- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Composite Method of Post-stroke Epilepsy Induction
Published: Vol 15, Iss 14, Jul 20, 2025 DOI: 10.21769/BioProtoc.5387 Views: 1948
Reviewed by: Mayank GautamAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1804 Views
Utilizing EdU to Track Leukocyte Recruitment to the Brain
Zoie K. Lipfert [...] David P. Sullivan
Dec 5, 2025 1555 Views

Station Holding During Rheotaxis: A Sensitive Assay of Lateral Line Function in Larval Zebrafish
Sophie Cohen-Bodénès [...] Lavinia Sheets
Dec 20, 2025 754 Views
Abstract
The global burden of stroke has increased in the past several decades, and post-stroke epilepsy (PSE) is a common complication. Contrasted with the advancement in knowledge of stroke pathophysiology, the exact pathogenesis of PSE is unclear. Various animal stroke models have been utilized to investigate the underlying mechanisms of PSE, but the success rate of PSE induction is low. To address this limitation, a novel PSE model was established in the rat by inducing status epilepticus using lithium-pilocarpine one week after photothrombotic stroke. Successful indication of status epilepticus and mortality rate at three days after status epilepticus were the main measurements. Potential usefulness of this model was also illustrated by preliminary results on locomotor activity, exploratory behavior, and anxiety level evaluated using the open-field test, as well as mossy fiber sprouting (MFS) in the hippocampal dentate granule cells using Zinc transporter 3 immunofluorescence staining at 8 weeks after PSE induction. This novel composite method of PSE induction may facilitate future studies on the pathogenesis and treatment of PSE.
Key features
• We developed a new PSE model in the rat by combining the photothrombotic model of stroke and lithium-pilocarpine (LIP) model of temporal lobe epilepsy (TLE).
• In our novel rat PSE model, 94% of rats achieved status epilepticus, and mortality rate at 3 days was 25%.
• The PSE rats appeared to have a decreased anxiety level on the open-field test and MFS in the hippocampal dentate granule cells.
Keywords: Post-stroke epilepsyGraphical overview
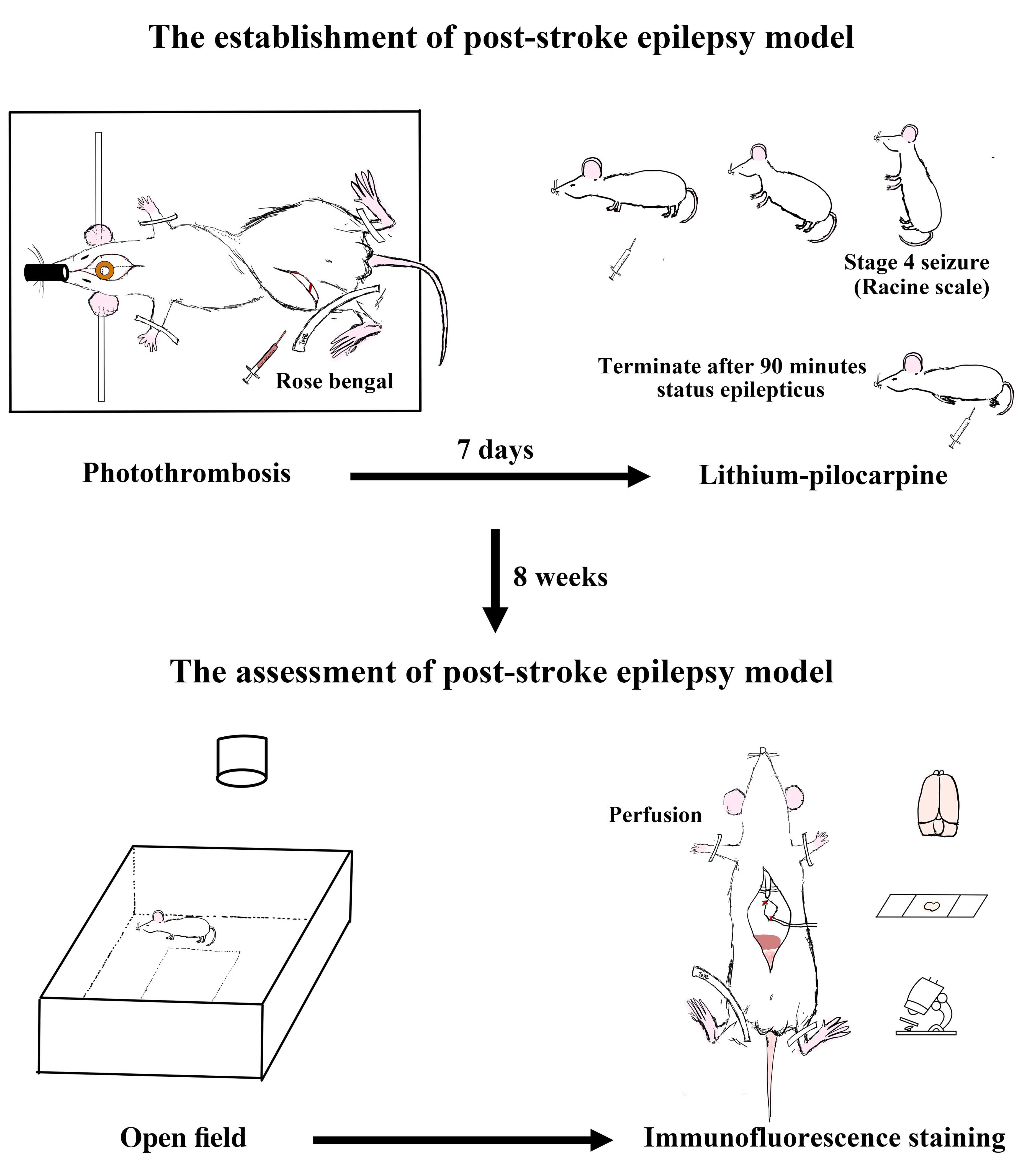
A novel composite method of post-stroke epilepsy (PSE) induction. The PSE model was established in the rat by inducing status epilepticus using lithium-pilocarpine one week after a photothrombotic stroke. Successful indication of status epilepticus and mortality rate at three days were the main measurements. Potential usefulness of this model was also illustrated by preliminary results on locomotor activity, exploratory behavior, and anxiety level evaluated using the open-field test, as well as mossy fiber sprouting (MFS) in the hippocampal dentate granule cells using Zinc transporter 3 immunofluorescence staining at 8 weeks after PSE induction.
