- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Metabolite Production and Extraction of Indole Compound From the Tomato Endophyte Streptomyces sp. VITGV100
Published: Vol 15, Iss 14, Jul 20, 2025 DOI: 10.21769/BioProtoc.5386 Views: 2401
Reviewed by: Samik BhattacharyaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Extraction of Small Molecules from Fecal Samples and Testing of Their Activity on Microbial Physiology
Eduardo S. Alves [...] L. Caetano M. Antunes
Apr 20, 2018 7984 Views

Visualization of the Evolution and Transmission of Circulating Vaccine-Derived Poliovirus (cVDPV) Outbreaks in the African Region
D. Collins Owuor [...] Anfumbom K.W. Kfutwah
Jul 5, 2025 1859 Views
A Rapid and Cost-Effective Pipeline to Identify and Capture BGCs From Bacterial Draft Genomes
Marco A. Campos-Magaña [...] Luis Garcia-Morales
Dec 20, 2025 760 Views
Abstract
Endophytic actinomycetes, particularly Streptomyces species, have gained significant attention due to their potential to produce novel bioactive compounds. In this study, we isolated and characterized an endophytic Streptomyces sp. VITGV100 from the tomato plant (Lycopersicon esculentum), employing the direct streak method and whole-genome sequencing. A genome analysis was done to uncover its biosynthetic potential and identify indole-type compounds. The strain's secondary metabolite production was evaluated through GC–MS analysis, and its antimicrobial activity was tested against selected human pathogenic bacteria. Our protocol outlines a comprehensive approach, describing the isolation and extraction of metabolites and genome mining for indole-type compounds. This isolate has potential pharmaceutical applications, accelerating the discovery of novel indole-type bioactive compounds.
Key features
• A systematic approach for isolating Streptomyces sp. VITGV100 from Lycopersicon esculentum, including surface sterilization and selective culturing on ISP2 medium.
• Whole-genome sequencing and annotation: High-throughput Illumina sequencing followed by genome assembly, annotation, and functional characterization to explore biosynthetic potential and metabolic pathways.
• Secondary metabolite profiling and bioactivity screening: GC–MS-based identification of bioactive metabolites, followed by antibacterial activity assessment against human pathogens using well-diffusion assays.
• Identification of biosynthetic gene clusters (BGCs) using antiSMASH 6.0.
Keywords: Endophytic StreptomycesGraphical overview
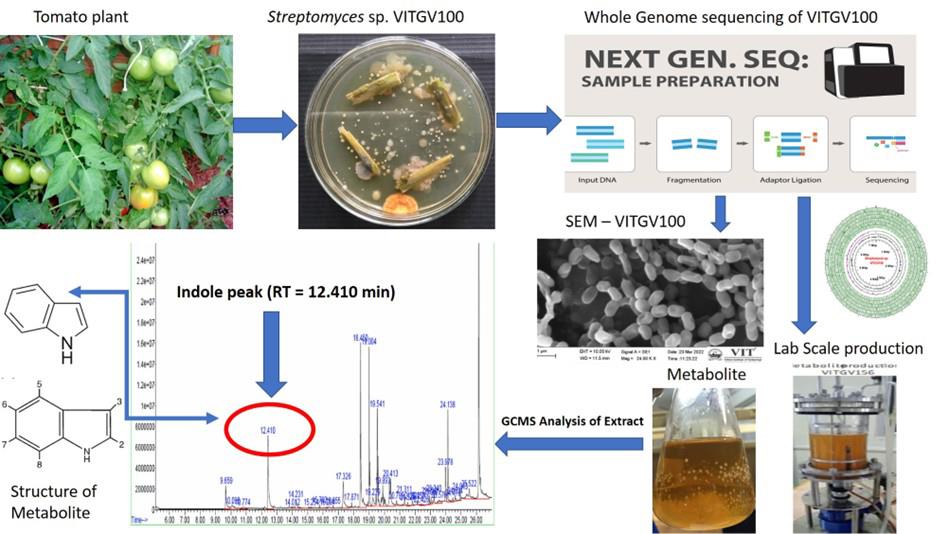
Genomic and metabolomic insights into the isolation of endophytic Streptomyces sp. VITGV100: a potential source of bioactive indole compounds
Background
Actinomycetes, particularly those belonging to the genus Streptomyces, are a prolific source of bioactive secondary metabolites with significant applications in medicine, agriculture, and industry. Among them, Streptomyces species have garnered attention for their potential to produce novel antimicrobial, anticancer, and even plant growth-promoting compounds [1]. Microbial strains offer a sustainable and environmentally friendly alternative to synthetic agrochemicals, playing a crucial role in biocontrol and plant health enhancement [2]. Traditional methods for isolating Streptomyces from environmental sources involve selective culturing techniques using specific growth media, followed by morphological and biochemical characterization [3]. However, recent advances in molecular biology and whole genome sequencing (WGS) have revolutionized the identification and functional analysis of these microorganisms [4]. WGS enables comprehensive insights into the genetic architecture of Streptomyces, facilitating the discovery of biosynthetic gene clusters (BGCs) responsible for secondary metabolite production [5]. This genomic approach overcomes the limitations of conventional screening methods, which often fail to detect cryptic or silent gene products under standard laboratory conditions [6]. The protocol described herein focuses on the isolation, genome sequencing, and functional characterization of Streptomyces sp. VITGV100, an endophytic actinomycete strain isolated from Lycopersicon esculentum (tomato) plants [5]. Compared to traditional bioactivity-guided screening, this genomic approach enhances the efficiency of novel bioactive compound discovery by directly linking genetic information to metabolite production [7]. A key advantage of this protocol is its ability to systematically identify and analyze BGCs involved in secondary metabolite biosynthesis using tools such as antiSMASH [8] and ARTS [9]. This computational approach significantly accelerates the identification of novel antibiotics [10]. Furthermore, comparative genomic analyses using ANI and dDDH facilitate the taxonomic positioning and evolutionary relationships of the newly isolated strain, contributing to a deeper understanding of Streptomyces diversity and genetic variability [11]. Beyond antibiotic discovery, the protocol has broad applications in biotechnology and agriculture. Endophytic Streptomyces strains have been reported to enhance plant growth through phytohormone production, nitrogen fixation, and antagonism against phytopathogens [12]. In summary, this protocol represents a comprehensive strategy for isolating, characterizing, and harnessing the biosynthetic potential of endophytic Streptomyces strains.
