- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Culture of Ferret Airway Stem Cells
Published: Vol 15, Iss 14, Jul 20, 2025 DOI: 10.21769/BioProtoc.5399 Views: 2408
Reviewed by: Wendy Leanne HempstockAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
An In Vitro Model of Murine Osteoclast-Mediated Bone Resorption
Xiaoyue Sun [...] Lingxin Zhu
Nov 5, 2024 2204 Views
Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3903 Views
Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 193 Views
Abstract
Well-differentiated airway epithelial cultures are commonly used to study airway stem cell lineages, ion and fluid transport, respiratory virus infection and replication, and disease mechanisms in vitro. This culture model involves the isolation and expansion of airway stem cells followed by their differentiation at an air–liquid interface (ALI), a process that has been previously documented in humans and mice. Domestic ferrets (Mustela putorius furo) have gained considerable importance in respiratory disease research due to their notable susceptibility to these conditions and their anatomical similarities to humans. Here, we present a comprehensive description of the isolation and culture of stem/progenitor cells from the ferret airway, along with a protocol for their differentiation at the ALI. Our findings have demonstrated that this ferret culture system not only supports the differentiation of the predominant airway epithelial cell types but also facilitates the generation of rare airway epithelial subpopulations, including pulmonary ionocytes, tuft cells, and pulmonary neuroendocrine cells. Additionally, we provide a detailed procedure for measuring transepithelial ion transport relevant to airway diseases, particularly cystic fibrosis. The ability to isolate and culture ferret airway stem cells, combined with ALI differentiation and functional assessment of transepithelial ion transport, offers a powerful platform for evaluating genetic and pharmacologic interventions related to cystic fibrosis.
Key features
• A protocol for isolating ferret airway basal cells and generating air–liquid interface (ALI) cultures for electrophysiologic research.
• Detailed procedures for propagating ferret airway basal cells and culturing in vitro well-differentiated airway epithelium.
• A protocol for measuring ion transport, conductance, and immunofluorescence to identify airway cell types.
Keywords: Transgenic ferretsGraphical overview
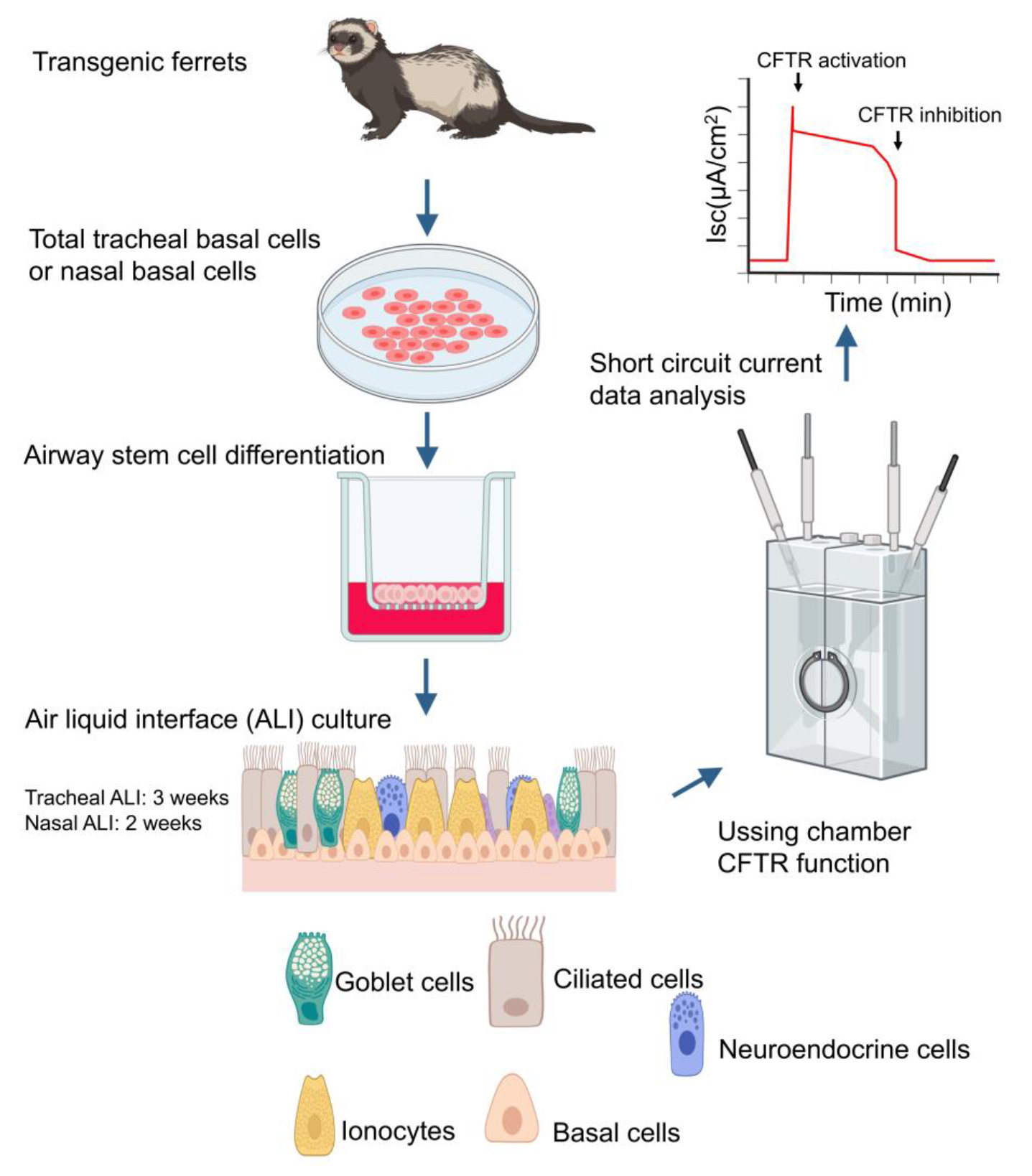
Background
The ferret, Mustela putorius furo, has proven to be an invaluable model for research into complex pulmonary conditions, including cystic fibrosis (CF) [1–4], idiopathic pulmonary fibrosis (IPF) [5], and chronic obstructive pulmonary disease (COPD) [6], the infection and transmission of respiratory viruses, such as influenza viruses [7,8], and neurological research [7,9]. Ferrets are a mid-sized experimental animal model with anatomical and physiological similarities to humans [10], particularly the respiratory system, offering a balance between practicality and biological relevance to bridge the gap between nonhuman primates and the widely utilized rodent models [4,11,12]. In the airway anatomy, ferrets and humans have submucosal glands in the cartilaginous airways, similar distribution patterns of goblet cells in the proximal airway, and club cells across the distal airway and respiratory bronchioles [10].
Human airway epithelium (HAE) air–liquid interface (ALI, HAE-ALI) cultures, which comprise multiple epithelial cell types and recapitulate the pseudostratified structure of the proximal airway epithelium, represent an invaluable in vitro model system for investigating the physiology and pathophysiology of human airways. In CF research, ALI cultures derived from CF patient donors enable the study of cystic fibrosis transmembrane regulator (CFTR) function, including chloride and bicarbonate transport and other aspects that are altered in CF, such as airway surface liquid (ASL) chemistry, pH, and hydration. In the past, human ALI cultures were a crucial resource in studying CF. However, the utility of these cultures encounters several limitations, including difficulties in obtaining adequate materials, limitations in airway basal cell passage capacity, and challenges related to airway basal cell expansion.
The successful establishment of CF ferret models provides a compelling alternative [1–3,13]. Polarized ferret airway epithelium (FAE) cultured at an ALI (FAE-ALI) differentiated from the base cells of CF animals comprises not only the predominant airway epithelial cell types but also rare airway epithelial subpopulations, including pulmonary ionocytes, tuft cells, and pulmonary neuroendocrine cells. Importantly, we have established culture conditions to enrich and expand the primary ferret airway stem/progenitor cells from the nose, trachea, and bronchi tissues. These stem/progenitor cells retain their differentiated capacity for up to 12 passages.
Notably, the pulmonary ionocyte has been identified as showing high levels of CFTR mRNA expression [11,14] and protein in the apical cap [15] in the airway epithelium. Their function in the airway has been recognized, including involvement in fluid absorption [15,16], secretion [15], pH regulation [15,17], and mucociliary clearance [15]. Pulmonary ionocytes represent a rare epithelial cell type, like tuft cells and pulmonary neuroendocrine cells (PNEC); their functions remain to be thoroughly explored. In this protocol, we aimed to create a method for isolating and culturing ferret airway stem cells from any airway location. Additionally, we sought to develop ALI cultures capable of continuously producing rare epithelial cell types and examining transepithelial ion transport functions. This protocol for culturing ferret airway stem cells is particularly valuable for investigating CF and exploring potential therapeutic interventions.