Background
Both stroke and epilepsy are common. Their burdens are rising, especially with the aging of the global population. Epilepsy is a common complication of stroke, referred to as post-stroke epilepsy (PSE). Various animal stroke models have been developed to advance our knowledge of stroke pathophysiology and treatment. In the photothrombotic stroke model, a highly consistent and reproducible infarct is achieved by illuminating the brain after the injection of Rose Bengal to produce cortical thrombosis [1]. Epileptic seizures after photothrombotic stroke with small or larger infarct size over various cortical regions were monitored with video and EEG recordings in F344 and Sprague-Dawley (SD) rats of different ages [2]. PSE was recorded in post-mature rats 2 months after larger photothrombotic infarcts in the frontoparietal cortex extending to the cortical-subcortical interface. Small and parietal cortical lesions were relatively ineffective in producing PSE. In another study using SD rats, video and EEG recordings were conducted at 2, 4, 6, 8, and 10 months after photothrombosis [3]. Only 18% of rats developed PSE. In addition, epileptic rats had bilateral mossy fiber sprouting (MFS) in hippocampal dentate granule cells. Although photothrombotic stroke and other stroke models per se can be used to investigate the underlying pathogenic mechanisms of PSE, a low PSE rate is a major limitation. Furthermore, the PSE occurrence is unpredictable and delayed. The lithium-pilocarpine (LIP) model has been widely used to study temporal lobe epilepsy (TLE) in rodents. Strokes in the temporal lobe are associated with a high risk of post-stroke TLE. Here, we set up a new rat PSE model by combining the photothrombotic model of stroke and the LIP model of TLE, with successful indication of status epilepticus and mortality rate at three days after status epilepticus as the main measurements. Preliminary results on locomotor activity, exploratory behavior, and anxiety level evaluated using the open-field test, as well as MFS in the hippocampal dentate granule cells using Zinc transporter 3 immunofluorescence staining at 8 weeks after PSE induction, were shown to illustrate the potential usefulness of this model.
Materials and reagents
Reagents
1. Skin prepping antiseptic solution (Povidone Iodine) (3M, catalog number: IA420100272)
2. Rose Bengal dye (Sigma-Aldrich, catalog number: 632-69-9, stable at room temperature)
3. Scopolamine methyl bromide (Tokyo Chemical Industry, catalog number: 155-41-9, stable for 5 years at room temperature)
4. Lithium chloride (Sigma-Aldrich, catalog number: 7447-41-8, stable for 3 years at room temperature)
5. Pilocarpine hydrochloride (Sigma-Aldrich, catalog number: 54-71-7, stable for 5 years at room temperature)
6. Pamlin solution (Diazepam) (Ceva Animal Health, catalog number: 544-18, store at room temperature)
7. Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: 7558-79-4, stable for 3 years at room temperature)
8. Sodium phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: 7558-80-7, stable for 3 years at room temperature)
9. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 7647-14-5, stable for 3 years at room temperature)
10. Sodium hydroxide solution (NaOH) (Sigma-Aldrich, catalog number: 1310-73-2, store at room temperature)
11. Hydrochloric acid solution (HCl) (Sigma-Aldrich, catalog number: 7647-01-0, store at room temperature)
12. Paraformaldehyde (Sigma-Aldrich, catalog number: 30525-89-4, store at room temperature)
13. Sucrose (Sigma-Aldrich, catalog number: 57-50-1, store at room temperature)
14. Sodium citrate (Sigma-Aldrich, catalog number: 6132-04-3, stable for 5 years at room temperature)
15. Tween 20 (Sigma-Aldrich, catalog number: 9005-64-5, store at room temperature)
16. Triton X-100 (Sigma-Aldrich, catalog number: 9036-19-5, stable for 2 years at room temperature)
17. Rabbit anti-ZnT3 (Synaptic system, catalog number: 197002, store at -20 °C in the dark)
18. Normal goat serum (Thermo Fisher Scientific, catalog number: PCN5000, store at -20 °C)
19. Alexa Fluor® 568 goat anti-rabbit (Thermo Fisher Scientific, catalog number: A11011, stable for 2 years at 4 °C, store in the dark)
20. DietGel 76A [Clear H2O, catalog number: 72-07-5022; ingredients: purified water, molasses, animal protein (milk), vegetable oil, food acids, cane sugar, mineral mix, hydrocolloids, citrus fiber, vitamin mix, electrolyte blend, L-methionine]
21. O.C.T. Compound (Tissue-Tek, catalog number: 4583)
Solutions
1. 0.9% sterile saline solution (see Recipes)
2. Rose Bengal solution (see Recipes)
3. Scopolamine stock solution (see Recipes)
4. Scopolamine working solution (see Recipes)
5. Lithium working solution (see Recipes)
6. Pilocarpine working solution (see Recipes)
7. 4% formalin solution (see Recipes)
8. 10% sucrose solution (see Recipes)
9. 30% sucrose solution (see Recipes)
10. 10 mM citrate buffer (see Recipes)
Recipes
1. 0.9% sterile saline solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 9 g/L | 4.5 g |
| Double-distilled water (DDW) | n/a | quantity sufficient (Q.S.) 500 mL |
| Total | n/a | 500 mL |
2. Rose Bengal solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Rose Bengal | 10 mg/mL | 50 mg |
| 0.9% sterile saline solution | n/a | Q.S. 5 mL |
| Total | n/a | 5 mL |
Caution: Stable for 4 h at 4 °C, protected from light; 5 mL adequate for 5 rats (around 300 g).