Materials and reagents
Biological materials
1. Healthy 4-week-old tomato plants grown in natural soil under conditions (Lycopersicon esculentum) (Arka Rakshak)
2. Streptomyces sp. VITGV100 (MCC 4961)
4. Staphylococcus aureus (MTCC 737)
5. Escherichia coli (MTCC 1687)
Reagents
1. International Streptomyces Project -2 agar (Himedia, CAS number: 8002-48-0)
2. Tetracycline (Himedia, CAS number: 64-75-5)
3. Cetyltrimethylammonium bromide (Himedia, AS number: 57-09-0)
4. Cyclohexamide (Himedia, CAS number: 66-81-9)
5. Nystatin (Himedia, CAS number: 1400-61-9)
6. Streptomyces DNA Purification kit (Himedia, CAS number: MBA527)
7. Ethanol (Himedia, CAS number: 64-17-5)
8. NaHCO3 (Himedia, CAS number: 144-55-8)
9. Ethyl acetate (Himedia, CAS number: 141-78-6)
10. Methanol (Himedia, CAS number: 67-56-1)
11. Glycerol (Himedia, CAS number: 56-81-5)
12. Sodium hypochlorite (Himedia, CAS number: 7681-52-9)
13. Mueller Hinton agar (MHA) (Himedia, CAS number: M1084)
Solutions
1. Yeast malt agar - ISP2 agar (see Recipes)
2. MHA agar (see Recipes)
Recipes
1. Yeast malt agar - ISP2 agar
| Ingredients | Final concentration |
|---|---|
| Peptone | 5 g/L |
| Yeast extract | 3 g/L |
| Malt extract | 3 g/L |
| Dextrose (glucose) | 10 g/L |
| Agar | 20 g/L |
2. MHA agar
| Ingredients | Final concentration |
|---|---|
| HM infusion solids B # (from 300 g) | 2 g/L |
| Acicase | 17.5 g/L |
| Starch | 1.5 g/L |
| Agar | 17. g/L |
Laboratory supplies
1. Erlenmeyer flasks (Borosil Scientific, catalog number: 4980)
2. Separating funnel (Borosil Scientific, catalog number: 6403)
3. Round-bottom flask (Borosil Scientific, catalog number: 4380)
4. Storage bottle (Borosil Scientific, catalog number: 1519)
5. Sample vials (Borosil Scientific, catalog number: 9913)
6. Petri plates (Borosil Scientific, catalog number: 3165)
7. Cork borer (HiMedia, India: LA737)
Equipment
1. Incubator (Thermo Scientific, catalog number: 50125590)
2. Hot air oven (Thermo Scientific, catalog number: PR305225M)
3. Biological safety cabinet, HEPA filter (Thermo Scientific, catalog number: 51025411)
4. Rotary vacuum evaporator (Fisher Scientific, catalog number: 05-405-195)
5. Gas chromatography and mass spectrophotometry (Thermo Scientific, model: TOEXGFTH)
6. Nanodrop (Thermo Scientific, model: ND-2000C)
7. Centrifuge (Remi, model: R-24)
8. Cooling centrifuge (Remi, model: CM-12)
9. Zone measuring scale (HiMedia, India, model: PW297)
10. GC–MS (Thermo Scientific Trace GC Ultra & ISQ Single Quadrupole MS, USA)
Software and datasets
1. antiSMASH 6.0 (Manufacturer, Version - https://antismash.sondarymetabolites.org)
2. Alignment Search Tool (BlastX): https://blast.ncbi.nlm.nih.gov/Blast.cgi
3. Kyoto Encyclopedia of Genes and Genomes (KEGG):
3. KEGG Automatic Annotation Server: https://www.genome.jp/tools/kaas/
4. CGView server 1.0: https://proksee.ca/
5. Blast2GO platform: https://www.blast2go.com
6. Web Gene Ontology Annotation plot (http://wego.genomics.org.cn/cgi-bin/wego/index.pl)
7. MegaX 6.0: https://www.megasoftware.net/
8. Antimicrobial Resistant Target Seeker: https://arts.ziemertlab.com
9. Prokka (version 1.12): https://github.com/tseemann/prokka
10. SPAdes assembler (v-3.13.0): https://github.com/ablab/spades
11. Trimmomatic v0.38: http://www.usadellab.org/cms/?page=trimmomatic
12. Streptomyces sp. VITGV100: https://trace.ncbi.nlm.nih.gov/Traces/?view=run_browser&acc=SRR15294182&display=analysis
Procedure
A. Isolation of endophytic Streptomyces sp.
1. Collect healthy tomato plants (Lycopersicon esculentum). In our work, healthy 4-week-old tomato plants (L. esculentum), from Madurai (9.9420° N, 77.9724° E), India, grown in natural soil in an agricultural field (37 ± 2 °C, 12 h light/dark photoperiod), were used for endophyte isolation (lower stem 3–5 cm, plant measured upward from the soil surface).
2. Perform surface sterilization of plant samples using stems collected from a 4-week-old tomato plant. Cut the stems into 4–5 cm long segments using sterile surgical blades and do the following:
a. Immerse in 70% ethanol for 1 min.
b. Immerse in 90% ethanol for 1 min.
c. Treat with 0.9% sodium hypochlorite for 4 min.
d. Rinse in 70% ethanol for 30 s.
e. Soak in 10% NaHCO3 for 5 min.
f. Rinse thoroughly three times with sterile distilled water.
3. Cut the surface-sterilized lower stems in a cross direction and streak on ISP2 agar (pH 7.2 before autoclaving at 121 °C, 15 psi for 15 min) medium supplemented with cyclohexamide and nystatin (each used at 50 μg/mL in ISP2 agar). Stock solutions (10 mg/mL) were prepared in ethanol and filter-sterilized before use.
4. Then, incubate the plates at 30 °C for 15 days and isolate individual microbial colonies. All incubation steps should be carried out in a light/dark cycle at 30 °C to support endophyte growth. From this point onward, all procedures should be conducted under a clean bench/laminar flow hood to maintain sterility
5. After 15 days, pick distinct colonies with a powdery morphology and subculture onto fresh ISP2 agar plates to ensure purity and morphological consistency (Figure 1).
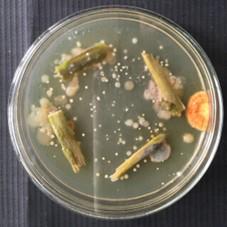
Figure 1. Isolation of endophytic Streptomyces sp. VITGV100
B. Whole-genome sequencing
1. Grow subcultures in ISP2 broth at 30 °C for 7 days with shaking at 120 rpm.
2. Extract genomic DNA using the CTAB method (Veilumuthu et al., 2022).
3. Assess DNA quality and quantity using NanoDrop. Typical DNA concentration is 146.9 ng/μL.
4. Prepare paired-end sequencing libraries using Illumina TruSeq Nano DNA Library Prep kit. In our case, paired-end sequencing was performed with 2 × 150 bp reads, achieving ~100× coverage. (Genome sequence deposited in NCBI; SRA accession number SRS9635924.)