Materials and reagents
Biological materials
1. Ferret airway tissue (National Ferret Resource and Research Center)
2. 804G cell line (a rat bladder carcinoma cell line, a gift from Jayaraj Rajagopal Laboratory) [18]
Reagents
1. Pronase (Roche, catalog number: 11459643001)
2. Accutase (StemCell Technology, catalog number: 07920)
3. Hydrocortisone stock solution (StemCell Technology, catalog number: 07925)
4. Heparin sodium salt (StemCell Technology, catalog number: 07980)
5. Penicillin-streptomycin (Gibco, catalog number: 15140122)
6. Protease from Streptomyces griseus, type XIV (Millipore Sigma, catalog number: P5147)
7. CryoStor® CS10 (StemCell Technology, catalog number: 07930)
8. PneumaCultTM-Ex Plus basal medium (StemCell Technology, catalog number: 05041)
9. PneumaCultTM-Ex Plus 50× supplement (StemCell Technology, catalog number: 05042)
10. PneumaCultTM-ALI basal medium (StemCell Technology, catalog number: 05002)
11. PneumaCultTM-ALI 10× supplement (StemCell Technology, catalog number: 05003)
12. PneumaCultTM-ALI maintenance 100× supplement (StemCell Technology, catalog number: 05006)
13. F-12 medium (Gibco, catalog number: 11765-054)
14. RPMI medium 1640 (Gibco, catalog number: 11875-093)
15. GlyH101 (TOCRIS, catalog number: 5485)
16. Amiloride hydrochloride hydrate (Millipore Sigma, catalog number: A7410-1G)
17. DIDS disodium salt (Millipore Sigma, catalog number: 309795)
18. 3-Isobutyl-1-methylxanthine (Millipore Sigma, catalog number: I5879)
19. Forskolin (Millipore Sigma, catalog number: F6886)
20. DPBS without Ca2+ and Mg2+ (Gibco, catalog number: 14190144)
21. Donkey serum non-sterile (Equitech-Bio, catalog number: DS-05-1000)
22. Minimum essential medium (Gibco, catalog number: 11095080)
23. Fetal bovine serum (FBS) (Gibco, catalog number: 26140079)
24. Primaxin® (imipenem/cilastatin, prescription drug at the pharmacy)
25. Amphotericin B (Millipore Sigma, catalog number: A2942)
26. Ceftazidime (Millipore Sigma, catalog number: C3809)
27. Gentamicin solution (IBI Scientific, catalog number:1405-41-0)
28. Paraformaldehyde solution (PFA), 4% in PBS (Thermo Scientific, catalog number: J19943-K2)
29. CaCl2 (Research Products International, catalog number: C20010)
30. NaCl (Research Products International, catalog number: S23025)
31. MgCl2 (Research Products International, catalog number: M25000)
32. NaHCO3 (Research Products International, catalog number: S25060)
33. Dextrose (Research Products International, catalog number: G32045)
34. HEPES, free acid (Research Products International, catalog number: H75030)
35. KH2PO4 (Research Products International, catalog number: P41200)
36. K2HPO4 (Research Products International, catalog number: P41425)
37. Mg-gluconate (Millipore Sigma, catalog number: G9130)
38. Na-gluconate (Millipore Sigma, catalog number: G9005)
39. Ca-gluconate (Millipore Sigma, catalog number: G4625)
40. Sodium citrate (Research Products International, catalog number: S23045)
41. Aqua-mount (Epredia, catalog number: 13800)
42. ProLong glass antifade mountant (Invitrogen, catalog number: P36980)
43. DAPI and Hoechst nucleic acid stains (Thermo Scientific, catalog number: 62249)
44. TritonTM X-100 (Millipore Sigma, catalog number: X100-5ML)
45. TWEEN 20 (Millipore Sigma, catalog number: P6585-10ML)
46. Anti-NKA (DSHB UIOWA, catalog number: a5, working dilution: 1/100, ionocyte marker)
47. Anti-CFTR (cftrantibodies.web.unc.edu, catalog number: 596, working dilution: 1/200, ionocyte marker)
48. Anti-acetylated tubulin (Sigma-Aldrich, catalog number: T7451, working dilution: 1/1000, ciliated cell marker)
49. Anti-SYP (Santa Cruz Biotechnology, catalog number: sc-17750, working dilution: 1/200, pulmonary neuroendocrine cell marker)
50. Anti-BSND (Abcam, catalog number: ab196017, working dilution: 1/400, ionocyte marker)
51. Anti-ATP6V1G3 (Sigma-Aldrich, catalog number: HPA028701, working dilution: 1/300, ionocyte marker)
Solutions
1. 804 G culture media (see Recipes)
2. PneumaCultTM-Ex Plus basal cell culture media (see Recipes)
3. PneumaCultTM-ALI airway differentiation media (see Recipes)
4. High chloride buffer (see Recipes)
5. Low chloride buffer (see Recipes)
6. K- buffer (see Recipes)
7. Bicarbonate buffer (see Recipes)
8. Donkey serum blocking buffer (see Recipes)
9. Donkey serum dilution buffer (see Recipes)
10. 4× MEM wash media with antibiotics (see Recipes)
11. F12 wash media with antibiotics (see Recipes)
12. Pronase digestion media (see Recipes)
13. Protease digestion media (see Recipes)
14. Sodium citrate buffer (see Recipes)
Recipes
1. 804 G culture media
Note: The media is filtered in a Corning 500 mL filter system and stored at -20 °C for a year; it can be warmed up at 37 °C in a Thermo Scientific Precision water bath.
| Reagent | Final concentration | Amount |
|---|---|---|
| RPMI medium 1640 | n/a | 445 mL |
| FBS | n/a | 50 mL |
| Penicillin-streptomycin | n/a | 5 mL |
| Total | n/a | 500 mL |
2. PneumaCultTM-Ex Plus basal cell culture media
Note: The media is filtered in a Corning 500 mL filter system and stored at 4 °C for a month; it can be warmed up at 37 °C in a Thermo Scientific Precision water bath.
| Reagent | Final concentration | Amount |
|---|---|---|
| PneumaCultTM-Ex Plus basal medium | n/a | 484.5 mL |
| PneumaCultTM-Ex Plus 50× supplement | n/a | 10 mL |
| Penicillin-streptomycin | n/a | 5 mL |
| Hydrocortisone stock solution | n/a | 0.5 mL |
| Total | n/a | 500 mL |
3. PneumaCultTM-ALI airway differentiation media
Note: The media is filtered in a Corning 500 mL filter system and stored at 4 °C for a month; it can be warmed up at 37 °C in a Thermo Scientific Precision water bath.
| Reagent | Final concentration | Amount |
|---|---|---|
| PneumaCultTM-ALI basal medium | n/a | 439 mL |
| PneumaCultTM-ALI 10× supplement | n/a | 50 mL |
| PneumaCultTM-ALI maintenance 100× supplement | n/a | 5 mL |
| Heparin sodium salt | n/a | 1 mL |
| Penicillin-streptomycin | n/a | 5 mL |
| Total | n/a | 500 mL |
4. High chloride buffer
Note: Use ddH2O to dissolve. Adjust to pH 7.4 with NaOH, filter, and store at 4 °C for one month.
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | n/a | 7.89 g |
| HEPES Acid | n/a | 1.192 g |
| Dextrose | n/a | 1.3572 g |
| MgCl2 (1 M stock solution) | 1.2 mM | 1.2 mL |
| CaCl2 (1 M stock solution) | 1.2 mM | 1.2 mL |
| KH2PO4 (1 M stock solution) | 0.6 mM | 0.6 mL |
| K2HPO4 (1 M stock solution) | 2.4 mM | 2.4 mL |
| Total | n/a | 1,000 mL |
5. Low chloride buffer
Note: Use ddH2O to dissolve. Adjust to pH 7.4 with NaOH, filter, and store at 4 °C for one month.
| Reagent | Final concentration | Amount |
|---|---|---|
| Na-gluconate | n/a | 29.95 g |
| HEPES acid | n/a | 1.192 g |
| Dextrose | n/a | 1.3572 g |
| MgCl2 (1 M stock solution) | 1.2 mM | 1.2 mL |
| CaCl2 (1 M stock solution) | 1.2 mM | 1.2 mL |
| KH2PO4 (1 M stock solution) | 0.6 mM | 0.6 mL |
| K2HPO4 (1 M stock solution) | 2.4 mM | 2.4 mL |
| Total | n/a | 1,000 mL |
6. K- Buffer
Note: Use ddH2O to dissolve. Adjust to pH 7.4 with HCl, filter, and store at 4 °C for one month.