3. Scopolamine stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Scopolamine | 10 mg/mL | 100 mg |
| 0.9% sterile saline solution | n/a | Q.S. 10 mL |
| Total | n/a | 10 mL |
Caution: Stable for 1 week at 4 °C, protected from light.
4. Scopolamine working solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Scopolamine stock solution | n/a | 1 mL |
| 0.9% sterile saline solution | n/a | 9 mL |
| Total | n/a | 10 mL |
Caution: Generally safe, use gloves and eye protection; freshly prepared, protected from light; 10 mL adequate for 30 rats (around 300 g).
5. Lithium working solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Lithium chloride | 3 mEq/mL | 30 mEq |
| 0.9% sterile saline solution | n/a | Q.S. 10 mL |
| Total | n/a | 10 mL |
Caution: Generally safe, use gloves and eye protection; freshly prepared; 10 mL adequate for 15 rats (around 300 g).
6. Pilocarpine working solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Pilocarpine | 30 g/L | 0.3 g |
| 0.9% sterile saline solution | n/a | Q.S. 10 mL |
| Total | n/a | 10 mL |
Caution: Generally safe, use gloves and eye protection; freshly prepared; 10 mL adequate for 15 rats (around 300 g).
7. 4% formalin solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Paraformaldehyde | 40 g/L | 80 g |
| NaOH (5 M) | n/a | 0.3 mL |
| Na2HPO4 | 0.2 M | 22.71 g |
| NaH2PO4 | 0.2 M | 4.80 g |
| DDW | n/a | Q.S. 2 L |
| Total (pH 7.4) | n/a | 2 L |
Caution: Stable for 1 month at 4 °C.
Hazardous: The preparation of formalin solution should be performed in the ventilated fume hood.
8. 10% sucrose solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 300 g/L | 15 g |
| DDW | n/a | Q.S. 50 mL |
| Total (pH 7.4) | n/a | 50 mL |
Caution: Stable for 2 weeks at 4 °C.
9. 30% sucrose solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 300 g/L | 15 g |
| DDW | n/a | Q.S. 50 mL |
| Total (pH 7.4) | n/a | 50 mL |
Caution: Stable for 2 weeks at 4 °C.
10. 10 mM citrate buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium citrate | 2.5 mM | n/a |
| Tween 20 | n/a | 1 mL |
| HCl (1 N) | n/a | n/a |
| DDW | n/a | Q.S. 200 mL |
| Total (pH 6.0) | n/a | 200 mL |
Caution: Stable for 3 months at room temperature.
Laboratory supplies
1. Masks (Medicom, catalog number: 206415)
2. Gloves (Medicom, catalog number: OPXL-BIO)
3. 1 mL syringe (Terumo, catalog number: 22290018)
4. 27 G × 1/2” needle (Terumo, catalog number: NN2713R)
5. Blunt 15 G Needle for transcardiac perfusion (Strategic Applications, catalog number: NC0604239)
6. Superfrost Plus slides (Epredia, catalog number: 22-042-941)
7. Heating pad with a rectal thermometer (Stoelting, catalog number: 50300)
8. Orion Pro Star PH211 pH/ORP bench meter (Thermo Fisher Scientific, catalog number: PSTAR2119)
Equipment
1. CLS150 H-LED cold light source (Leica Biosystems, model: CLS150)
2. Compact stereotaxic frame (Harvard Apparatus, model: A0801)
3. Digital USB 2.0 CMOS camera and Lens Vari-focal 2.8–12 mm (Stoelting, model: 60516)
4. Cryostat (Leica Biosystems, model: CM1850)
5. Microscope (Nikon, model: Nikon ECLIPSE Ni)
6. Ventilated fume hood (Dynaflow, model: 2000GRP)
7. Video-tracking system, ANY-maze (Stoelting Co., model: 60000)
Software and datasets
1. ImageJ software (NIH, ImageJ Ver.1.36 b, 03/20/2012)
2. Prism (GraphPad Version 9.5.1, 02/07/2023)
Procedure
A. PSE model
1. Calculate the required volume of Rose Bengal for injection and dilute it with sterile saline (10 mg/mL).
Caution: Always prepare the solution freshly and keep it away from light exposure.
2. Before performing the surgery, weigh the rat (Sprague-Dawley rats, 7 weeks old) and administer the anesthesia (ketamine at 67 mg/kg and xylazine at 6 mg/kg intraperitoneally). Test the consciousness of the rat with stimuli; the absence of pedal reflex (withdrawal of the foot when stimulated) is considered as under a deep level of anesthesia. Shave the fur over the scalp and left thigh. Disinfect the skin using iodophor and 70% ethanol.
3. Place the rat in a stereotaxic apparatus (Figure 1B) and maintain its body temperature (36.5 °C ± 0.5 °C) using a heating pad and a rectal probe to limit the variability of infarct volume. Apply eye ointment to prevent the eyes from drying out.
4. Make an incision (around 3 cm) of the scalp along the midline from the ear level to the neck and remove some of the periosteum.
5. Locate the bregma (0,0) and mark the stereotaxic coordinates [4] relative to the bregma at the following points: (-5.2, -1.8), (0.8, -1.8), (-2.2, -1.2), (-2.2, -4.8). Circle the region of interest, which is round and centered at 1.8 mm caudal and 2.2 mm left to the bregma.
6. Adjust the distance between the cold light source and the skull (1.8 mm caudal and 2.2 mm left to the bregma) to ensure the final illumination intensity of approximately 14,000–15,000 lux.
7. Apply a brass shim stencil with a circular diameter of 6 mm over the center of the circle we drew to cover the adjacent region.