5. Process sequencing data using Trimmomatic v0.38 to obtain high-quality reads. Perform quality trimming of raw reads using Trimmomatic v0.38 to remove adapter sequences and low-quality regions. Recommended command line (paired-end data): https://github.com/usadellab/Trimmomatic.
6. Assemble genome using SPAdes assembler v3.13.0. Assemble the cleaned reads using SPAdes v3.13.0 with default settings and careful flags to reduce mismatches: https://github.com/ablab/spades.
7. Perform annotation using Prokka v1.12 and identify rRNA genes. Annotate the assembled genome using Prokka v1.12 for gene prediction and identification of rRNA, tRNA, and CDS: https://github.com/tseemann/prokka.
8. Conduct functional annotation using the NCBI non-redundant protein database (BlastX) and KEGG database (KAAS). Do pathway mapping using KEGG Automatic Annotation Server: https://www.genome.jp/tools/kaas/.
9. Construct a circular genome using CGView server 1.0 (Figure 2).
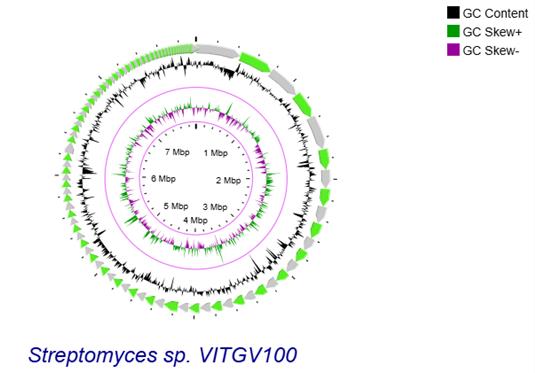
Figure 2. Genomic landscape of VITGV100. Circular genomic map of Streptomyces sp. VITGV100 assembled contigs are depicted as green arrows, accompanied by positive and negative GC skews indicated in the inner circle.
10. All analyses were run in Ubuntu Linux, with Trimmomatic and SPAdes via command line, and Blast2GO on a Java-supported desktop client. The genome sequencing was done with Eurofins Genomics Pvt. Ltd, and results were compiled.
C. Secondary metabolite gene cluster analysis
1. Detect biosynthetic gene clusters (BGCs) using antiSMASH 6.0 (Figures 3 and 4).
2. The biosynthetic potential of Streptomyces sp. VITGV100 was analyzed using antiSMASH 6.0, which identified multiple secondary metabolite biosynthetic gene clusters, as summarized in Table 1.
3. Utilize ARTS for antibiotic-specific genome mining.
Table 1. Secondary metabolite cluster of Streptomyces sp. VITGV100 obtained using antiSMASH 6.0
| S.No | Region | Type | Most similar known cluster | Similarity |
| 1 | Region 1.1 | NRPS | Coelichelin | 100% |
| 2 | Region 1.2 | NRPS, lanthipeptide-class-I | Coelibactin | 90% |
| 3 | Region 1.3 | lanthipeptide-class-III | Sapb | 100% |
| 4 | Region 2.1 | Siderophore | Paulomycin | 9% |
| 5 | Region 2.2 | NRPS | Cda1b/Cda2aCda3a | 72% |
| 6 | Region 3.1 | Terpene | Lysolipin I | 4% |
| 7 | Region 4.1 | T3PKS | Herboxidiene | 8% |
| 8 | Region 5.1 | Terpene | Hopene | 100% |
| 9 | Region 5.2 | T1PKS | Streptovaricin | 31% |
| 10 | Region 9.1 | Siderophore | – | – |
| 11 | Region 10.1 | NRPS-like | Streptothricin | 95% |
| 12 | Region 11.1 | Melanin | Melanin | 60% |
| 13 | Region 11.2 | Siderophore | Desferrioxamin B/Desferrioxamine E | 83% |
| 14 | Region 12.1 | Lanthipeptide-ClassIII | Catenulipeptin | 60% |
| 15 | Region 14.1 | RiPP-like | – | – |
| 16 | Region 15.1 | T2PKS | Spore Pigment | 66% |
| 17 | Region 16.1 | NRPS-like | Alanylclavam/2 | 12% |
| 18 | Region 28.1 | PKS-like, class-V | Methylenomycin A | 9% |
| 19 | Region 31.1 | T1PKS, NRPS, NRPS like | Candicidin | 90% |
| 20 | Region 32.1 | Terpene | Versipelostatin | 5% |
| 21 | Region 32.2 | RiPP-like | Informatipeptin | 42% |
| 22 | Region 33.1 | Terpene | Geosmin | 100% |
| 23 | Region 35.1 | T1PKS | Nystatin A1 | 31% |
| 24 | Region 37.1 | Indole | 7-Prenylisatin | 100% |
| 25 | Region 37.2 | Terpene | Isorenieratene | 100% |
| 26 | Region 40.1 | Ectoine | Ectoine | 100% |
| 27 | Region 1.1 | PKS-like, T2PKS, Butyrolactone | Fluostatins M-Q | 69% |
| 28 | Region 49.1 | Terpene | Isorenieratene | 18% |
| 29 | Region 50.1 | Terpene | Carotenoid | 45% |
| 30 | Region 70.1 | Indole | 5-Isoprenylindole-3-Carboxylate Β-D Glycosyl Ester | 23% |
| 31 | Region 72.1 | Terpene | Albaflavenone | 100% |
| 32 | Region 86.1 | hglE-KS | 2'-Chloropentostatin | 6% |
| 33 | Region 106.1 | T1PKS | Griseochelin | 53% |
| 34 | Region 111.1 | T1PKS | Sanglifehrin A | 11% |
| 35 | Region 114.1 | T1PKS | – | – |
NRPS: non-ribosomal peptide synthetase.
T1PKS: Type 1 Polyketide synthases.
T2PKS: Type 2 Polyketide synthases.
T3PKS: Type 3 Polyketide synthases.
RiPP: Ribosomally synthesized and post-translationally modified peptides.