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | n/a | 6.94 g |
| NaHCO3 | n/a | 2.1 g |
| Dextrose | n/a | 0.091 g |
| MgCl2 (1 M stock solution) | 1.2 mM | 1.2 mL |
| CaCl2 (1 M stock solution) | 1.2 mM | 1.2 mL |
| KH2PO4 (1 M stock solution) | 0.6 mM | 0.6 mL |
| K2HPO4 (1 M stock solution) | 2.4 mM | 2.4 mL |
| Total | n/a | 1,000 mL |
7. Bicarbonate buffer
Note: Use ddH2O to dissolve. Adjust to pH 7.4 with H2SO4, filter, and store at 4 °C for one month.
| Reagent | Desired concentration | Amount |
|---|---|---|
| Na-gluconate | 118.9 mM | 25.94 g |
| NaHCO3 | 25 mM | 2.1 g |
| Dextrose | 5 mM | 0.901 g |
| Mg-gluconate | 1 mM | 0.415 g |
| Ca-gluconate | 5 mM | 2.152 g |
| KH2PO4 solution (1 M) | 0.6 mM | 0.6 mL |
| K2HPO4 solution (1 M) | 2.4 mM | 2.4 mL |
| Total | n/a | 1,000 mL |
8. Donkey serum blocking buffer
Note: Filter and freeze aliquots (5 mL per aliquot) at -20 °C. Aliquots can be stored at -20 °C for a year or for ~2 weeks at 4 °C.
| Reagent | Final concentration | Amount |
|---|---|---|
| Donkey serum | n/a | 10 mL |
| CaCl2 | 2.5 M | 20 μL |
| 10% Triton X | n/a | 250 μL |
| DPBS | n/a | 39.730 mL |
| Total | n/a | 50 mL |
9. Donkey serum dilution buffer
Note: Filter and freeze aliquots (5 mL per aliquot) at -20 °C. Aliquots can be stored -20 °C for a year or for ~2 weeks at 4 °C.
| Reagent | Final concentration | Amount |
|---|---|---|
| Donkey serum | n/a | 500 μL |
| CaCl2 (2.5 M stock solution) | 1 mM | 20 μL |
| 10% Triton X | n/a | 250 μL |
| DPBS | n/a | 49.230 mL |
| Total | n/a | 50 mL |
10. 4× MEM wash media with antibiotics
Note: Filter and freeze aliquots at -80 °C or -20 °C. Aliquots can be stored at -20 °C for a year.
| Reagent | Final concentration | Amount |
|---|---|---|
| Minimum essential medium | n/a | 473.75 mL |
| Primaxin (5 mg/mL stock solution) | 0.1 mg/mL | 10 mL |
| Amphotericin B (125 μg/mL stock) | 1 μg/mL | 4 mL |
| Ceftazidime (50 mg/mL stock solution) | 0.1 mg/mL | 1 mL |
| Gentamicin (50 mg/mL stock solution) | 0.125 mg/mL | 1.25 mL |
| Penicillin-streptomycin | n/a | 10 mL |
| Total | n/a | 500 mL |
11. F12 wash media with antibiotics
Note: Filter and store for ~1 month at 4 °C.
| Reagent | Final concentration | Amount |
|---|---|---|
| F-12 medium | n/a | 44.5 mL |
| FBS | n/a | 5 mL |
| Penicillin-streptomycin | n/a | 500 μL |
| Total | n/a | 50 mL |
12. Pronase digestion media
| Reagent | Final concentration | Amount |
|---|---|---|
| F-12 medium | n/a | 10 mL |
| Pronase | n/a | 100 mg |
| Total | n/a | 10 mL |
13. Protease digestion media
| Reagent | Final concentration | Amount |
|---|---|---|
| 4× MEM wash media | n/a | 10 mL |
| Protease, type XIV | n/a | 15 mg |
| Total | n/a | 10 mL |
14. Sodium citrate buffer
Note: Adjust pH to 6.0 with HCl.
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium citrate | n/a | 2.94 g |
| ddH2O | n/a | 1,000 mL |
| Tween 20 | n/a | 0.5 mL |
| Total | n/a | 1,000 mL |
Laboratory supplies
1. Corning 6.5 mm Transwell® with 0.4 μm pore polyester membrane insert (Corning, catalog number: 3470)
2. Corning cell strainer 100 μm nylon (Corning, catalog number: CLS431752)
3. Premium touch nitrile gloves (Laboratory Products Sales, catalog numbers: 1720-PF, 1721-PF, 1722-PF)
4. FisherbrandTM sterile polystyrene disposable serological pipettes with magnifier stripe (Thermo Fisher Scientific, catalog numbers: 1367811, 1367811D, 1367811E)
5. InvitrogenTM CountessTM cell counting chamber slides and holder, disposable (Invitrogen, catalog number: T10282)
6. Trypan blue stain (Invitrogen, catalog number: T10282)
7. FisherbrandTM SureOneTM aerosol barrier pipette tips (Thermo Fisher Scientific, catalog number: 02707430)
8. Centrifuge tube, 50 mL (Thermo Fisher Scientific, catalog number: 14955239)
9. Centrifuge tube, 15 mL (Thermo Fisher Scientific, catalog number: 14955237)
10. Cell culture dish 100 mm diameter (Thermo Fisher Scientific, catalog number: 130182)
11. Corning 500 mL filter system (Corning, catalog number: 431097)
12. Sterile surgical blades (Cincinnati Surgical, No. 22 and No. 11)
13. Kelly hemostat (Supply Clinic, catalog number: HTK145)
14. 2.0 mL Corning cryogenic vials with washer (Corning, catalog number: CLS431417)
15. Cryo-safe cooler -1 °C freezer (Bel-Art Products, catalog number: F18844-0000)
16. Scalpel blade (Millipore Sigma, catalog number: S2771)
17. Oxygen (Linde Gas & Equipment Inc., catalog number: OX M-K)
Equipment
1. Thermo Scientific Precision water bath GP 15D, 5 L and 10 L (Thermo Fisher Scientific, catalog number: TSGP15D)
2. Beckman Coulter Allegra GS-6R refrigerated benchtop centrifuge (Beckman, model: GS-6R Centrifuge)
3. P2300 multi-channel Ussing chamber system with integrated VCC MC8 voltage/current clamp (Physiologic Instruments, Inc)
4. Type A2 biological safety cabinet (Thermo Scientific, model: 1300 series A2)
5. Caron Oasis CO2 incubator (Caron, model: Oasis 6404-1)
6. Orion Star A211 pH meter (Thermo Scientific, model: STARA2110)
7. Confocal laser scanning microscope (either Zeiss LSM 880 or Zeiss LSM 980 will be used during imaging)
8. Invitrogen Countess 3 automated cell counter (Thermo Fisher Scientific, catalog number: A49865)
Software and datasets
1. Fiji software (free, open-source image processing package, a distribution of ImageJ + ImageJ2)
2. Acquire & Analyze (bioelectrical properties data acquisition and analysis, Physiologic Instruments Inc.)
3. Prism 10 (a biostatistical analysis software for data analysis, GraphPad Software Inc.)
Procedure
A. Preparation of 804 G conditioned medium
1. Thaw a vial of 804G cells (frozen stock in CryoStor® CS10 at a liquid nitrogen tank) and seed the cells at a density of 1 × 106 cells per dish on 100 mm × 15 mm Petri dishes with 804 G culture media.
2. Maintain cultures at 37 °C with 5% CO2.
3. Split cells at a 1:5 ratio and store one part in CryoStor CS10 at a liquid nitrogen bank.
4. Expand 804G cells in 804 G culture media in 100 mm × 15 mm Petri dishes.
5. Once cells are near 100% confluency, collect the supernatant 804G-conditioned media every 48 h, filter in a Corning 500 mL filter system, and store at -20 °C.
B. Isolation of total tracheal basal cells
Day 1: Washing and digesting tracheal tissue
1. Place the 2-cm trachea into a 15 mL conical tube with 10 mL of 4× MEM wash media with antibiotics. Place in a rotating shaker and change every 30 min. Repeat washing three times. Biopsy of tracheal samples is indicated in Figure 1A.
