, 10 mM ROCK inhibitor, and 1% penicillin/streptomycin) and transfer to the uncoated T25 cell culture flasks.
Note: Include 10 µg/mL gentamicin and 1×/0.1% Fungin to avoid contamination.
18. Incubate at 37 °C and 5% CO2 in a humidified incubator.
Note: Monitor regularly for growth and exchange medium every 2 days (compare growth to Figure 2).
19. Once the cultures reach ~70% confluence, proceed to section B.
B. Transfer to three-dimensional FT organoid culture
A tissue fragment of ~5 cm3 normally gives rise to a confluent monolayer of FT epithelial progenitors of 8× 106 cells within 5 days. If routinely transferred to three-dimensional organoid culture, these cells could result in ≥ 320 wells, or ≥ fourteen 24-well plates, with 25,000 cells per ECM droplet. With an average expansion rate of approximately 9× between passages, these cultures can yield almost 1 × 108 cells within 3 weeks of isolation.
B1. Before starting, prepare the following:
1. Thaw an appropriate amount of ECM (up to 50 mL) on ice for subsequent usage.
Note: Depending on the aliquot volume of the ECM, thawing can take several hours. If 10 mL aliquots are used, thaw the ECM on ice inside a refrigerator overnight. Account for 1 mL of ECM for each 24-well plate.
Critical: ECM needs to be kept on ice at all times. Prewarm 24-well plates at 37 °C.
Note: The aim is to seed 25,000 cells per well. The number of 24-well plates necessary for this procedure is dependent on the confluence in section A.
2. Prepare a bucket with ice.
Note: Handling cells, medium, and ECM on ice allows for easier handling and reduces the risk of the ECM polymerizing.
3. Cool down a centrifuge to 4 °C for the subsequent steps.
4. Prepare ADF+++ and FT expansion medium.
5. Coat all 15 mL Falcon tubes with sterile 0.1% BSA in DPBS. Apply the solution to all surfaces that come into contact with cells by up-and-down pipetting or decanting.
B2. For the transfer:
1. Aspirate the medium from the flask(s) containing the cells.
2. Wash the cells in each flask with 10 mL of DPBS.
3. Aspirate the DBPS.
4. Detach the cells by incubating cells for 5–10 min with 2 mL of TrypLE Express.
Note: Monitor the detachment of cells and keep the incubation time to a minimum, as extended incubation damages the cells.
5. Harvest cells by adding 10 mL of ADF+++ and transfer the cell suspension to a 15 mL Falcon tube.
Note: The trypsin-replacement TrypLE Express does not require deactivation by FCS.
6. Pellet the collected cells by centrifugation at 300× g for 7 min at 4 °C.
7. Aspirate the supernatant.
8. Resuspend the cell pellet in an appropriate volume of ADF++ to reach a total volume of 10 mL.
9. Count the number of cells using a Neubauer Chamber or an automated cell counter.
10. Calculate the number of wells, plates, and volume of ECM needed for subsequent seeding.
Note: The aim is to seed 25,000 cells per 50 μL droplet of ECM per well. Following this, the aim is to seed 600,000 cells in 1,200 μL of ECM/cell/media suspension per 24-well plate. It is best to add 200 μL of cell/media suspension to 1 mL of ECM per plate. The higher the ratio of ECM to cell/media, the longer the ECM droplets will be stable. Try to maintain a 1:5 ratio of ECM to cells/media.
11. Pellet the collected cells by centrifugation at 300× g for 7 min at 4 °C.
12. Aspirate the supernatant.
13. Resuspend the pellet in an appropriate amount of ice-cold expansion media.
Note: Add 200 μL of medium per 600,000 cells, if following the calculations above.
14. Add an appropriate amount of ECM to the cell/media suspension.
Note: Add 1,000 μL of ECM per 600,000 cells, if following the calculations above.
15. Distribute the ECM/cell/media suspension in 50 μL droplets in each well of the prewarmed 24-well plates.
16. Incubate the plates at 37 °C for 30–45 min.
17. Prewarm the expansion medium in a water bath to 37 °C.
18. After polymerization of the ECM, overlay the droplets with 500 μL of prewarmed expansion medium.
19. Incubate at 37 °C and 5% CO2 in a humidified incubator and change medium every 2–4 days. Monitor by standard light microscopy (Figure 2).