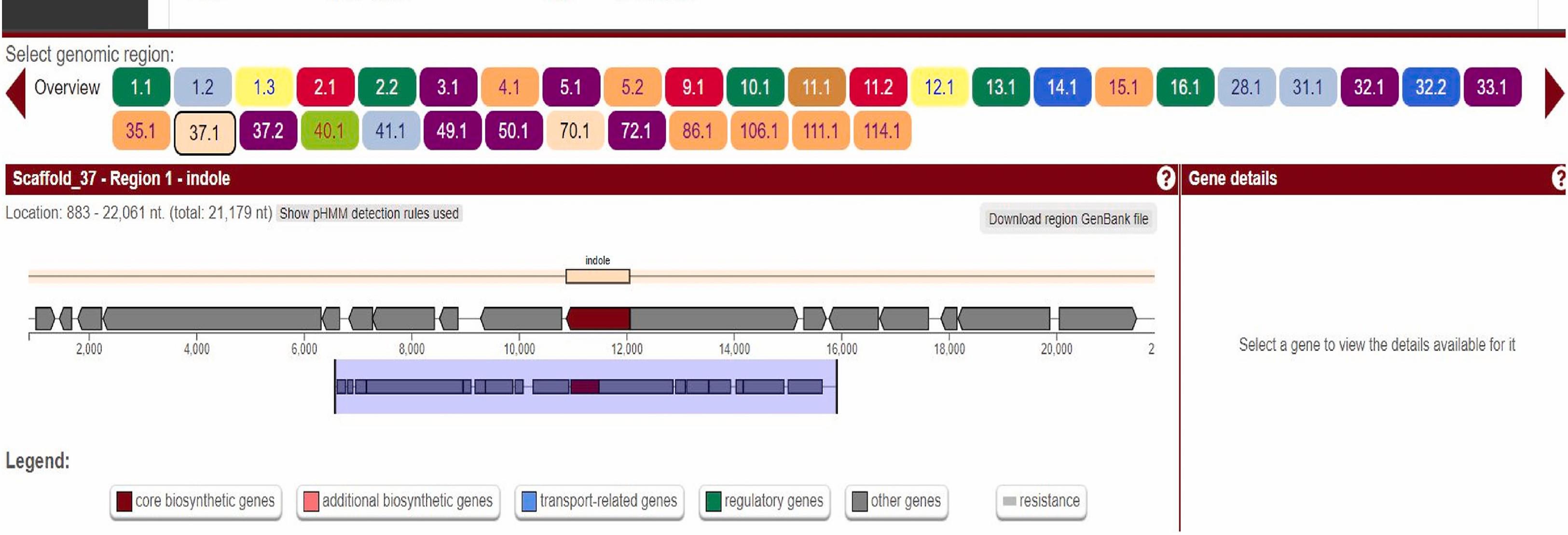
Figure 3. Screenshot highlighting two major stions: core gene table prioritization and visualization of the BGC of indole located in scaffold 37.1 region of the genome.
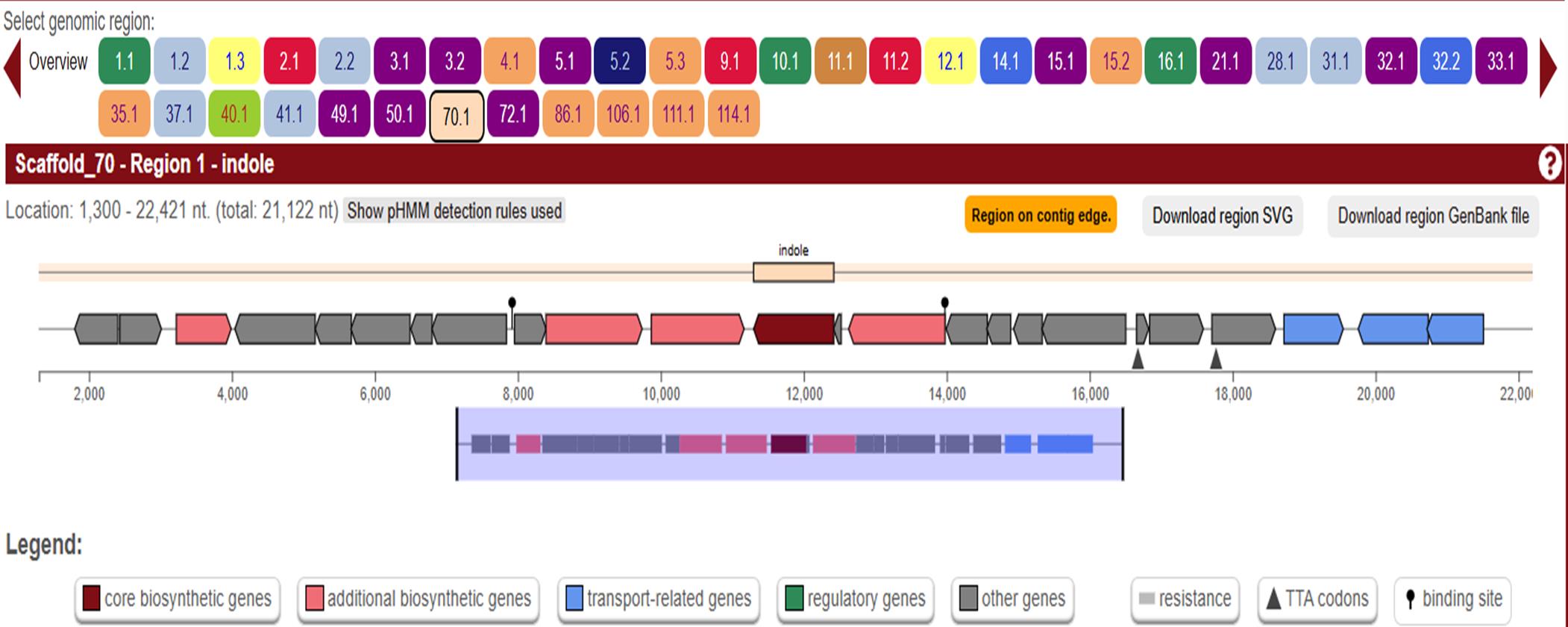
Figure 4. Screenshot highlighting two major stions: core gene table prioritization and visualization of BGC of indole located in scaffold 70.1 region of the genome.
D. Gene ontology analysis
1. Perform GO annotations using Blast2GO platform. Gene ontology enrichment visualization: http://wego.genomics.org.cn/.
2. Categorize gene functions into biological process (BP), molecular function (MF), and cellular component (CC).
3. Retrieve GO terms using WEGO portal and Blast2GO.
E. Metabolite extraction
1. Grow subcultures in ISP2 broth at 30 °C for 15 days with a 1:50 inoculum ratio and shaking at 120 rpm.
2. Confirm cellular growth by visual inspection of pellets and aggregates (Figure 5).

Figure 5. Visual inspection of pellets and aggregates of Streptomyces sp. VITGV100
3. Centrifuge the culture broth at 100 × g for 20 min at 4 °C and collect the supernatant in a separate tube. Discard cells.
4. To the supernatant, add an equal volume of ethyl acetate and shake the mixture vigorously for 10 min.
5. Incubate the flask in a rotary shaker at 200 rpm for 24 h at room temperature to enhance the extraction process. Bead beating is not required, but may enhance extraction efficiency.
6. Allow the mixture to settle undisturbed for 30 min, enabling phase separation.
7. Carefully collect the upper organic phase containing the metabolites and concentrate it using a rotary evaporator at 54 °C and 80 rpm until a dry or semi-solid residue is obtained. Collect organic extracts in sterile glass vials to avoid solvent interaction with plastic.. After drying the solvent in a rotary evaporator, weigh the resulting residue.