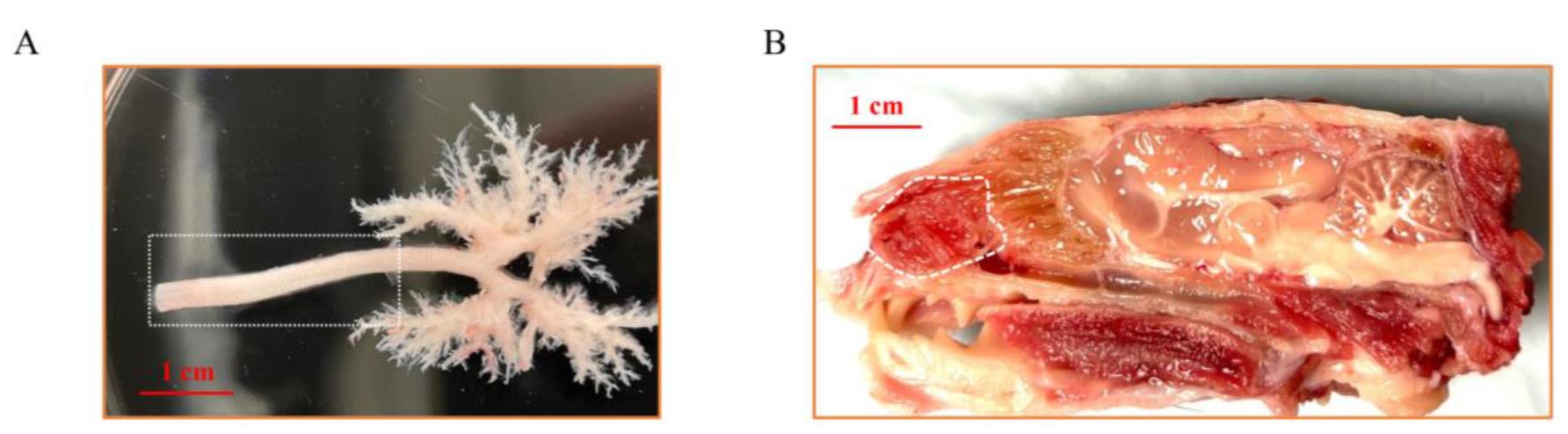
Figure 1. Gross anatomy of the ferret trachea and nasal cavity. (A) Dissection of ferret trachea and intrapulmonary airway. The white rectangle indicates the area utilized for total tracheal basal cell isolation. (B) Longitudinal section of adult ferret head. The white curved area indicates the nasal turbinates used for the isolation of nasal basal cells.
2. Open the trachea lengthwise along the muscle and add 4 mL of protease digestion media.
3. Leave overnight at 4 °C without agitation.
Day 2: Cell collection
4. Add 0.4 mL of FBS to the trachea in protease digestion media (with trachea); the desired final concentration of FBS is 10%.
5. Pour the entire solution and trachea into a sterile 100 mm dish.
6. Cut the trachea lengthwise into two pieces.
7. Scrape the inside of the trachea (~20 times per piece) using the blunt side of a sterile surgical blade attached to a hemostat. The scraped-off cell suspension should be cloudy. To confirm airway epithelial cells in the scraped-off cell suspension, transfer 200 μL into a Petri dish. Ciliated epithelial cells can be observed under a microscope.
8. Cut the trachea into small pieces using scissors. By the end of this step, it should resemble a paste. This may not be possible with older animals. Mince the tissue as thoroughly as possible.
9. Rinse the tools, plate F12 wash media with antibiotics, and filter the minced tissue and solution through a cell strainer into a 50 mL tube.
10. Use F12 wash media to rinse a 100 cm dish thoroughly and filter it to the cell strainer.
11. Transfer the filtered solution to a 15 mL conical tube.
12. Centrifuge at 514× g for 5 min in a Beckman Coulter Allegra GS-6R refrigerated benchtop centrifuge.
13. Decant the supernatant without disturbing the cell pellet.
14. Resuspend the pellet with 5 mL of F12 media (no FBS).
15. Centrifuge at 514× g for 5 min.
16. Aspirate and resuspend the pellet with PneumaCultTM-Ex Plus basal cell culture media and plate in a 6-well plate.
Days 3–5
17. Wash the plate three times with cold F12 to remove dead cells or debris around 18 h after plating.
18. Culture the cells in the PneumaCultTM-Ex Plus basal cell culture media. Airway stem cells can be frozen in CryoStor® CS10 and stored in liquid nitrogen.
19. Submit the culture media for a mycoplasma test.
Troubleshooting: A mycoplasma test is necessary because mycoplasma can influence airway stem cell differentiation. The reduced proliferation of airway stem cells occurs because mycoplasma competes with stem cells for nutrients, leading to altered shapes or structures in airway stem cells. Those will indicate mycoplasma contamination. If the test is positive, we can either redo the experiment or use the mycoplasma elimination kit (plasmocin treatment; Invivogen, catalog code: ant-mpt-1, ant-mpt).
C. Isolation of nasal basal cells
1. Dissect the nasal epithelium into 4× MEM wash media with antibiotics. Wash the tissue and change the media three times (once every 30 min). A biopsy of nasal turbinate samples is indicated in Figure 1B.
2. Cut the tissue several times and digest it at 37 °C with agitation for 1 h in pronase digestion media (5 mL).
3. After the first hour of pronase digestion, try to knock the surface airway epithelial (SAE) cells away from the tissues. If cells are mostly off of the tissue, proceed to step C4; alternatively, continue digestion for another hour.
Tip: Break a 5 mL FisherbrandTM SureOneTM aerosol barrier pipette so that the tissue can pass through the pipette. When the tissue is small enough to pass through a pipette, pipette it up and down in the pronase solution about 10 times to knock off as many SAE cells as possible. If the tissue pieces are too large or sticky, vortex them in the tube for 1 min.
4. To collect SAE cells, first filter the cells through a 100 μm cell strainer into a 50 mL conical tube (only the liquid containing the liberated SAE will pass through, while larger tissue pieces will remain behind). Next, wash the remaining tissue pieces caught in the strainer with some F12 wash media, following the same method as in step C3 to knock off and liberate the SAE cells. Finally, filter the liquid through the strainer again to gather the liberated SAE cells.
5. Spin the SAE cells down at 514× g for 5 min in a Beckman Coulter Allegra GS-6R refrigerated benchtop centrifuge, aspirate the buffer, and resuspend the cell pellet in F12 wash media. Centrifuge and wash in F12 wash media again, and resuspend the cell pellet in PneumaCultTM-Ex Plus basal cell culture media. Finally, spin the cells at 514× g for 5 min and seed onto 804G-conditioned media-coated plates.
Tip: One side of the ferret's nasal epithelium can be plated into two wells of a 6-well plate.
6. Nasal basal cell culturing and differentiation will follow the airway stem cell culturing and differentiation protocol. Nasal basal cells can be frozen in CryoStor® CS10 and stored in liquid nitrogen.
7. Submit the culture media for a mycoplasma test.
D. Ferret airway stem cell culture methods
1. Coat plates with 804 G conditioned media at 37 °C for at least 1 h. Then, remove 804 G conditioned media from the plates and use them immediately (5 mL of 804 G conditioned media for a 100 mm dish).
2. For freshly harvested primary tracheal epithelial cells or nasal epithelial cells, seed the cells in 804G-coated plates at 1 × 106 per 100 mm dish. For frozen cells, quickly thaw the frozen ferret stem cells and spin them at 514× g for 5 min at room temperature (RT).
3. Resuspend the cell pellet with prewarmed PneumaCultTM-Ex Plus media to propagate the epithelial cells on the 804 G-coated plates.
4. Change the growth media every other day.
5. When cells reach 100% confluency, use Accutase detach solution to detach the cells. It will take 20–30 min for the stem cells to detach from the plates. Use a volume of 20 μL of cell suspension for calculating cell concentration. Measure airway stem cell concentration using the trypan blue method. First, mix the cell suspension with trypan blue at a 1:1 ratio and load into a chamber slide. Evaluate cell concentration using the Invitrogen Countess 3 automated cell counter. Add an equal volume of growth media to the detached cells and centrifuge at 514× g for 5 min in a Beckman Coulter Allegra GS-6R refrigerated benchtop centrifuge. Aspirate the supernatant and resuspend the cell pellet in growth media. Split the cells at a ratio of 1:4. We typically split the cells from one 100 mm dish into four 100 mm dishes.
E. Ferret airway stem cell differentiation protocol (air–liquid interface cultures)
1. Coat Transwells® with 804 G conditioned media at 37 °C in a Caron Oasis CO2 incubator for at least 1 h (150 μL of 804 G conditioned media per Transwell®).