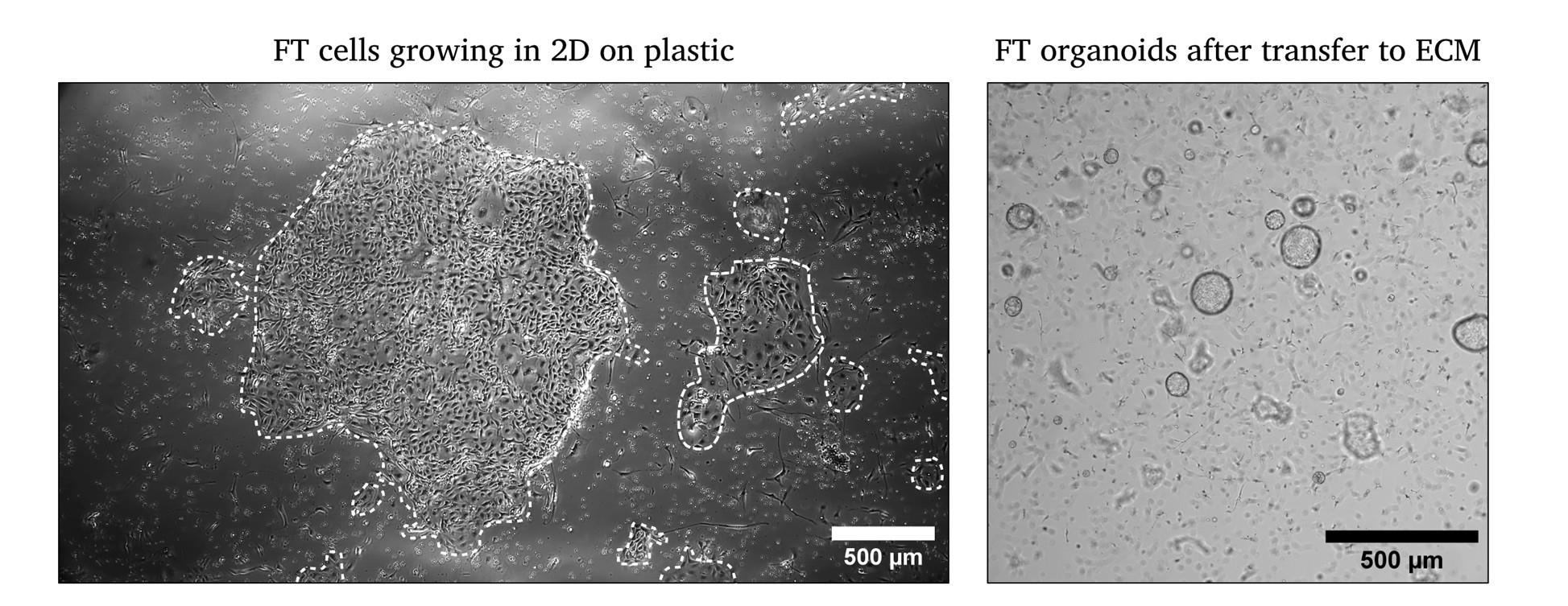
Figure 2. Representative images of FT cells after isolation in 2D and after transfer to ECM on day 3. White outlines mark the growth of epithelial cells in 2D.
C. Long-term maintenance of FT organoid cultures
The medium is exchanged every 2–4 days, depending on its color, and organoids are passaged every 14–21 days. Growth should be regularly monitored by microscopy (Figure 3). The organoids can be passaged at least 20 times without growth deficiencies. After successful establishment of cultures, organoids can be passaged/expanded with a 1:5 ratio.