8. Dissolve the dried crude extract in 200 μL of acetonitrile to ensure proper solubility and store it at -20 °C for further use.
9. Blank media extract was included as an ISP2 control to validate compound specificity during GC–MS analysis, as shown in Figure 6.
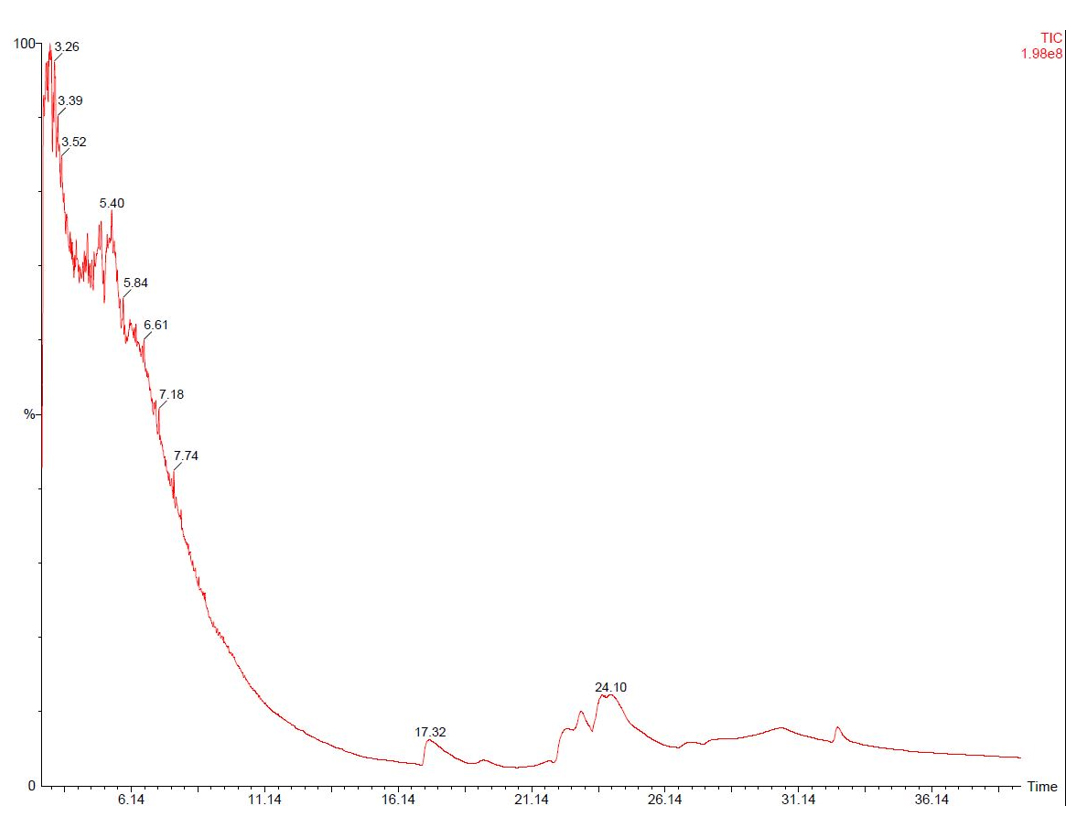
Figure 6. Blank media extract was run as an ISP2 control during GC–MS analysis
F. GC–MS analysis
1. Analyze crude extract using GC–MS.
2. Use a TG-5MS fused silica capillary column (30 m × 0.25 mm × 0.1 mm film thickness).
3. Set electron ionization at 70 eV and helium flow at 1 mL/min.
4. Maintain the injector and MS transfer line temperature at 280 °C.
5. Use the following temperature program:
a. 50 °C (2 min hold).
b. Increase to 150 °C at 7 °C/min.
c. Increase to 270 °C at 5 °C /min.
d. Increase to 310 °C at 3.5 °C/min.
The major chemical constituents present in the crude extract of Streptomyces sp. VITGV100, as identified by GC–MS analysis, are listed in Table 2. GC–MS analysis revealed that the ethyl acetate extract of Streptomyces sp. VITGV100 contained 35 metabolites. Indole was detected at 12.410 min. The identity of each compound was confirmed using the NIST mass spectrometry database. Figure 7 presents the GC–MS chromatogram obtained from the crude extract. Figure 8 shows the mass spectrum of indole obtained from the crude extract.
Table 2. Major chemical compounds identified by GC–MS for Streptomyces sp. VITGV100
| S. No | Chemical compound | RT | Molecular weight | Molecular formula | Area % | Activity | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Ethanol, 2-(2-butoxyethoxy) | 9.659 | 162.23 | C8H18O3 | 2.54 | - | |
| 2 | Butyl 2-(2-(2-ethoxyethoxy) ethoxy) acetate | 10.095 | 248.3160 | C12H24O5 | 1.02 | - | |
| 3 | 3-Aminobenzhydrazide | 10.774 | 151.1659 | C7H9N3O | 0.4 | Antimicrobial | [1] |
| 4 | Indole | 12.410 | 117.148 | C8H7N | 7.05 | Antimicrobial | [2] |
| 5 | Bicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid | 17.024 | 104.1491 | C8H8 | 0.16 | - | - |
| 6 | Benzeneethanol, 4-hydroxy | 14.231 | 138.1638 | C8H10O2 | 1.33 | Antimicrobial | [3] |
| 7 | 1-Penten-3-yne, 2-methyl | 15.254 | 80.1277 | C6H8 | 0.31 | - | - |
| 8 | 5-Eicosene, (E) | 15.782 | 280.5316 | C20H40 | 0.24 | - | - |
| 9 | 3-Methylbut-2-enoic acid, 8-chlorooctyl ester | 16.294 | 128.171 | C13H23ClO2 | 0.18 | - | - |
| 10 | Acetophenone, 4'-amino- | 16.655 | 135.1632 | C8H9NO | 0.21 | Antimicrobial | [4] |
| 11 | Benzoic acid, 4-(methylamino)- | 17.326 | 151.16 | C8H9NO2 | 3.26 | Antimicrobial | [5] |