2. Aspirate out 804 G conditioned media.
3. Seed 3 × 105 ferret airway stem cells in 150 μL of PneumaCultTM-Ex plus media onto the supportive membrane of the Transwell®. This seeding density ensures 100% confluency immediately.
Tip: If the cells are not confluent the next day, expand the cells using airway stem cell culturing medium (add the growth medium to both the upper and lower chambers) until the cells achieve 100% confluency (no more than two days of expansion on the membrane). If stem cells do not reach confluency in this timeframe, it suggests either slow growth of the stem cells or inaccurate determination of cell concentration.
4. Culture the cells in with PneumaCultTM-Ex Plus media for 24 h in the Transwell® inserts, with 500 μL of PneumaCultTM-Ex Plus medium in the basal chamber, and 150 μL of PneumaCultTM-Ex Plus medium in the apical chamber.
5. Pre-ALI treatment (1 day): Around 24 h after airway stem cells seeding, replace the PneumaCultTM-Ex Plus media in apical and basal chambers with PneumaCultTM-ALI airway differentiation medium. The media volumes for the apical and basal sides are 150 μL and 500 μL, respectively.
6. After 24 h pre-ALI treatment, aspirate out the PneumaCultTM-ALI medium in the apical chamber, leaving the cells exposed to air (at an ALI) from this point on. Check the cultures every day and aspirate any medium residue from the apical chamber.
Tip: In the early days (usually 1–5 days) after air lift, the medium may come up from the basal chamber to the apical chamber. With cell differentiation and the formation of epithelial junctions, such liquid leakage stops.
7. Change PneumaCultTM-ALI airway differentiation media in lower chambers every other day. We usually do it on Mondays, Wednesdays, and Fridays.
8. Establish mucociliary differentiation on ALI (Figure 2). By standard protocol, we typically culture tracheal ALI for 21 days and nasal ALI for 14 days.
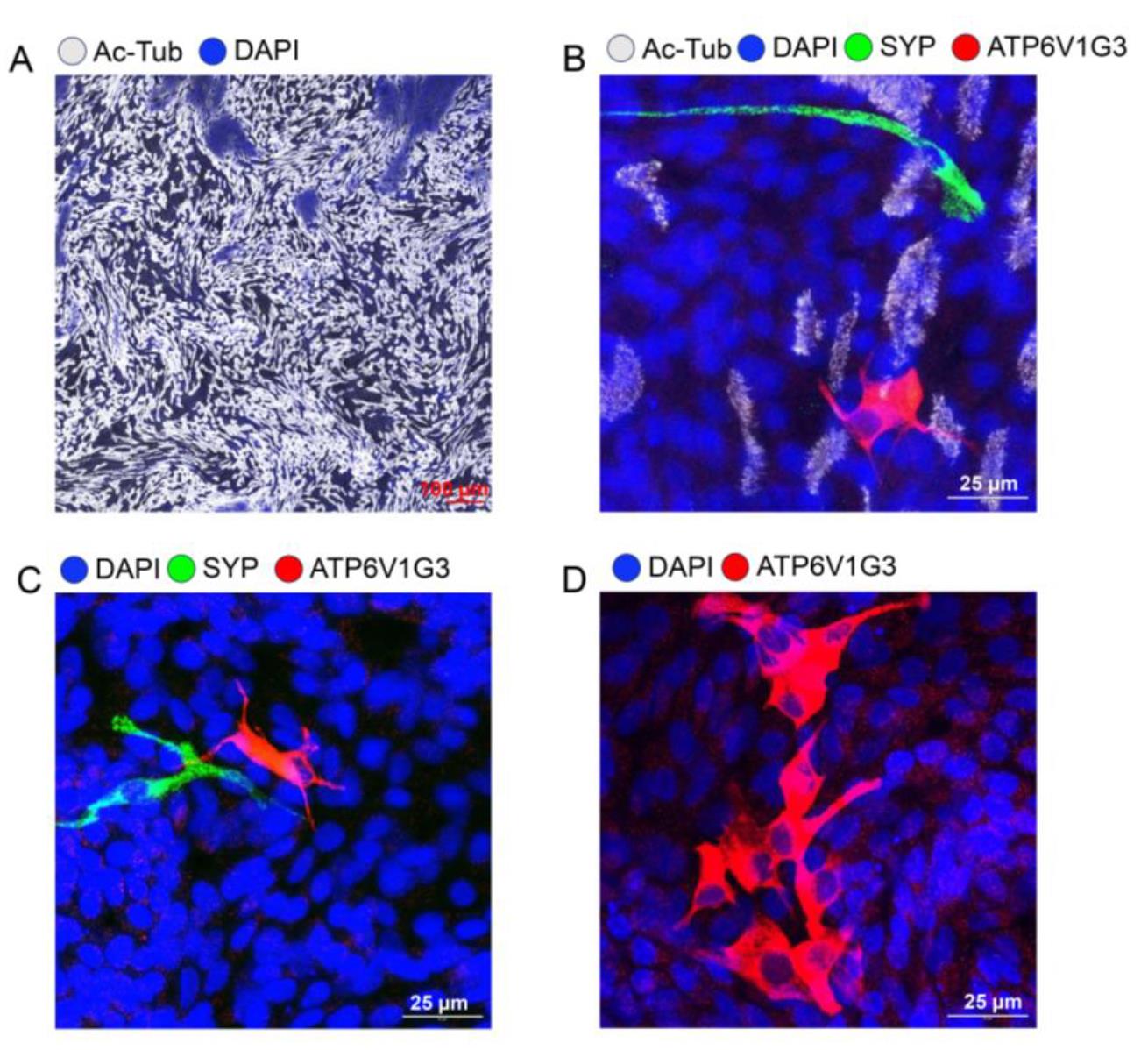
Figure 2. Immunofluorescence staining of ferret airway epithelial cells cultured at tracheal air–liquid interface (ALI). (A) Ciliated cells were stained with acetylated tubulin (gray) in tracheal ALI culture. (B) High-resolution image of pulmonary ionocyte staining by ATP6V1G3 (red), pulmonary neuroendocrine staining by synaptophysin (SYP) (green), and ciliated cell staining by acetylated tubulin (gray) in tracheal ALI culture. (C) High-resolution image of pulmonary ionocyte staining by ATP6V1G3 (red) and pulmonary neuroendocrine cell staining by SYP (green) in tracheal ALI culture. (D) High-resolution image of pulmonary ionocyte cluster staining by ATP6V1G3 (red) in tracheal ALI culture.
9. Mucociliary movement can be observed under the microscope to indicate ALI culture differentiation.
F. Banking of ferret airway stem cells
1. Resuspend the ferret airway stem cells pellet in CryoStor® CS10 at 1 × 106 cells/mL.
2. Aliquot 1 mL cell suspension into a 2.0 mL Corning cryogenic vial with washer, secure the cap, and ensure proper labeling of the stem cell genotype and the date.
3. Transfer Corning cryogenic vials with ferret airway stem cells to a cryo-compatible freezing container (allowing for temperature drop -1 °C/min) and save the container in a -80 °C freezer overnight. The next day, transfer the freezing vials to the liquid nitrogen tank for long-term banking.
Note: Stem cells can be stored at -80 °C for up to three months.
G. Immunofluorescence staining of ferret airway cultures
1. Fix differentiated ferret airway ALI cultures in 4% PFA in PBS for 30 min at RT.
2. Wash ALI cultures in DPBS three times for 5 min each at RT. Samples can be stored in DPBS at 4 °C for a week.
3. Antigen retrieval: Place samples in citrate buffer at 65 °C for 1 h in a water bath.
4. Cool down to RT and wash ALI culture in DPBS three times for 5 min each at RT.
5. Block samples in donkey serum blocking buffer (D-BB) for 1 h at RT.
6. Remove D-BB and incubate with the desired primary antibody, such as anti-CFTR (1:200–1:300), anti-ATP6V1G3 (1:300), anti-NKA primary antibody (1:100), or anti-BSND primary antibody (1:400), in donkey serum dilution buffer (D-DB) overnight at 4 °C.
7. Wash ALI cultures in DPBS three times for 10 min each at RT.
8. Apply anti-mouse and anti-rabbit secondary antibodies at 1:250 and Hoechst at 1:1,000 for 1 h at RT.
9. Wash ALI cultures in DPBS three times for 10 min each at RT. The transwell membrane is cut using a scalpel blade.
10. Mount the transwell membrane with Prolong glass antifade mountant and coverslip.
11. Image samples using Zeiss LSM 880 or Zeiss LSM 980 confocal laser scanning microscope. Ionocyte staining images are shown in Figures 3 and 4.