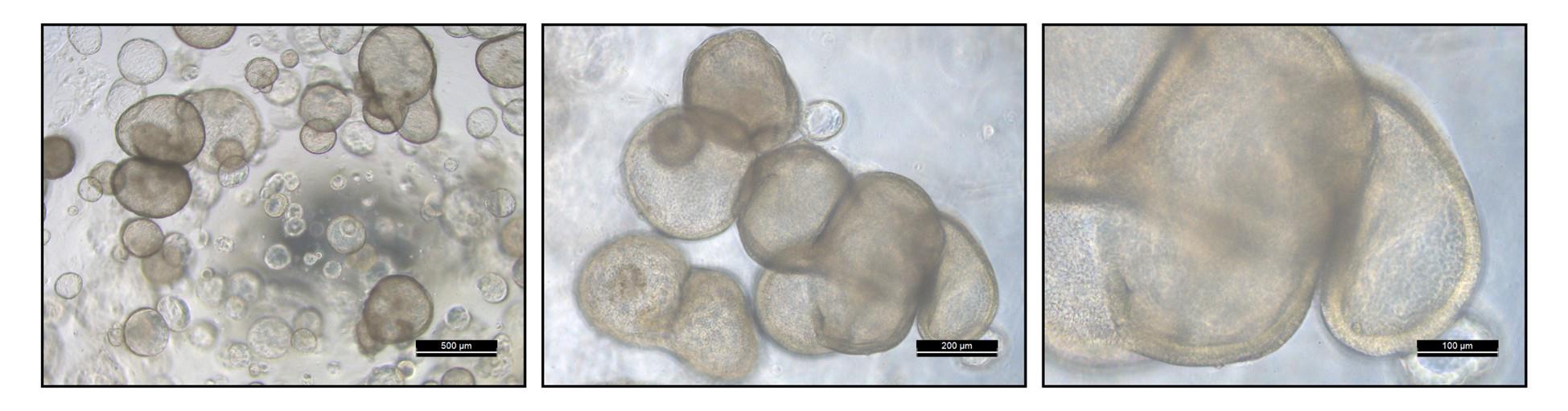
Figure 3. Fully matured passage 1 FT organoids ready for passaging. Scale bars are indicated for every image at the lower right corner. The third image is a zoomed-in inset of the second image. Refer to Figure 2 for non-matured organoids for comparison.
C1. Before starting, prepare the following:
1. Thaw an appropriate amount of the ECM on ice for subsequent usage.
Note: Depending on the aliquot volume of the ECM, thawing can take several hours. If 10 mL aliquots are used, thaw the ECM on ice inside a refrigerator overnight. Thaw 1 mL of ECM for each new 24-well plate.
Critical: ECM needs to be kept on ice at all times.
2. Prewarm an appropriate number of 24-well plates at 37 °C.
3. Prepare a bucket with ice.
Note: Handling cells, medium, and ECM on ice allows for easier handling and reduces the risk of the ECM polymerizing.
4. Cool down a centrifuge to 4 °C for the subsequent steps.
5. Prepare ADF+++ and FT expansion medium.
6. Coat all 15 mL Falcon tubes, needles, and syringes with sterile 0.1% BSA in DPBS. Apply the solution to all surfaces that come into contact with cells by up-and-down pipetting or decanting.
C2. For passaging:
1. Disrupt the ECM inside the medium in each well using a P1,000 pipette tip by scraping the ECM droplet inside the medium.
Note: It is faster to disrupt all wells first and then harvest the solution inside the 15 mL Falcon tubes. For beginners, it is best to monitor their progress with each well by disrupting and then directly harvesting.
2. Collect up to 12 wells in a single 15 mL Falcon tube.
Note: The inclusion of more wells per tube will result in incomplete pelleting of cells and ECM. Repeated washing steps are advisable in case large amounts of ECM are still visible on top of the cell pellet after centrifugation.
3. Add an appropriate amount of ice-cold ADF+++ to each 15 mL Falcon tube up to a total volume of 12 mL. In case of the recommended 12 wells, use 6 mL of ADF+++.
4. Close the tubes and invert the 15 mL Falcon tubes to mix the suspension.
5. Pellet the collected cells by centrifugation at 300× g for 5 min at 4 °C.
6. Aspirate the supernatant.
Note: ECM aspiration is not necessary as digestion and fragmentation, followed by washing, will remove the remaining ECM.
7. Add 1 mL of TrypLE Express and resuspend.
8. Incubate for 5 min at 37 °C in a water bath.
Note: A shaking water bath increases the efficacy of the digestion.
9. Fragment the organoids by up-and-down pipetting using a coated syringe with an 18 G needle.
Note: The amount of up-and-down pipetting is dependent on the handler and the needle's gauge. Generally, three to six times is sufficient for fragmentation.
10. Add an appropriate amount of ice-cold ADF+++ to each 15 mL Falcon tube up to a total volume of 12 mL.
11. Pellet the collected cells by centrifugation at 300× g for 5 min at 4 °C.
12. Aspirate the supernatant.
13. Add an appropriate amount of ice-cold ADF+++ and resuspend the pellet.
14. Add an appropriate amount of ECM to the cell/media suspension.
15. Distribute the ECM/cell/media suspension in 50 μL droplets in each well of the prewarmed 24-well plates.
16. Incubate the plates at 37 °C for 30–45 min.
17. Prewarm the expansion medium in a water bath to 37 °C.
18. Overlay the droplets with 500 μL of prewarmed expansion medium.
19. Incubate at 37 °C and 5% CO2 in a humidified incubator.
D. Cryopreservation of human fallopian tube organoids
D1. Organoid freezing
Let organoids grow for 5–7 days to have proliferative organoids that are not too large. The size should be approximately 100–200 μm.