| 12 | 5-Eicosene, (E)- | 17.871 | 280.5316 | C20H40 | 0.24 | Antimicrobial | [6] |
| 13 | n-Hexadecanoic acid | 18.450 | 256.4241 | C16H32O2 | 10.34 | Antimicrobial | [7] |
| 14 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 19.004 | 278.3435 | C16H22O4 | 7.16 | Antimicrobial | [8] |
| 15 | (p-Hydroxyphenyl) glyoxal | 19.239 | 150.13 | C8H6O3 | 1.32 | Antimicrobial | [9] |
| 16 | Dibutyl phthalate | 19.541 | 278.3435 | C16H22O4 | 6.07 | Antimicrobial | [10] |
| 17 | Phthalic acid, isobutyl octyl ester | 19.893 | 334.456 | C20H30O4 | 2.80 | Antimicrobial | [11] |
| 18 | 1,2-Benzenedicarboxylic acid, butyl octyl ester | 20.413 | 334.456 | C20H30O4 | 4.65 | Antimicrobial | [12] |
| 19 | 1,2-Benzenedicarboxylic acid, bis(1-methylethyl) ester | 20.799 | 250.2903 | C14H18O4 | 0.44 | - | - |
| 20 | Hexadecanamide | 21.311 | 311.5 | C16H33NO | 0.89 | - | - |
| 21 | Cyclotetracosane | 21.529 | 336.6380 | C24H48 | 0.20 | - | - |
| 22 | cis-11-Hexadecenal | 21.822 | 238.41 | C16H30O | 0.46 | - | - |
| 23 | 4-s-Butylaniline | 22.158 | 149.233 | C10H15N | 0.24 | - | - |
| 24 | 1,6-Dibromo-2-cyclohexylpentane | 22.426 | 444.12 | C11H20Br2 | 0.16 | - | - |
| 25 | 1H-Indole, 3-methyl- | 22.930 | 174.2423 | C11H14N2 | 0.63 | Antimicrobial | |
| 26 | Octadecanamide | 23.047 | 536.0 | C18H37NO | 0.39 | - | - |
| 27 | Phenol, 2-propyl- | 23.240 | 136.1910 | C9H12O | 0.73 | - | - |
| 28 | Octadecanoic acid, octadecyl ester | 23.576 | 536.9557 | C36H72O2 | 0.91 | - | - |
| 29 | Octadecanoic acid, 2,3-dihydroxypropyl ester | 23.978 | 358.5558 | C21H42O 4 | 2.14 | - | - |
| 30 | Diisooctyl phthalate | 24.138 | 390.56 | C24H38O4 | 4.09 | - | - |
| 31 | 8-Quinolinamine | 24.473 | 144.17 | C9H8N2 | 0.30 | Antimicrobial | |
| 32 | cis-11-Eicosenamide | 24.624 | 309.53 | C20H39NO | 0.45 | - | - |
| 33 | Dodecanoic acid, 2-butoxyethyl ester | 24.943 | 285.49 | C18H36O3 | 0.57 | - | - |
| 34 | Methyl 11-oxo-9-undecenoate | 25.522 | 212.28 | C12H20O3 | 2.60 | - | - |
| 35 | 9-Octadecenamide, (Z)- | 26.159 | 3281.476 | C18H35NO | 35.47 | - | - |
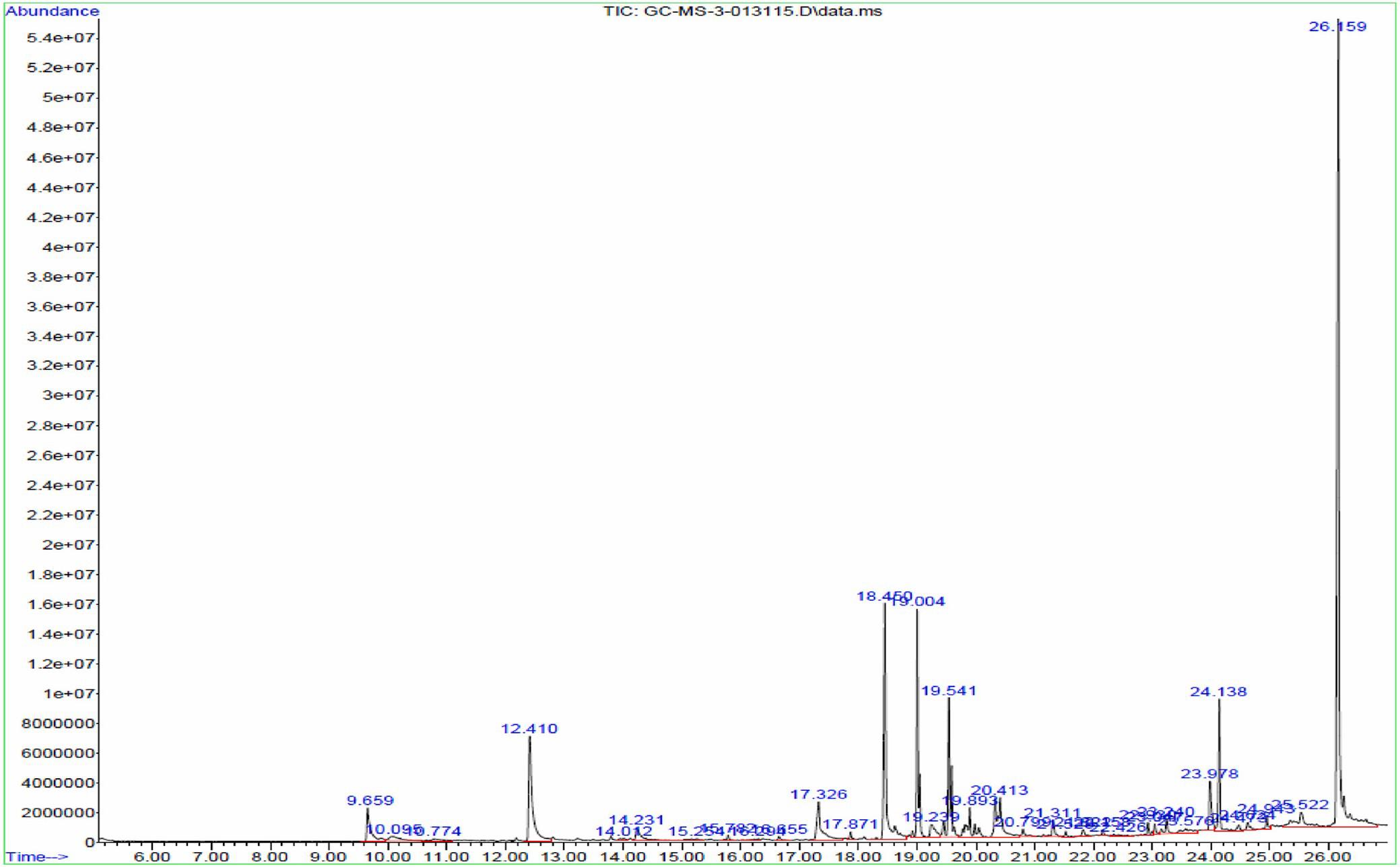
Figure 7. GC–MS chromatogram obtained from the crude extract of Streptomyces sp. VITGV100
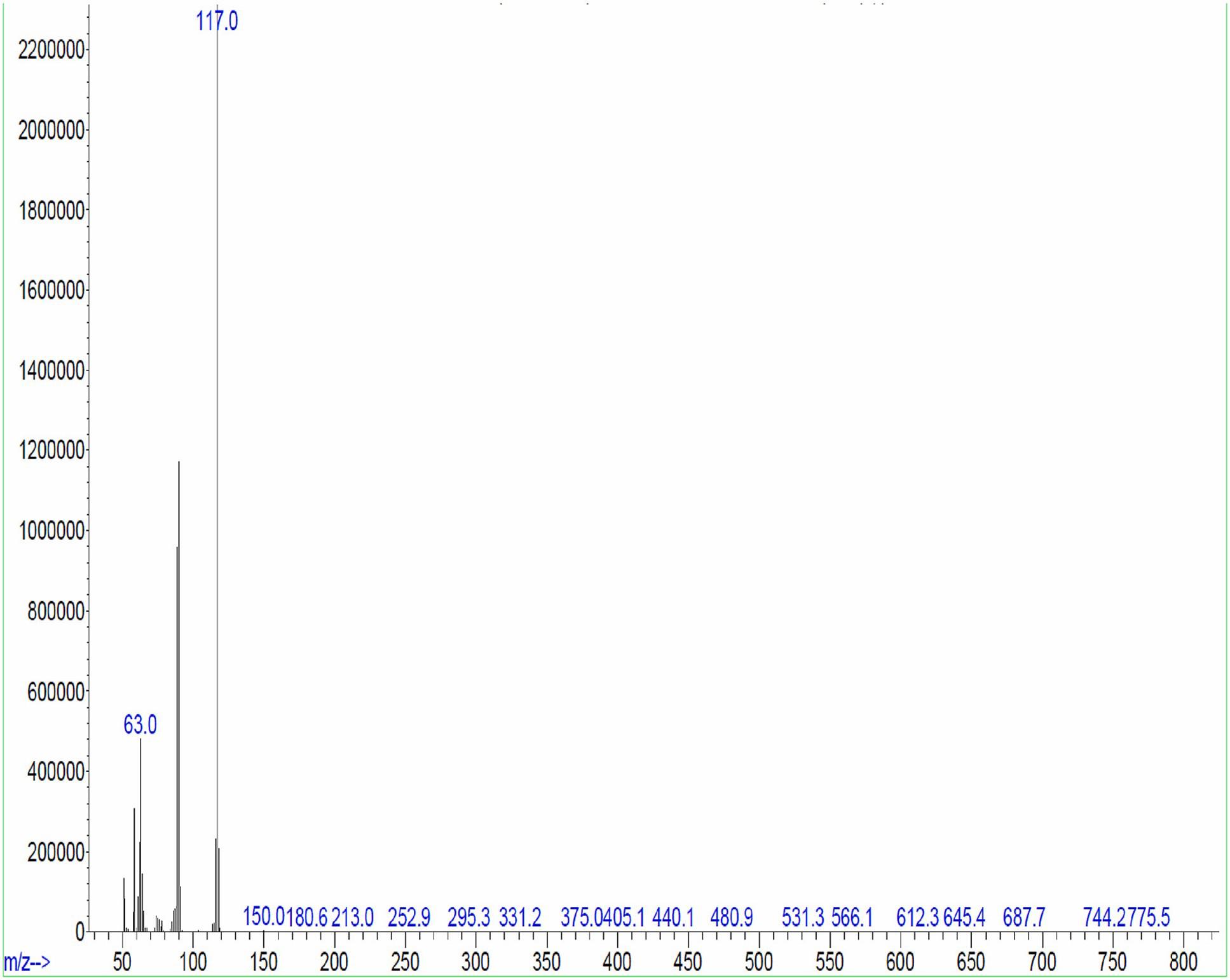
Figure 8. Indole massspectrum obtained from the crude extract of Streptomyces sp. VITGV100 at 12.40 min
G. Secondary screening against human pathogens
1. Cut 6 mm diameter wells in agar plates using a sterile steel cork borer.
2. Compare the antibacterial activity of the crude extract with tetracycline (positive control).