8. Make an incision in the middle of the left thigh and expose the femoral vein with blunt dissection.
9. Load the Rose Bengal solution in a 1 mL syringe and slowly inject it using a 27 G needle into the femoral vein with a dose of 3 mL/kg of body weight over 2 min. Remove the needle and apply pressure to the wound with a sterilized cotton swab to stop bleeding. Disinfect and suture the wound on the thigh immediately.
10. Five minutes after the injection, switch on the cold light source and illuminate the region of interest.
11. After 15 min of illumination, turn off the cold light source.
12. Take away the brass slim stencil. Gently remove the rat from the stereotaxic apparatus. Disinfect and suture the wound on the scalp.
13. Return the rat to its cage.
Critical: Avoid direct light exposure for 2 h after the surgery; feed the rat with DietGel and 5 mL of saline twice per day to ensure adequate nutrition.
14. The sham group undergoes sham surgery without the injection of RB.
15. On the subsequent day, use the modified Neurological Severity Score (mNSS) [5] to measure neurological functioning, including a composite of motor, sensory, reflex, and balance tests. Rats that fail to reach a mNSS score of 4 are excluded from the study.
16. One week after photothrombosis (Figure 1A), subject the rat to the lithium-pilocarpine (LIP)-induced status epilepticus [6].
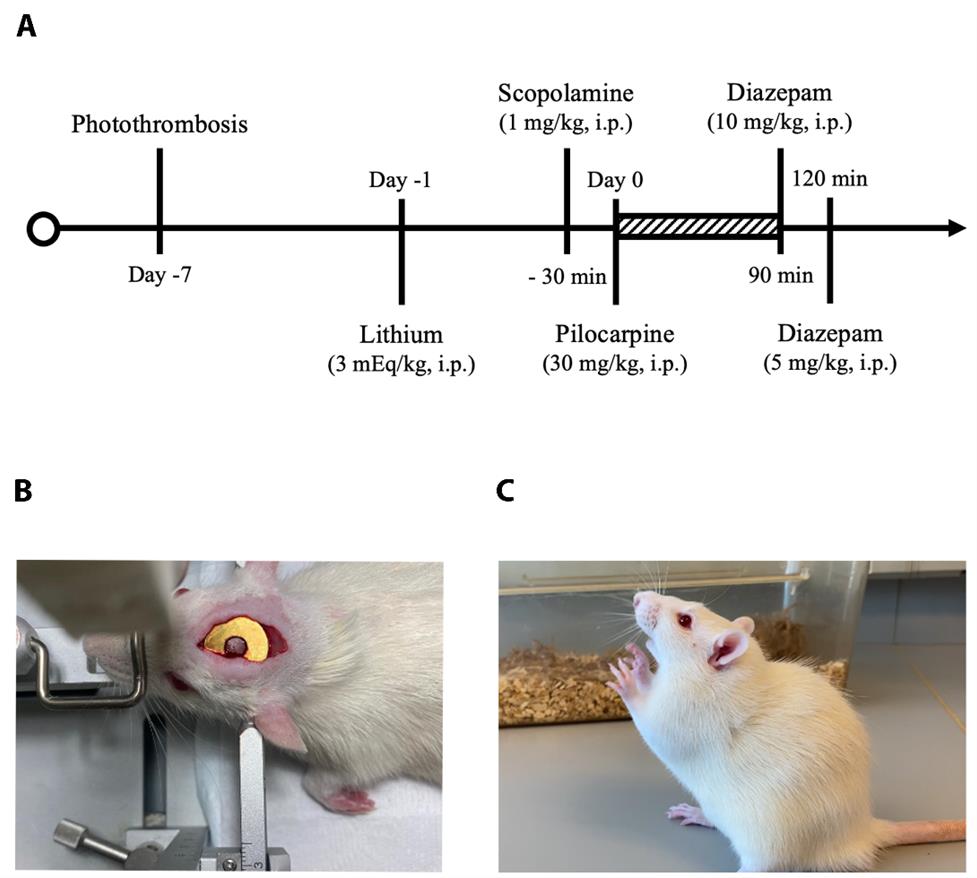
Figure 1. Establishment of the post-stroke epilepsy (PSE) model. (A) A flow diagram of the experimental design and the timeline of photothrombosis and induction of status epilepticus. (B) Surgical photograph during photothrombosis. (C) A rat with Racine scale 4 seizure.
17. Inject lithium chloride (3 mEq/kg and 1 mL/kg intraperitoneally) 24 h before administering pilocarpine (30 mg/kg and 1 mL/kg intraperitoneally). To prevent peripheral effects, a single injection of scopolamine (1 mg/kg and 1 mL/kg intraperitoneally) will be administered 30 min before the pilocarpine.
Caution: Use gloves and eye protection.
18. The seizures will be monitored and classified according to the Racine scale [7] as follows: 1) mouth and facial movement, 2) head nodding, 3) forelimb clonus, 4) rearing with forelimb clonus, 5) rearing and falling with forelimb clonus, and 6) wild running, jumping, or vocalizations.
19. Only rats that reach stage 4 or 5 for more than 30 min (Figure 1C) are considered to have developed status epilepticus; rats that reach stage 3 or below or over stage 6 [8] are excluded from the study and euthanized to avoid pain and distress.
20. Terminate persistent or prolonged seizures at 90 min, if necessary, by administering diazepam (10 mL/kg intraperitoneally). Thirty minutes later, administer a second injection of diazepam (5 mL/kg intraperitoneally) to completely halt seizures if necessary to reduce mortality.
21. Give the rat an intraperitoneal injection of 5 mL of saline immediately after the procedure and return the rat to an individual cage. Monitor the rat for at least three days, as it may have difficulty accessing food and water because of the persistent seizures.
Critical: Feed the rat with DietGel and 5 mL of saline twice per day to ensure adequate nutrition.