D2. Before starting, prepare the following:
1. Prepare a bucket with ice.
Note: Handling cells, medium, and ECM on ice allows for easier handling and reduces the risk of the ECM polymerizing.
2. Cool down a centrifuge to 4 °C for the subsequent steps.
3. Coat all 15 mL Falcon tubes with sterile 0.1% BSA in DPBS. Apply the solution to the inner walls, either by up-and-down pipetting or decanting.
4. Follow section C up to step 6.
5. Add 1 mL of CryoSFM cryo medium for every 4 wells of organoids and resuspend the suspension.
6. Aliquot the suspension in cryovials with 1 mL per vial.
7. Place in -80 °C in an appropriate cryodevice (e.g., Mr. Frosty).
8. Transfer to liquid nitrogen the next day.
D3. Organoid thawing
Before starting, prepare the following:
1. Thaw an appropriate amount of ECM on ice for subsequent usage.
2. Prewarm an appropriate number of 24-well plates at 37 °C.
3. Prepare a bucket with ice.
Note: Handling cells, medium, and ECM on ice allows for easier handling.
4. Cool down a centrifuge to 4 °C for the subsequent steps.
5. Prepare ADF+++ and FT expansion medium.
6. Coat all 15 mL Falcon tubes with sterile 0.1% BSA in DPBS. Apply the solution to the inner walls, either by up-and-down pipetting or decanting.
7. Transfer organoids into the cryovial on ice to the laboratory.
8. Thaw the vial in a water bath at 37 °C.
Critical: Do not extend this period unnecessarily, as it decreases cell viability.
9. Add 11 mL of ice-cold ADF+++ into a coated 15 mL Falcon tube.
10. Resuspend the CryoSFM/organoid mixture in the cryovial and transfer to the 15 mL Falcon tube.
11. Pellet the collected cells by centrifugation at 300× g for 5 min at 4 °C.
12. Aspirate the supernatant.
13. Add an appropriate amount of ice-cold ADF+++ and resuspend the pellet.
14. Add an appropriate amount of ECM to the cell/media suspension.
15. Distribute the ECM/cell/media suspension in 50 μL droplets in each well of the prewarmed 24-well plates.
16. Incubate the plates at 37 °C for 30–45 min.
17. Prewarm the expansion medium in a water bath to 37 °C.
18. Overlay the droplets with 500 μL of prewarmed expansion medium.
20. Incubate at 37 °C and 5% CO2 in a humidified incubator.
E. Enrichment of secretory and ciliated cells in fallopian tube organoids