3. Prepare four different concentrations (25, 50, 75, and 100 μg/mL) of the crude extract in ethyl acetate and add to 4 different wells (100 μL each). Include negative controls (ethyl acetate) and positive controls (tetracycline) in all experiments.
4. Spread 100 µL of bacterial culture (OD600 ~0.6) evenly using a sterile cotton swab to create a confluent lawn. Air-dry plates for 10 min before well diffusion.
5. Prepare each test plate with a uniform lawn of the respective test bacteria, including Escherichia coli and Staphylococcus aureus, to evaluate the antibacterial activity of the crude extract.
6. Incubate plates at 37 °C for 24 h.
7. The antimicrobial activity of the crude extract was evaluated against selected test organisms (Figure 9), and the results, expressed as mean inhibition zone diameters (± SD, n = 3), are presented in Table 3.

Figure 9. Secondary screening of culture extract from Streptomyces sp. VITGV100 against (a) S. aureus and (b) E. coli at different concentrations (25, 50, 75, and 100 µg) by the well-diffusion method
Table 3. Antimicrobial activity of crude extract and controls (mean ± SD, n = 3)
| Strain | Treatment | 25 µg/mL | 50 µg/mL | 75 µg/mL | 100 µg/mL |
|---|---|---|---|---|---|
| E. coli | VITGV38 extract | 15 ± 0.47 | 16 ± 0.94 | 17 ± 0.47 | 18 ± 0.82 |
| Tetracycline | 23 ± 1.41 | 24 ± 0.82 | 24 ± 0.47 | 25 ± 1.25 | |
| Ethyl acetate | – | – | – | – | |
| S. aureus | VITGV38 extract | 10 ± 0.94 | 14 ± 0.47 | 15 ± 0.82 | 17 ± 0.94 |
| Tetracycline | 16 ± 0.47 | 18 ± 1.25 | 19 ± 0.94 | 19 ± 1.25 | |
| Ethyl acetate | – | – | – | – |
Data analysis
All experimental data were processed using standardized statistical and bioinformatics approaches. Data points deviating by more than 2 standard deviations from the mean were considered outliers and excluded. Only data from experiments with at least three independent biological replicates were included. Technical replicates with >10% variation was removed from further analysis. Biological replicates: Minimum of three independent replicates per experimental condition. Technical replicates: Minimum of three technical replicates per sample for reproducibility.
Data analysis was performed using the following software tools:
MEGA X: Used for phylogenetic analysis.
Linux (Ubuntu 20.04): Required for running bioinformatics pipelines such as SPAdes genome assembly, Prokka annotation, and antiSMASH BGC detection.
Validation of protocol
This protocol has been rigorously tested to ensure reproducibility and robustness across multiple independent experiments.