22. The sham group receives the same injections of lithium chloride and scopolamine but without pilocarpine.
B. Success rate and mortality
1. Calculate the percentage of rats having Racine scale 4 or 5 seizures as successful induction of status epilepticus.
2. Record the percentage of deaths occurring at or before three days after status epilepticus as the mortality rate.
C. Open field
1. Prepare an open-field apparatus (Figure 2) consisting of a box (100 cm × 100 cm × 60 cm) with an outer zone (periphery) and an inner square (center, 50 cm × 50 cm) [9].
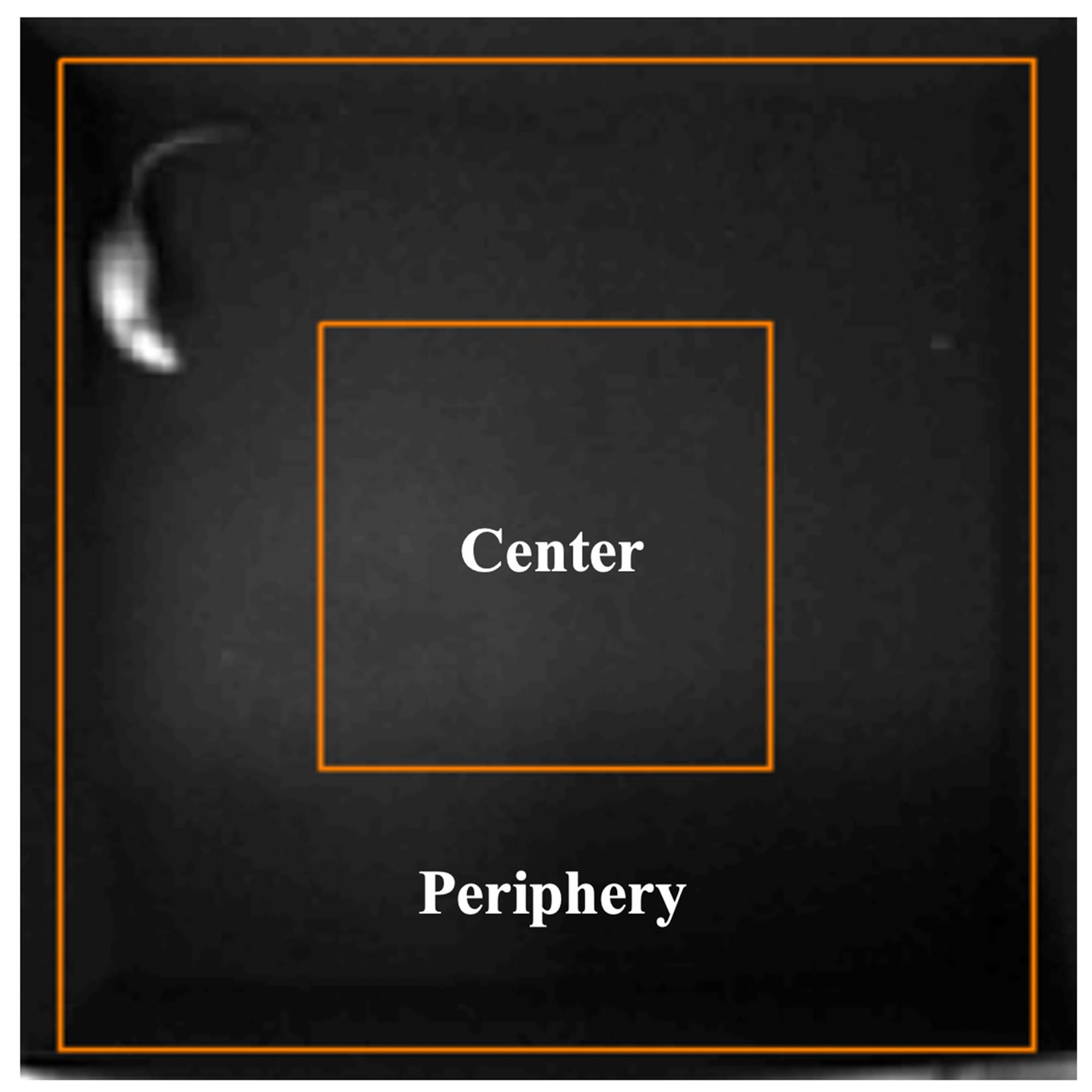
Figure 2. Open-field test. The open-field apparatus consists of a box (100 cm × 100 cm × 60 cm) with an outer zone (periphery) and an inner square (center).
2. For this analysis, use ANY-maze software to track and evaluate movements.
3. Conduct the test in a quiet room with a stable light source.
4. Bring the rat to the testing room at least 30 min before the test.
5. Wipe the cage with 70% ethanol every time before placing the rat inside to eliminate any scent clues.
6. Gently grasp the rat’s tail and place it in the center of the cage.
7. The ANY-maze software will automatically start 3 s after the placement. Allow the rat to explore the cage for 10 min.
8. Record the time spent in the center, the number of entries into the center, and the total distance travelled by the rat.
9. If seizures occur during the test, return the rat to its home cage and repeat the trial 1 h later.
10. After the trial, gently remove the rat from the cage. Remove any fecal pellets and wipe the cage with 70% ethanol again.
11. Allow the ethanol solution to dry completely and repeat the trial with another rat.
D. Brain collection
1. Anaesthetize the rat (sodium pentobarbital at 150 mg/kg intraperitoneally). Whilst under deep anesthesia, open the chest to expose the heart. Carefully insert a 16 G blunt needle into the left ventricle and make a small incision (around 1.5 mm) in the right atrium to allow for smooth outflow. Perfuse the rat transcardially first with ice-cold PBS at a rate of 60 mL/min for 250 mL and then with ice-cold 4% PFA at 40 mL/min for 250 mL.
Caution: The perfusion of PFA (which is hazardous) should be performed in a ventilated fume hood.