FT organoids are comprised of epithelial progenitors, secretory, and ciliated cells under standard growth conditions. Exemplary fluorescent images of fallopian tube organoids with differentiated cells are given in Figure 4. FT organoids can be further enriched with secretory cells using hormonal treatment and ciliated cells using dibenzazepine (via NOTCH signaling inhibition). We have provided an overview of expected magnitudes of differentiation and representative images in Figures 2, 3, 5, and 6 of Kessler et al. [11].
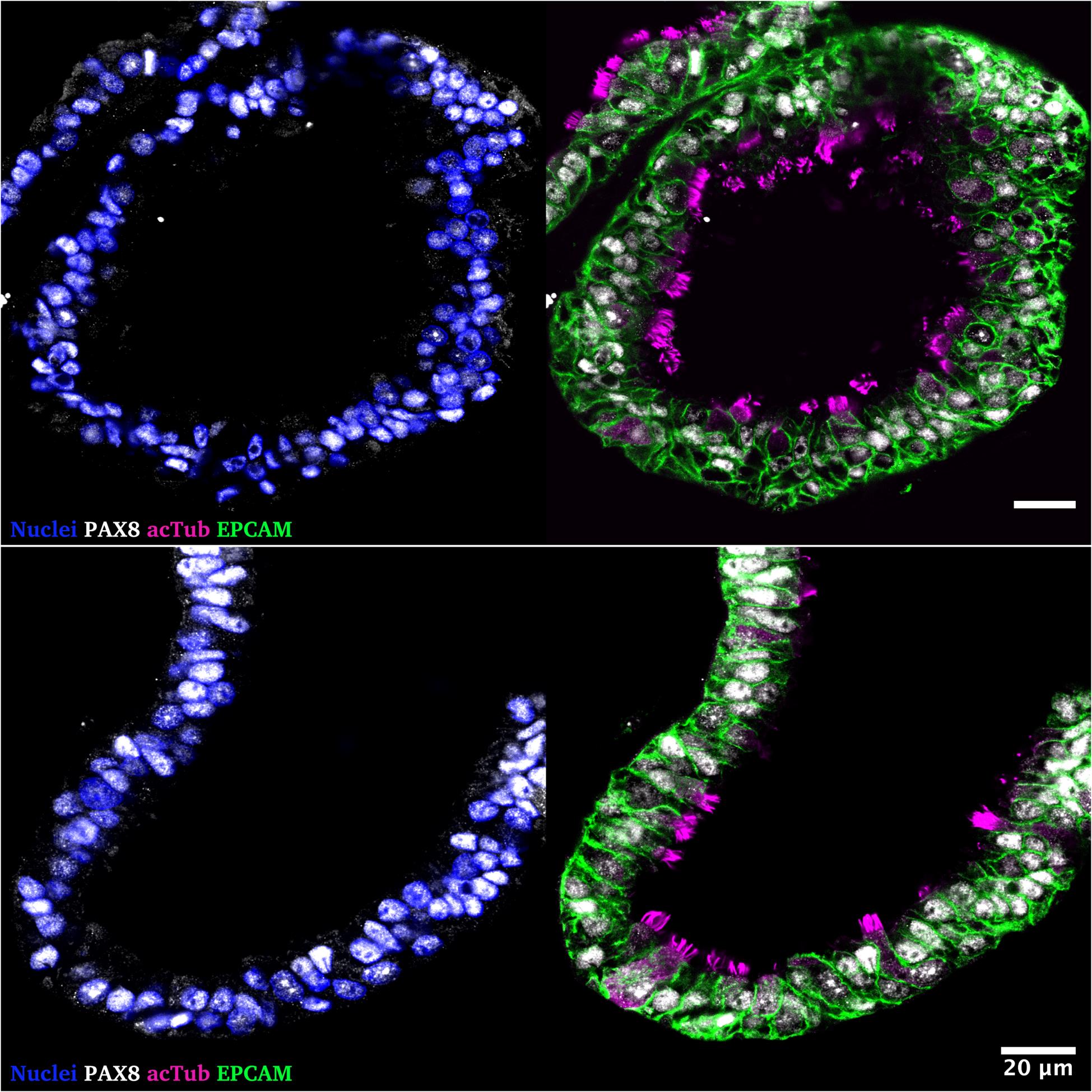
Figure 4. Representative immunofluorescence staining of human fallopian tube organoids cultured for 14 days (passage 8) in expansion medium. Left: Nuclear staining with DAPI (blue) and the Müllerian epithelial lineage marker PAX8 (white). Right: PAX8 (white) co-stained with epithelial cell marker EPCAM (green) and cilia marker acetylated Tubulin (magenta), highlighting luminal epithelial polarization and differentiation. Scale bars represent 20 μm. The following antibodies were used in this experiment: goat anti-EPCAM (1:200, Af960, R&D Systems), mouse anti-acetylated Tubulin (1:1,000, T7451-25, Sigma), rabbit anti-PAX8 (1:200, 10336-1-AP, Proteintech), donkey anti-goat Alexa Fluor Plus 488 (1:1,000, A32814, Invitrogen), donkey anti-mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 555 (1:1,000, A32773, Invitrogen), and donkey-anti-rabbit IgG (H+L) Alexa Fluor Plus 647 (1:1,000, A32795, Invitrogen). Images were taken using a Leica SP8 confocal microscope. Organoids were stained following the guidelines by Trillsch et al. [21].
1. Seed FT organoids as described in section C.
2. To further enrich secretory or ciliated cells in the fallopian tube organoids, add 1 μM dibenzazepine (DBZ, for ciliated cells), 500 pM β-estradiol, 50 ng/mL progesterone (both secretory cells), and DMSO or pure ethanol as mock treatment to the expansion media.
3. Overlay the ECM droplets with the respective differentiation media.
4. Harvest cells after 7 days of DBZ-treatment and 14 days of hormonal treatment for subsequent analysis.
Data analysis
Fallopian tube organoids can be used for several purposes, e.g., fallopian tube physiology and tissue architecture, infection studies with gynecological pathogens like Human papilloma virus or Chlamydia trachomatis, studies of WNT- and Notch-signaling, or cancer initiation and progression; they can also serve as toxicity control in personalized medicine purposes like drug sensitivity screens.