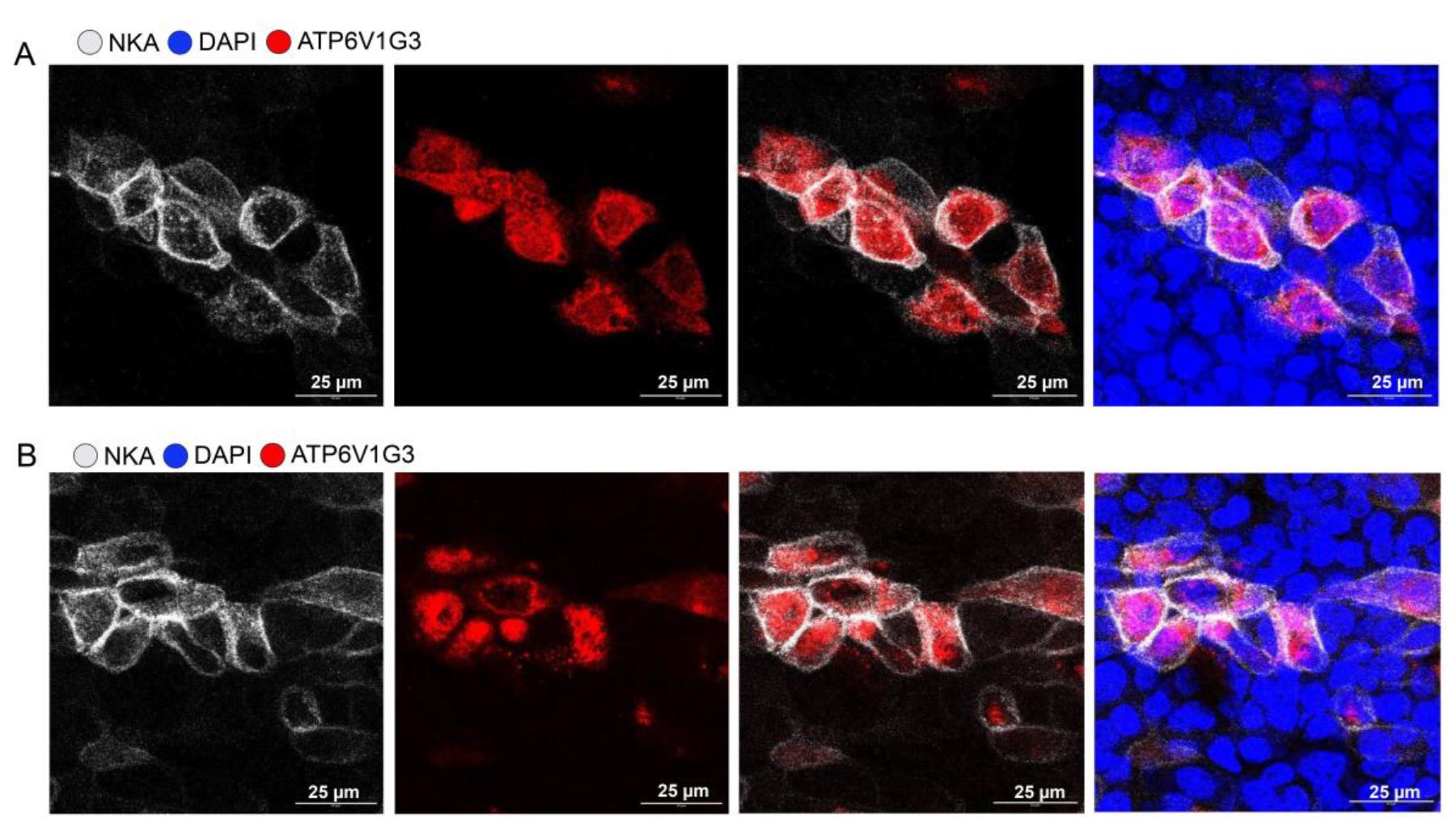
Figure 3. Ferret pulmonary ionocytes are enriched in NKA (Na+/K+-ATPase). (A, B) High-resolution image of pulmonary ionocyte cluster staining by ATP6V1G3 (red) and NKA (gray) in tracheal air–liquid interface (ALI) culture.
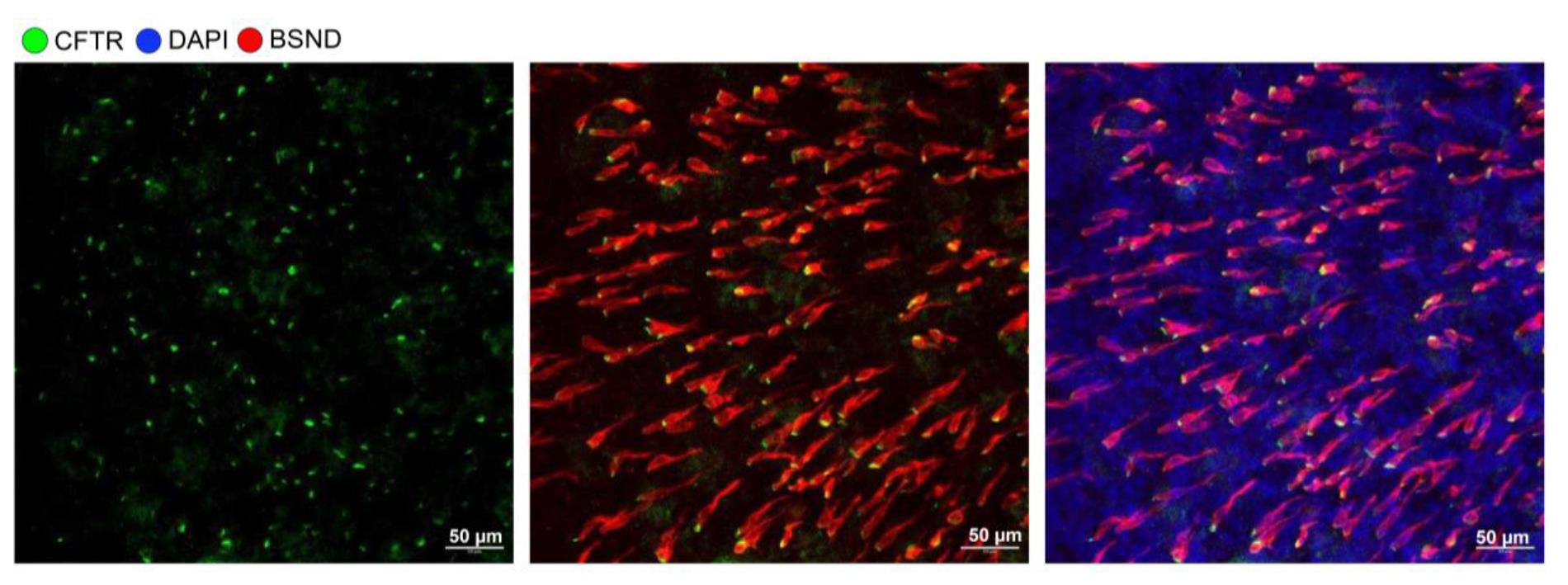
Figure 4. Ferret pulmonary ionocytes are enriched in cystic fibrosis transmembrane regulator (CFTR). High-resolution image of pulmonary ionocyte staining by BSND and CFTR in nasal air–liquid interface (ALI) culture.
H. Electrophysiology of ferret airway cultures
1. Turn on the circulating water bath and warm up the high chloride buffer, low chloride buffer, and K- buffer to 37 °C. Bring all electrodes to room temperature.
2. Assemble the P2300 Ussing chamber and P2302T EasyMount Ussing chamber slider into the clamp and fill the apical and basal chambers with K- buffer. Buffer-filling the Ussing chamber system will need around 15–30 min to equilibrate before making corrections.
3. For measuring the cultures on Transwell® on P2302T EasyMount Ussing chamber slider, set the Gain to 10 and FRC RANGE to 20. For measuring tracheal on P2304 EasyMount Ussing chamber slider, set the Gain to 10 and FRC RANGE to 50. FRC stands for fluid resistance compensation.
4. Make sure all the wire connectors (input module and DM-MC6) between the device and clamp chambers are at the Operate position. Then, turn on the multi-channel device.
5. Calibrate electrodes. Push the reading to current I and push the PUSH TO ADJ button for reading. The electrode reading should be around 65 (60–65 is acceptable). Adjust electrodes if reading is off the range or change to new ones if necessary.