2. Remove the brain from the skull and place it in a 50 mL tube containing 4% PFA solution overnight.
3. On the next day, replace the 4% PFA with 10% sucrose solution. Wait until the brain sinks to the bottom of the tube and replace the solution with 30% sucrose.
4. When the brain sinks to the bottom again, remove it from the tube and embed it with OCT compound (mold composed of tinfoil). Freeze with liquid nitrogen and store at -80 °C.
5. Use a cryostat to obtain 10 mm coronal brain sections 2.64 mm posterior to the bregma at -18 °C. Thaw-mount the sections onto Superfrost Plus slides and store the slides at -80 °C.
E. MFS staining
1. Take brain sections (2.64 mm posterior to the bregma, one section per rat) from the fridge and allow them to dry completely at room temperature.
2. For antigen retrieval, boil the brain sections in citrate buffer for 10 min using a microwave.
3. Cool down the brain section with tap water for 30 min.
4. Draw a circle on the slides around the tissue with a hydrophobic barrier pen.
5. Incubate the slides with 0.3% Triton X-100 for 10 min.
6. Wash the slides in PBS three times for 5 min.
7. Block the slides with 5% goat serum for 1 h (room temperature).
8. Incubate the slides with a rabbit polyclonal anti-ZnT3 antibody diluted 1:500 in 3% goat serum (overnight, 4 °C).
9. The next day, wash slides in PBS three times for 5 min.
10. Incubate the slides with a secondary antibody diluted 1:1,000 in 3% goat serum for 1 h (room temperature; avoiding light exposure).
11. Wash slides in PBS three times for 5 min.
12. Apply one drop of mounting medium to the slide.
13. Place a coverslip on the tissue and allow it to dry while limiting slide exposure to light.
14. Capture ZnT3 staining regions (ipsilateral side) from the midpoint of the upper and lower blades of the dentate gyri with the microscope (Figure 3C).
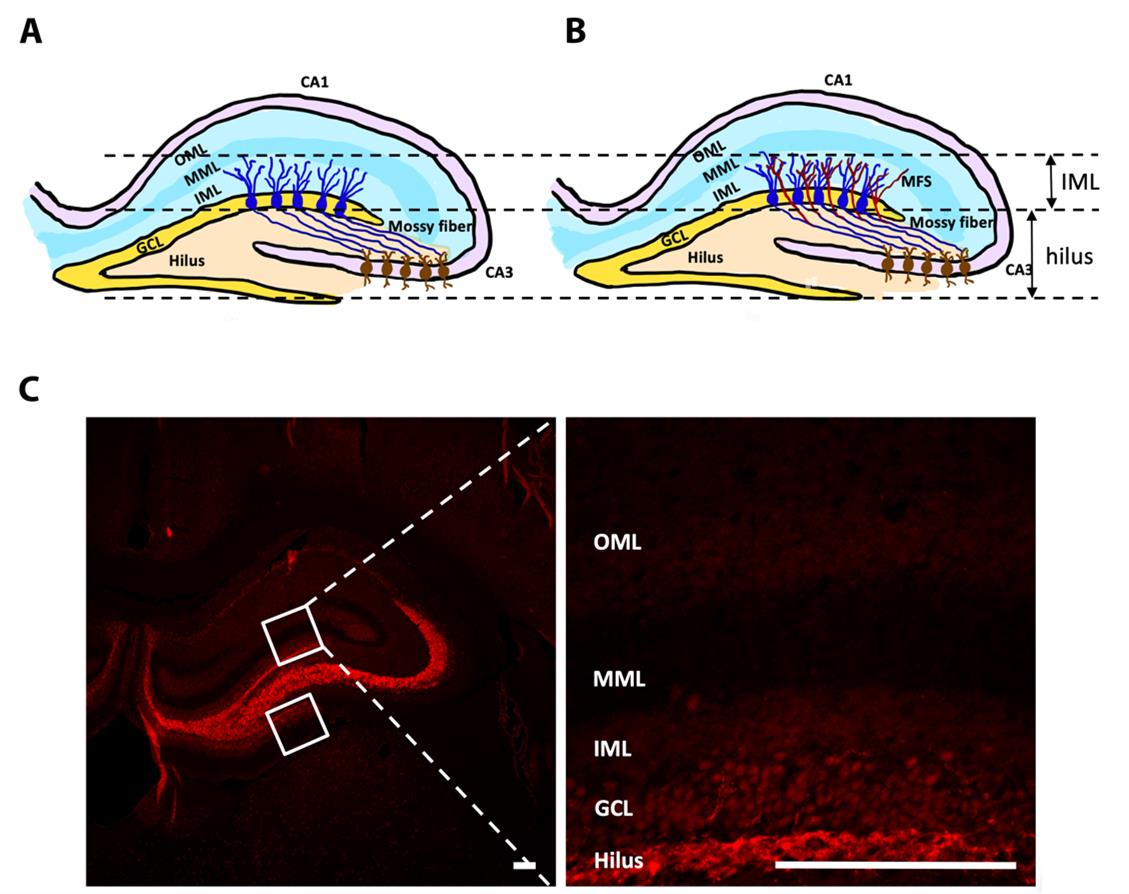
Figure 3. Mossy fiber sprouting (MFS) in the post-stroke epilepsy (PSE) model. (A) Left hippocampal formation in the normal brain. (B) Left hippocampal formation in the epileptic brain. (C) Representative pictures of the ZnT3-stained coronal brain section over the left hippocampus after sham procedures. Boxes represent the placement of measuring frames. Abbreviations: CA: cornu ammonis; GCL: granular cell layer; IML: inner molecular layer; MML: middle molecular layer; OML: outer molecular layer. Scale bar = 200 μm.