Downstream analyses may include but are not limited to RNA sequencing, mutational profiling by DNA sequencing, epigenetic profiling, western blotting, RT-qPCR, and immunofluorescence or immunohistochemistry assays.
Validation of protocol
This protocol or parts of it has been used and validated in the following research articles:
Kessler et al. [11]. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. Figures 1–6.
Kessler et al. [14]. Chronic Chlamydia infection in human organoids increases stemness and promotes age-dependent CpG methylation. Nat Commun. Figures 1–2.
Hoffmann et al. [15]. Stable expansion of high-grade serous ovarian cancer organoids requires a low-Wnt environment. The EMBO Journal. Figures 3–4.
General notes and troubleshooting
General notes
1. Work on ice whenever handling ECM.
2. Controlled production and subsequent quality control of conditioned media is essential before their use as media components. Closely follow published [19,20,22] or commercial protocols (ATCC, CRL-3276 and CRL-2647; ENZO LIFE SCIENCES, ENZ-61002) for production and testing.
Troubleshooting
Problem 1: Few epithelial cells after digestion.
Possible cause: Insufficient digestion of tissue.
Solution: Cut the tissue into a smaller size; increase incubation time.
Problem 2: Organoids are overcrowded, and medium turns yellow in color.
Possible cause: Excess number of epithelial cells seeded.
Solution: Seed an appropriate number of cells per ECM droplet. Higher density of organoids in the ECM may induce stress to epithelial cells and sudden death of organoids due to inadequate availability of growth components from the medium. Collect and reseed at a lower density.
Problem 3: Collapsed ECM and adherent cells.
Possible cause 1: ECM was not adequately mixed during aliquoting.
Solution 1: Completely thaw the ECM stock overnight at 4 °C in ice. Mix properly before aliquoting.
Possible cause 2: Ratio of ECM/Medium is too low.
Solution 2: Add more ECM to the cell/medium mixture before seeding.
Problem 4: No organoid growth.
Possible cause 1: Cells were diluted at a ratio too high.
Solution 1: Seed a higher density of epithelial cells in the initial culture.
Possible cause 2: Growth factor missing in the 3D medium or inactive.
Solution 2: Ensure that the growth factors were reconstituted at the recommended concentration and used within the expiry date, and double-check each growth factor addition during 3D medium preparation.
Possible cause 3: Conditioned media was not prepared well or lacks activity.
Solution 3: Test conditioned media with cell lines such as LEADING LIGHT® Wnt reporter cell line (ENZO LIFE SCIENCES, ENZ-61002) or other protocols [19].
Problem 5: Fewer organoids after harvest.
Possible cause: Organoids lost during aspiration; organoids stuck to the tube or pipette tip.
Solution: Coat all plastic with 0.1% BSA and/or use low-binding tubes.
Problem 6: Organoids suspended in the ECM after centrifugation (see also Figure 5 for comparison).
Possible cause 1: ECM was not cold during harvest.
Solution 1: Use ice-cold Advanced DMEM/F12 to harvest organoids and mix ECM three to four times with ADF.

Figure 5. Exemplary images of successful pelleting of cells during passaging. “Before” images taken after step C5 and “After” images taken after C11. White arrows indicate the border of ECM/supernatant, and black arrows indicate the border of cells/supernatant. The aspiration of ECM is not necessary, as the digestion, fragmentation, and washing will remove the remaining ECM.
Possible cause 2: ECM sticking to the tubes.
Solution 2: Coat all plastic with 0.1% BSA and/or use low-binding tubes.
Possible cause 3: Collected more than 12 wells in one 15 mL Falcon tube.
Solution 3: Divide the suspension into multiple 15 mL Falcon tubes. Collect less than 12 wells per tube.
Possible cause 4: Insufficient digestion.
Solution 4: Increase incubation time with TrypLE Express or up-and-down pipetting with the syringe.
Acknowledgments
Author contributions:
Methodology: M.K., T.F.M.; Visualization: DH; Resources: I.A., H.D.L., N.H., T.F.M.; Conceptualization: D.H., M.K., T.F.M.; Investigation: D.H., M.K., S.T.G.; Writing—Original Draft: D.H.; Writing—Review & Editing: all authors; Funding acquisition: D.H., N.H., M.K., T.F.M.; Supervision: N.H., M.K., T.F.M.