6. Set the voltage, V to 0. For each clamp channel, set the Function to open; the Mode and Meter should be set to voltage, V. Based on the reading from the meter, press the OFFSET switch to either a + or - polarity. Adjust the dial nut until the reading is 0.00; next, adjust the resistance to 0 by going to the Fluid Re Comp, pressing the PUSH TO ADJ button, and turning the nut above the pushbutton to set the reading to -0.00. Now, both voltage and resistance are calibrated to 0. If the device and the electrode are well calibrated, the meter reading for V should remain steady at 0.00.
7. After calibration, remove all the K- buffer, assemble the EasyMount Ussing chamber slider with the transwell containing the epithelium to be tested, and place them into the clamps. Fill the chambers with 5 mL of warmed 37 °C low chloride buffer in the apical chamber and 5 mL of high chloride buffer in the basal chamber. Turn on the oxygen gas bubbling.
Tip: Either asymmetrical buffering (with a low chloride buffer on the apical side and a high chloride buffer on the basal side) or symmetrical buffering (using a high chloride buffer on both sides or a bicarbonate buffer on both sides) can be utilized based on the experimental design.
8. Turn on the computer and the software Acquire & Analyze.
9. Open a new file for each new experiment; data will be automatically saved once the program starts running.
10. Update the Transwell® insert area to 0.33 cm2 if using Corning 6.5 mm Transwell® with 0.4 μm pore polyester membrane insert.
11. From the master control unit, set all the channels to Function = clamp; Mode = remove (REM) and voltage (V); Meter = voltage (V).
12. Click on Acquire and conduct the reference check. If the V and I calibrations in steps H4 and H5 were performed correctly, the I values from all channels in the reference table will fall between -0.1 and 0.1, while the V values will range from -0.1 to 0.1. Click OK. If the reference shows any “red” readings in V or I—for instance, I or V values exceeding 0.5 or below -0.5—please return to verify the V and I electrodes, respectively. Check for any gas bubbles in the chambers or on the electrodes, and ensure that the electrode connections are secure, as adjustments may be necessary.
Tip: The reference turns yellow when the V and I values range from -0.5 to 0.5. We can then click OK to process. If it turns red, please stop and check the electrodes.
13. Start the short circuit current measurement by clicking the running man icon in the software. Set the monitor at the intermediate speed icon and run for 5 min before proceeding with the next step.
14. Sequentially add the below chemicals from the apical side (all 1,000× stock); mark the event before adding, with a ~5–6 min interval between each addition:
a. 6 μL of amiloride (final concentration: 100 μM, from a 100 mM stock) (inhibition of epithelial sodium conductance (ENaC).
b. 6 μL of DIDS (final concentration: 100 μM, from a 100 mM stock) (inhibition of all the non-CFTR chloride channels).
c. 6 μL of IBMX/6 μL of Forskolin (final concentration: 100 μM/10 μM, from a 100 mM/10 mM stock) (to activate the CFTR channels).
d. 6 μL of GlyH101 (final concentration: 50 μM, from a 50 mM stock) (CFTR inhibitor) (blocks all the transepithelial Cl- secretion).
Tip: If gas bubbles occur during measurement and block the short circuit connection, the current will drop dramatically, and the data from that chamber should be excluded.
Troubleshooting: Tracheal ALI transepithelial resistance is between 500 and 2,000 Ω·cm2, while nasal ALI transepithelial resistance is approximately 250 Ω·cm2. If gas bubbles occur at the beginning of data acquisition, resistance will be out of range (>10K Ω·cm2). The best solution is to disassemble the chamber and reassemble it. If gas bubbles form while adding chemicals and block the short circuit connection, the current will drop dramatically, and the data in that chamber should then be excluded. Making oxygen bubbles at a consistent speed can help prevent gas bubbles in the short-circuit connection.
15. Stop and save the data.
Data analysis
Pulmonary ionocytes have been shown to regulate Cl-/fluid secretion and absorption [15]. In this protocol, we tested Cl- absorption in both wild-type (WT) and FOXI1 knockout (FOXI1-KO) tracheal basal cell-derived ALI epithelium, as indicated by Cl- moving from the apical to the basolateral side of the epithelium (negative CFTR currents) (Figure 5A, Table S1). The depletion of pulmonary ionocyte resulting from FOXI1-KO demonstrated a significant decrease in CFTR-mediated Cl- transport from the apical to the basolateral side (Figure 5B, C), alongside a reduction in transepithelial conductance (Figure 5D, E).
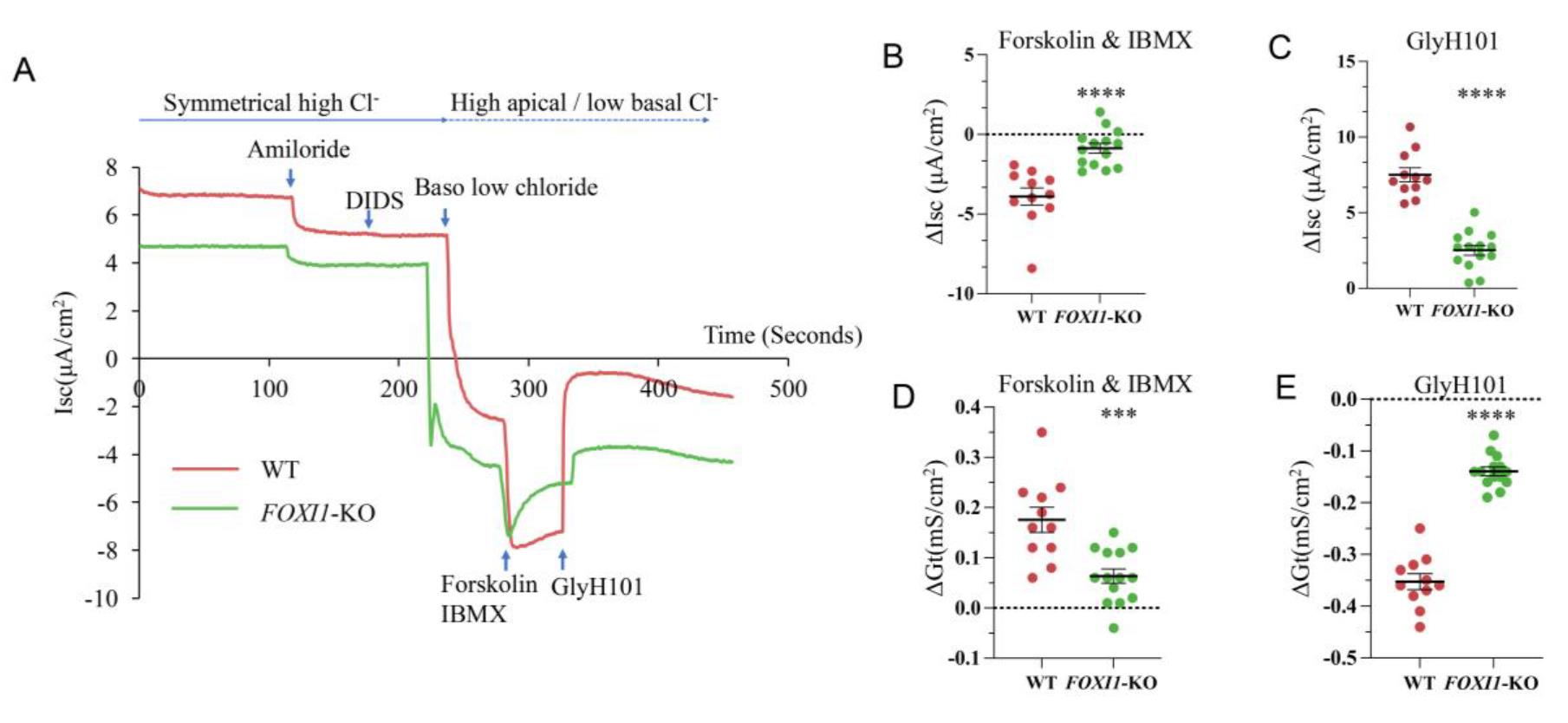
Figure 5. Reduced chloride absorption in FOXI1-KO tracheal air–liquid interface (ALI) culture. (A) Representative short-circuit current measurements. Experiments were conducted in Ussing chambers to assess chloride ion transport from the apical to the basolateral side of the wildtype (WT) and FOXI1-KO ALI cultures. (B, C) Changes of short circuit current (∆Isc) under cystic fibrosis transmembrane regulator (CFTR) activation by forskolin and IBMX (B), and CFTR inhibition by GlyH101 (C). (D, E) Changes of transepithelial conductance (∆Gt) under CFTR activation by forskolin and IBMX (D), and CFTR inhibition by GlyH101 (E). N = 11 ALI cultures in WT group, N = 14 ALI cultures in FOXI1-KO group, *** P < 0.001, **** P < 0.0001, statistical significance was determined by Student's t-test.