Data analysis
The percentage of rats that achieved status epilepticus was noted in the PSE and sham groups. Mortality rate at 3 days after real or sham PSE was calculated. For the open-field test, the time spent in the center, the number of entries into the center, and the total distance travelled were recorded. These data can interpret the rats’ exploratory activity, anxiety, and fear of the new environment. For the detection of MFS, only puncta with an intensity greater than twice the background and a diameter greater than 0.5 μm [10] were measured. The mean percentage of ZnT3 immunoreactive puncta’s area that covers the inner molecular layer in the upper and lower blades of the dentate gyri was calculated and determined as a score for MFS.
Validation of protocol
Among 17 rats of the PSE group, 16 rats (94%) achieved status epilepticus; with a mortality rate of 25%, 12 rats were alive at 3 days. In the sham group, none of the 12 rats had seizures, and all were alive at 3 days.
To illustrate the potential usefulness of this model, preliminary results of the open-field test and hippocampal MFS immunofluorescence at 8 weeks after PSE induction are shown in Figure 4. Locomotor activity, exploratory behavior, and anxiety level were evaluated using the open-field test. A PSE rat would spend more time in the center and make a higher number of entries into the center when compared to a rat after the sham procedures (Figure 4A, B). These behaviors suggest a decreased anxiety level (anxiolytic changes), which may be related to PSE activity [9].
Mossy fibers are the axons of hippocampal dentate granule cells. In the normal hippocampus, these axons innervate the hilar cells and CA3 pyramidal neurons (Figure 3A). In the epileptic hippocampus following severe seizures, however, these granule cell axons sprout and extensively innervate the inner molecular layer (Figure 3B) [11,12]. MFS was seen in a PSE rat to indicate synaptic reorganization (Figure 4D); MFS was not seen in a sham rat (Figure 4C).
Our composite model reflects the clinical reality that stroke patients are at increased risk of developing epilepsy [13]. The photothrombotic stroke mimics focal ischemic injury, and the LIP model induces epileptogenesis similar to TLE. Combining these captures the "multi-hit" processes of post-stroke epileptogenesis seen in humans, aligning with known disease mechanisms and clinical observations. This approach provides a relevant and translational model to study PSE development.
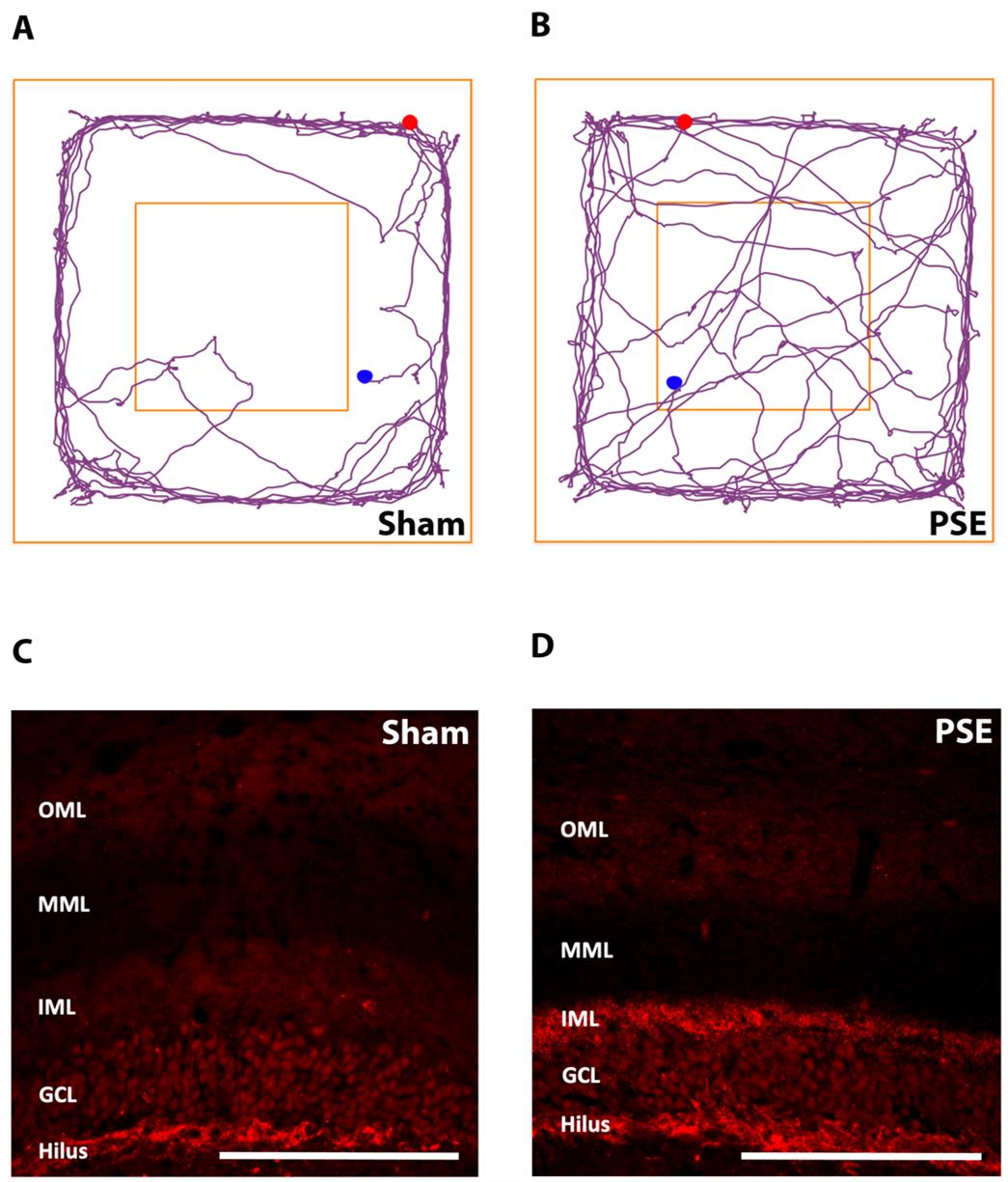
Figure 4. Representative results from the open-field test and mossy fiber sprouting (MFS) staining. (A) Representative moving trace of a rat after sham procedures in the open-field test. (B) Representative moving trace of a post-stroke epilepsy (PSE) rat in the open-field test. (C) Representative image of ZnT3-stained mossy fibers after sham procedures. (D) Representative image of ZnT3-stained mossy fibers after PSE. Abbreviations: CA: cornu ammonis; GCL: granular cell layer; IML: inner molecular layer; MML: middle molecular layer; OML: outer molecular layer. Scale bar = 200 μm.