TFM acknowledges funding from BMBF through the Infect-ERA project “CINOCA” (FK 031A409A) and the ERC Advanced grant “MADMICs” (ID 885008). MK is supported by ERA PERMED 2022-141 (BMBF Grant 01KU2302). NH and DH acknowledge support by the University Cancer Centre Schleswig-Holstein (UCCSH), the University Cancer Centre Hamburg (UCCH), and the University Clinic Schleswig Holstein (UKSH). The authors acknowledge financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
This work was originally described and validated in [11].
The authors thank Finja Grundt and Frauke Grohmann for valuable feedback during the writing of this protocol. We thank all other involved colleagues from the Department of Gynecology and Obstetrics at the University Hospital Schleswig-Holstein in Kiel for the provision of tissues.
The authors acknowledge support by Caroline Wiensauer, Farhana Ishrat Ghani, Aki Imai, Jörg Angermann, Ina Wagner, Christian Goosmann, and Jenny Kirsch for technical input and support, and thank Rike Zietlow for editing and Diane Schad for graphical assistance in the original research paper.
The graphical overview figure was created in https://BioRender.com and accessible via https://biorender.com/4vk88sq.
Competing interests
M.K. and T.F.M. are inventors of a patent describing the generation, maintenance, and use of ovarian cancer organoids (EP3755791). D.H., N.H., S.T.G., H.D.L., and I.A. declare no conflicts of interest.
Ethical considerations
Human fallopian tube samples were provided by the Department of Gynecology at the Charité University Hospital, Campus Virchow Clinic and the Department of Gynecology and Obstetrics at the University Hospital Schleswig-Holstein, Kiel, Germany. Scientific usage of the samples for experimental purposes was ethically approved by either the commission of the Charité, Berlin (EA1/002/07) or the commission of the medical faculty in Kiel (D631/24); all subjects gave informed consent to their tissue being used in scientific research.
References
- Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., van Es, J. H., Abo, A., Kujala, P., Peters, P. J., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459(7244): 262–265. https://doi.org/10.1038/nature07935
- Drost, J. and Clevers, H. (2018). Organoids in cancer research. Nat Rev Cancer. 18(7): 407–418. https://doi.org/10.1038/s41568-018-0007-6
- Clevers, H. (2016). Modeling Development and Disease with Organoids. Cell. 165(7): 1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
- Kayser, C., Brauer, A., Susanne, S. and Wandmacher, A. M. (2023). The challenge of making the right choice: patient avatars in the era of cancer immunotherapies. Front Immunol. 14: e1237565. https://doi.org/10.3389/fimmu.2023.1237565
- Battistini, C. and Cavallaro, U. (2023). Patient-Derived In Vitro Models of Ovarian Cancer: Powerful Tools to Explore the Biology of the Disease and Develop Personalized Treatments. Cancers (Basel). 15(2): 368. https://doi.org/10.3390/cancers15020368
- Lõhmussaar, K., Boretto, M. and Clevers, H. (2020). Human-Derived Model Systems in Gynecological Cancer Research. Trends Cancer. 6(12): 1031–1043. https://doi.org/10.1016/j.trecan.2020.07.007
- Sato, T., Stange, D. E., Ferrante, M., Vries, R. G., van Es, J. H., van den Brink, S., van Houdt, W. J., Pronk, A., van Gorp, J., Siersema, P. D., et al. (2011). Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett's Epithelium. Gastroenterology. 141(5): 1762–1772. https://doi.org/10.1053/j.gastro.2011.07.050
- Huch, M., Gehart, H., van Boxtel, R., Hamer, K., Blokzijl, F., Verstegen, M. M., Ellis, E., van Wenum, M., Fuchs, S. A., de Ligt, J., et al. (2015). Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell. 160: 299–312. https://doi.org/10.1016/j.cell.2014.11.050
- Huch, M., Bonfanti, P., Boj, S. F., Sato, T., Loomans, C. J. M., van de Wetering, M., Sojoodi, M., Li, V. S. W., Schuijers, J., Gracanin, A., et al. (2013). Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32(20): 2708–2721. https://doi.org/10.1038/emboj.2013.204