Validation of protocol
This protocol or parts of it have been used and validated in the following research articles:
Yuan et al. [15]. Transgenic ferret models define pulmonary ionocyte diversity and function. Nature 621, 857–867 (2023).
Yuan et al. [4]. Novel Cystic Fibrosis Ferret Model Enables Visualization of CFTR Expression and Genetic CFTR Reactivation. Human Gene Ther (2025).
Supplementary information
The following supporting information can be downloaded here:
1. Table S1. Electrophysiology data from WT and FOXI1-KO tracheal ALI cultures.
Acknowledgments
This work was funded by the following grants from the National Institute of Health (R01 HL165404, and NHLBI Federal Contract 75N92025C00007 to J.F.E.), the Cystic Fibrosis Foundation (YAN23G0 to Z.Y.; YUAN24G0 to F.Y.; ENGELH21XX0 to J.F.E.), the Cystic Fibrosis Research Institute (Grant #1267026 to F.Y.), and University of Alabama Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Research Development Fund for Feng Yuan. This protocol was used in [4,15].
Competing interests
The authors declare that there are no competing financial interests.
Ethical considerations
Ferret care and use were approved by the Institutional Animal Care and Use Committee of the University of Iowa (Animal Protocol 4111945).
References
- Sun, X., Sui, H., Fisher, J. T., Yan, Z., Liu, X., Cho, H. J., Joo, N. S., Zhang, Y., Zhou, W., Yi, Y., et al. (2010). Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 120(9): 3149–3160. https://doi.org/10.1172/jci43052
- Sun, X., Yi, Y., Yan, Z., Rosen, B. H., Liang, B., Winter, M. C., Evans, T. I. A., Rotti, P. G., Yang, Y., Gray, J. S., et al. (2019). In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci Transl Med. 11(485): eaau7531. https://doi.org/10.1126/scitranslmed.aau7531
- Yan, Z., Stewart, Z. A., Sinn, P. L., Olsen, J. C., Hu, J., McCray, P. B. and Engelhardt, J. F.(2015). Ferret and Pig Models of Cystic Fibrosis: Prospects and Promise for Gene Therapy. Hum Gene Ther Clin Dev. 26(1): 38–49. https://doi.org/10.1089/humc.2014.154
- Yuan, F., Sun, X., Park, S. Y., Tang, Y., Feng, Z., Ebadi, M., Yi, Y., Thompson, A. E., Karippaparambil, J. D., Engelhardt, J. F., et al. (2025). Novel Cystic Fibrosis Ferret Model Enables Visualization of CFTR Expression Cells and Genetic CFTR Reactivation. Hum Gene Ther.: e215. https://doi.org/10.1089/hum.2024.215
- Peabody Lever, J. E., Li, Q., Pavelkova, N., Hussain, S. S., Bakshi, S., Ren, J. Q., Jones, L. I., Kennemur, J., Weupe, M., Campos-Gomez, J., et al. (2024). Pulmonary Fibrosis Ferret Model Demonstrates Sustained Fibrosis, Restrictive Physiology, and Aberrant Repair. bioRxiv: e597198. https://doi.org/10.1101/2024.06.04.597198
- Raju, S. V., Kim, H., Byzek, S. A., Tang, L. P., Trombley, J. E., Jackson, P., Rasmussen, L., Wells, J. M., Libby, E. F., Dohm, E., et al. (2016). A ferret model of COPD-related chronic bronchitis. JCI Insight. 1(15): e87536. https://doi.org/10.1172/jci.insight.87536
- Albrecht, R. A., Liu, W. C., Sant, A. J., Tompkins, S. M., Pekosz, A., Meliopoulos, V., Cherry, S., Thomas, P. G. and Schultz-Cherry, S. (2018). Moving Forward: Recent Developments for the Ferret Biomedical Research Model. mBio. 9(4): e01113–18. https://doi.org/10.1128/mbio.01113-18
- Belser, J. A., Barclay, W., Barr, I., Fouchier, R. A., Matsuyama, R., Nishiura, H., Peiris, M., Russell, C. J., Subbarao, K., Zhu, H., et al. (2018). Ferrets as Models for Influenza Virus Transmission Studies and Pandemic Risk Assessments. Emerg Infect Dis. 24(6): 965–971. https://doi.org/10.3201/eid2406.172114
- Johnson, M. B., Sun, X., Kodani, A., Borges-Monroy, R., Girskis, K. M., Ryu, S. C., Wang, P. P., Patel, K., Gonzalez, D. M., Woo, Y. M., et al. (2018). Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature. 556(7701): 370–375. https://doi.org/10.1038/s41586-018-0035-0
- Rosen, B. H., Chanson, M., Gawenis, L. R., Liu, J., Sofoluwe, A., Zoso, A. and Engelhardt, J. F.(2018). Animal and model systems for studying cystic fibrosis. J Cyst Fibros. 17(2): S28–S34. https://doi.org/10.1016/j.jcf.2017.09.001
- Montoro, D. T., Haber, A. L., Biton, M., Vinarsky, V., Lin, B., Birket, S. E., Yuan, F., Chen, S., Leung, H. M., Villoria, J., et al. (2018). A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 560(7718): 319–324. https://doi.org/10.1038/s41586-018-0393-7
- Basil, M. C., Cardenas-Diaz, F. L., Kathiriya, J. J., Morley, M. P., Carl, J., Brumwell, A. N., Katzen, J., Slovik, K. J., Babu, A., Zhou, S., et al. (2022).Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature. 604(7904): 120–126. https://doi.org/10.1038/s41586-022-04552-0
- Yan, Z., Vorhies, K., Feng, Z., Park, S. Y., Choi, S. H., Zhang, Y., Winter, M., Sun, X. and Engelhardt, J. F. (2022). Recombinant Adeno-Associated Virus-Mediated Editing of the G551D Cystic Fibrosis Transmembrane Conductance Regulator Mutation in Ferret Airway Basal Cells. Hum Gene Ther. 33: 1023–1036. https://doi.org/10.1089/hum.2022.036
- Plasschaert, L. W., Žilionis, R., Choo-Wing, R., Savova, V., Knehr, J., Roma, G., Klein, A. M. and Jaffe, A. B. (2018). A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 560(7718): 377–381. https://doi.org/10.1038/s41586-018-0394-6
- Yuan, F., Gasser, G. N., Lemire, E., Montoro, D. T., Jagadeesh, K., Zhang, Y., Duan, Y., Ievlev, V., Wells, K. L., Rotti, P. G., et al. (2023). Transgenic ferret models define pulmonary ionocyte diversity and function. Nature. 621(7980): 857–867. https://doi.org/10.1038/s41586-023-06549-9
- Lei, L., Traore, S., Romano Ibarra, G. S., Karp, P. H., Rehman, T., Meyerholz, D. K., Zabner, J., Stoltz, D. A., Sinn, P. L., Welsh, M. J., et al. (2023).CFTR-rich ionocytes mediate chloride absorption across airway epithelia. J Clin Invest. 133(20): e1172/jci171268. https://doi.org/10.1172/jci171268
- Luan, X., Henao Romero, N., Campanucci, V. A., Le, Y., Mustofa, J., Tam, J. S. and Ianowski, J. P. (2024). Pulmonary Ionocytes Regulate Airway Surface Liquid pH in Primary Human Bronchial Epithelial Cells. Am J Respir Crit Care Med. 210(6): 788–800. https://doi.org/10.1164/rccm.202309-1565oc
- Mou, H., Vinarsky, V., Tata, P. R., Brazauskas, K., Choi, S. H., Crooke, A. K., Zhang, B., Solomon, G. M., Turner, B., Bihler, H., et al. (2016). Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell. 19(2): 217–231. https://doi.org/10.1016/j.stem.2016.05.012
Article Information
Publication history
Received: May 2, 2025
Accepted: Jun 24, 2025
Available online: Jul 13, 2025
Published: Jul 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Yan, Z., Engelhardt, J. F. and Yuan, F. (2025). Isolation and Culture of Ferret Airway Stem Cells. Bio-protocol 15(14): e5399. DOI: 10.21769/BioProtoc.5399.
Category
Stem Cell > Adult stem cell > Epithelial stem cell
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link