General notes and troubleshooting
To improve survival after induction of status epilepticus, manual feeding with mashed gel and water for at least three days is important. For the open-field test, a quiet environment and a consistent bright light source are important. Although it is not an ideal mimic of PSE in patients, this PSE model incorporates focal stroke as a pre-existing condition on top of the LIP-induced TLE model to allow further exploratory studies. This novel rat PSE model may be useful in studying the link between stroke and epilepsy, determining the risk of PSE, and exploring effective treatment and preventive measures for PSE. This PSE model in the rat may not apply to mice because of the high mortality in mice after LIP. The combination of photothrombotic stroke and kainic acid-induced epilepsy may be explored as a potential PSE model in mice.
Acknowledgments
This research was supported by matching and donation funds (UGC Matching Grant, HKU, Hong Kong; SHAC Fund, HKU, Hong Kong; Cerebrovascular Research Fund, HKU, Hong Kong; Dr. William Mong Research Fund, HKU, Hong Kong; CRCG Internal Research Fund, HKU, Hong Kong; and Lee Man-Chiu Professorship in Neuroscience, HKU, Hong Kong) awarded to Raymond Tak Fai Cheung.
Competing interests
None of the authors has any conflict of interest to disclose.
Ethical considerations
All procedures had been submitted to and were approved by the Centre for Comparative Medicine Research (CCMR) of the University of Hong Kong.
References
- Watson, B. D., Dietrich, W. D., Busto, R., Wachtel, M. S. and Ginsberg, M. D. (1985). Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 17(5): 497–504. https://doi.org/10.1002/ana.410170513
- Kelly, K. M., Kharlamov, A., Hentosz, T. M., Kharlamova, E. A., Williamson, J. M., Bertram, E. H., Kapur, J. and Armstrong, D. M. (2001). Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res. 47(3): 189–203. https://doi.org/10.1016/j.neuroscience.2007.05.047
- Karhunen, H., Bezvenyuk, Z., Nissinen, J., Sivenius, J., Jolkkonen, J. and Pitkänen, A. (2007). Epileptogenesis after cortical photothrombotic brain lesion in rats. Neuroscience. 148(1): 314–324. https://doi.org/10.1016/j.neuroscience.2007.05.047
- Paxinos, G. and Watson, C. (2007). The Rat Brain in Stereotaxic Coordinates. 6th Edition, Academic Press, San Diego.
- Chen, J., Sanberg, P. R., Li, Y., Wang, L., Lu, M., Willing, A. E., Sanchez-Ramos, J. and Chopp, M. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 32(11): 2682–2688. https://doi.org/10.1161/hs1101.098367
- Curia, G., Longo, D., Biagini, G., Jones, R. S. and Avoli, M. (2008). The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 172(2): 143–157. https://doi.org/10.1016/j.jneumeth.2008.04.019
- Racine, R. J. (1972). Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 32(3): 281–294. https://doi.org/10.1016/0013-4694(72)90177-0
- Long, Q., Upadhya, D., Hattiangady, B., Kim, D. K., An, S. Y., Shuai, B., Prockop, D. J. and Shetty, A. K. (2017). Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci USA. 114(17): e1703920114. https://doi.org/10.1073/pnas.1703920114
- Smolensky, I. V., Zubareva, O. E., Kalemenev, S. V., Lavrentyeva, V. V., Dyomina, A. V., Karepanov, A. A. and Zaitsev, A. V. (2019). Impairments in cognitive functions and emotional and social behaviors in a rat lithium-pilocarpine model of temporal lobe epilepsy. Behav Brain Res. 372: 112044. https://doi.org/10.1016/j.bbr.2019.112044
- Hester, M. S. and Danzer, S. C. (2013). Accumulation of Abnormal Adult-Generated Hippocampal Granule Cells Predicts Seizure Frequency and Severity. J Neurosci. 33(21): 8926–8936. https://doi.org/10.1523/jneurosci.5161-12.2013
- Buckmaster, P. S., Zhang, G. F. and Yamawaki, R. (2002). Axon Sprouting in a Model of Temporal Lobe Epilepsy Creates a Predominantly Excitatory Feedback Circuit. J Neurosci. 22(15): 6650–6658. https://doi.org/10.1523/jneurosci.22-15-06650.2002
- Cavarsan, C. F., Malheiros, J., Hamani, C., Najm, I. and Covolan, L. (2018). Is Mossy Fiber Sprouting a Potential Therapeutic Target for Epilepsy? Front Neurol. 9: e01023. https://doi.org/10.3389/fneur.2018.01023
- Ferlazzo, E., Gasparini, S., Beghi, E., Sueri, C., Russo, E., Leo, A., Labate, A., Gambardella, A., Belcastro, V., Striano, P., et al. (2016). Epilepsy in cerebrovascular diseases: Review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. 57(8): 1205–1214. https://doi.org/10.1111/epi.13448
Article Information
Publication history
Received: Apr 19, 2025
Accepted: Jun 12, 2025
Available online: Jul 3, 2025
Published: Jul 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Guo, Y. and Cheung, R. T. F. (2025). A Novel Composite Method of Post-stroke Epilepsy Induction. Bio-protocol 15(14): e5387. DOI: 10.21769/BioProtoc.5387.
Category
Neuroscience > Nervous system disorders > Stroke
Neuroscience > Behavioral neuroscience > Animal model
Neuroscience > Neuroanatomy and circuitry > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