- Bartfeld, S., Bayram, T., van de Wetering, M., Huch, M., Begthel, H., Kujala, P., Vries, R., Peters, P. J. and Clevers, H. (2015). In Vitro Expansion of Human Gastric Epithelial Stem Cells and Their Responses to Bacterial Infection. Gastroenterology. 148(1): 126–136.e6. https://doi.org/10.1053/j.gastro.2014.09.042
- Kessler, M., Hoffmann, K., Brinkmann, V., Thieck, O., Jackisch, S., Toelle, B., Berger, H., Mollenkopf, H. J., Mangler, M., Sehouli, J., et al. (2015). The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 6(1): e1038/ncomms9989. https://doi.org/10.1038/ncomms9989
- Leese, H., Tay, J., Reischl, J. and Downing, S. (2001). Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction. 121(3): 339–346. https://doi.org/10.1530/rep.0.1210339
- Crawford, A. J., Forjaz, A., Bons, J., Bhorkar, I., Roy, T., Schell, D., Queiroga, V., Ren, K., Kramer, D., Huang, W., et al. (2024). Combined assembloid modeling and 3D whole-organ mapping captures the microanatomy and function of the human fallopian tube. Sci Adv. 10(39): eadp6285. https://doi.org/10.1126/sciadv.adp6285
- Kessler, M., Hoffmann, K., Fritsche, K., Brinkmann, V., Mollenkopf, H. J., Thieck, O., Teixeira da Costa, A. R., Braicu, E. I., Sehouli, J., Mangler, M., et al. (2019). Chronic Chlamydia infection in human organoids increases stemness and promotes age-dependent CpG methylation. Nat Commun. 10(1): 1194. https://doi.org/10.1038/s41467-019-09144-7
- Hoffmann, K., Berger, H., Kulbe, H., Thillainadarasan, S., Mollenkopf, H., Zemojtel, T., Taube, E., Darb‐Esfahani, S., Mangler, M., Sehouli, J., et al. (2020). Stable expansion of high‐grade serous ovarian cancer organoids requires a low‐Wnt environment. EMBO J. 39(6): e2019104013. https://doi.org/10.15252/embj.2019104013
- Dai, Y., Xu, J., Gong, X., Wei, J., Gao, Y., Chai, R., Lu, C., Zhao, B. and Kang, Y. (2024). Human Fallopian Tube-Derived Organoids with TP53 and RAD51D Mutations Recapitulate an Early Stage High-Grade Serous Ovarian Cancer Phenotype In Vitro. Int J Mol Sci. 25(2): 886. https://doi.org/10.3390/ijms25020886
- Willert, K., Brown, J. D., Danenberg, E., Duncan, A. W., Weissman, I. L., Reya, T., Yates, J. R. and Nusse, R. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 423(6938): 448–452. https://doi.org/10.1038/nature01611
- Kim, K. A., Kakitani, M., Zhao, J., Oshima, T., Tang, T., Binnerts, M., Liu, Y., Boyle, B., Park, E., Emtage, P., et al. (2005). Mitogenic Influence of Human R-Spondin1 on the Intestinal Epithelium. Science. 309(5738): 1256–1259. https://doi.org/10.1126/science.1112521
- VanDussen, K. L., Sonnek, N. M. and Stappenbeck, T. S. (2019). L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem Cell Res. 37: 101430. https://doi.org/10.1016/j.scr.2019.101430
- Warschkau, D., Delgado-Betancourt, E., Holthaus, D., Müller, A., Kliem, G., Krug, S., Schulzke, J., Aebischer, T., Klotz, C., Seeber, F., et al. (2022). From 3D to 2D: Harmonization of Protocols for Two-dimensional Cultures on Cell Culture Inserts of Intestinal Organoids from Various Species. Bio Protoc. 12(2): e4295. https://doi.org/10.21769/bioprotoc.4295
- Trillsch, F., Czogalla, B., Kraus, F., Burges, A., Mahner, S. and Kessler, M. (2023). Protocol to optimize the biobanking of ovarian cancer organoids by accommodating patient-specific differences in stemness potential. STAR Protoc. 4(3): 102484. https://doi.org/10.1016/j.xpro.2023.102484
- Gurumurthy, R. K., Koster, S., Kumar, N., Meyer, T. F. and Chumduri, C. (2022). Patient-derived and mouse endo-ectocervical organoid generation, genetic manipulation and applications to model infection. Nat Protoc. 17(7): 1658–1690. https://doi.org/10.1038/s41596-022-00695-6
Article Information
Publication history
Received: May 5, 2025
Accepted: Jul 9, 2025
Available online: Jul 24, 2025
Published: Aug 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Holthaus, D., Hedemann, N., Geweniger, S. T., Le, H. D., Alkatout, I., Kessler, M. and Meyer, T. F. (2025). Establishing and Maintaining 3D Organoid Cultures From Human Fallopian Tube Epithelium. Bio-protocol 15(16): e5412. DOI: 10.21769/BioProtoc.5412.
Category
Cell Biology > Cell isolation and culture > 3D cell culture
Developmental Biology > Morphogenesis > Organogenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