1. Experimental validation
All experiments were performed with a minimum of three biological replicates and three technical replicates per sample. Negative controls (untreated samples) and positive controls (reference standards) were included in all experiments.
2. Reproducibility and consistency
Independent validation experiments were conducted under identical conditions, yielding consistent colony morphology, metabolite production, and genome sequencing results. Whole-genome sequencing quality metrics (e.g., N50 values, read coverage, and BUSCO completeness scores) confirmed the reliability of the sequencing and assembly steps. Metabolite profiling via GC–MS demonstrated a high degree of reproducibility, with consistent compound retention times and mass spectra across replicates.
General notes and troubleshooting
1. Sterility precautions: To prevent contamination, ensure all glassware, media, and reagents are sterilized before use.
2. DNA extraction products can be stored at -20 °C before sequencing.
3. Accurate measurement of reagents is essential for reproducibility.
4. If no microbial growth is observed, verify sterility of ISP2 medium and incubation conditions. Minor fluctuations in temperature (±2 °C) or humidity may affect microbial growth. Consistent incubation conditions are recommended for reproducibility.
5. Genetic variability: Differences in strain genetics may impact secondary metabolite production. If expected results are not obtained, validate the strain identity using 16S rRNA sequencing before proceeding.
6. Reagent preparation: Always prepare fresh sodium hypochlorite and ethanol solutions for surface sterilization to maintain efficiency.
7. ISP2 medium composition: The composition of ISP2 agar may vary by manufacturer, potentially influencing microbial growth. If growth is inconsistent, verify media components and adjust pH to 7.2 ± 0.2 before sterilization.
Troubleshooting
Problem 1: Poor or no growth of Streptomyces sp. on ISP2 medium.
Possible cause: Improper sterilization of plant material led to contamination.
Solution: Optimize surface sterilization by adjusting ethanol and sodium hypochlorite exposure times (see General Note 4). Ensure all steps are performed under aseptic conditions.
Possible cause: Medium pH is outside the optimal range for Streptomyces sp.
Solution: Adjust the pH to 7.2 ± 0.2 before autoclaving to maintain optimal growth conditions.
Problem 2: Low yield of genomic DNA.
Possible cause: Incomplete cell lysis during CTAB extraction.
Solution: Increase the incubation time at 65 °C during lysis and ensure the addition of RNase A to remove RNA contamination.
Problem 3: No secondary metabolite detection in GC–MS analysis.
Possible cause: Poor extraction efficiency.
Solution: Increase the extraction time (24–48 h) and ensure vigorous shaking during solvent extraction (see General Note 3).
Possible cause: Metabolites degraded due to improper storage.
Solution: Store crude extracts at -20 °C in amber vials to prevent degradation by light or oxidation.
Problem 4: Inconsistent antibacterial activity in bioassay.
Possible cause: Variation in crude extract concentration.
Solution: Use a rotary evaporator under controlled vacuum and temperature (54 °C, 80 rpm) to ensure uniform concentration before testing.
Possible cause: Inconsistent agar diffusion well sizes.
Solution: Use a standardized sterile cork borer (6 mm diameter) to create uniform wells for antibiotic diffusion.
Acknowledgments
The authors would like to thank the VIT University.
Specific contributions of each author: Conceptualization P.V., S.R., and S.M.; Investigation Writing—Original Draft, P.V., and J.C.; Writing—Review & Editing, R.S. and J.C.; Funding acquisition, J.C..; Supervision, J.C.
This work was funded by VIT SEED GRANT, Vellore Institute of Technology, India.
The graphical abstract was created with Microsoft Office.
This protocol is adapted from Veilumuthu et al. [1]
Competing interests
Dr. Shweta Panchal is an Associate Editor for Bio-protocol but did not participate in the editorial and peer review process of this article, except as an author. The authors declare no other conflict of interest.
Ethical considerations
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Veilumuthu, P., Nagaranjan, T., Sasikumar, S., Siva, S, Jose, S. and Christopher, J. G. (2022). Streptomyces sp. VITGV100: An endophyte from Lycopersicon esculentum as new source of indole type compounds. Biochem Syst Ecol. 105: 104523. https://doi.org/10.1016/j.bse.2022.104523
- Haq, I. U., Rahim, K., Yahya, G., Ijaz, B., Maryam, S. and Paker, N. P. (2024). Eco-smart biocontrol strategies utilizing potent microbes for sustainable management of phytopathogenic diseases. Biotechnol Rep,. 44: e00859. https://doi.org/10.1016/j.btre.2024.e00859
- Goodfellow, M. and Fiedler, H. P. (2010). A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie van Leeuwenhoek. 98(2): 119–142. https://doi.org/10.1007/s10482-010-9460-2
- Veilumuthu, P., Sathiyabama, R., Shah, M., A, S., Karuppasamy, R., Thirunavukkarasu, M. K., Samrot, A. V, K, D., Venugopal, S. and J, G. (2025). Streptomyces sp. VITGV156 secondary metabolite binds pathogenic protein PBP2a and Beta-lactamase. Front Bioinform. 5: 1544800. https://doi.org/10.3389/fbinf.2025.1544800
- Veilumuthu, P., Nagaranjan, T., Sasikumar, S., Siva, S, Jose, S. and Christopher, J. G. (2022). Streptomyces sp. VITGV100: An endophyte from Lycopersicon esculentum as new source of indole type compounds. Biochem Syst Ecol. 105: 104523. https://doi.org/10.1016/j.bse.2022.104523
- Pattaulavar, V., Siva, R. and Christopher, J. G. (2024). Activation of Silent Genes in Actinomycetes by Different Methods. Protocols of Actinomycetes. 216–220. https://doi.org/10.1201/9781003398400-32
- Veilumuthu, P., Nandana, K. and Christopher, J. G. (2023). Genomic Report on Lycopersene Producing Streptomyces sp. VITGV38. Curr Trends Biotechnol Pharm. 17(Supplement 3B), 1245–1251. https://doi.org/10.5530/ctbp.2023.3s.60
- Veilumuthu, P., Sathiyabama, R., Sekaran, M., Chandrasekaran, R., Panchal, S. and Christopher, G. (2025). Biosynthetic pathway of psi. psi. carotene from Streptomyces sp. VITGV38 (MCC 4869). Front Microbiol. 16: 1548894. https://doi.org/10.3389/fmicb.2025.1548894
- Mungan, M. D., Alanjary, M., Blin, K., Weber, T., Medema, M. H. and Ziemert, N. (2020). ARTS 2.0: feature updates and expansion of the Antibiotic Resistant Target Seeker for comparative genome mining. Nucleic Acids Res. 48: W546–W552. https://doi.org/10.1093/nar/gkaa374
- Veilumuthu, P. and Godwin, C. J. (2022). Antimicrobial compounds produced by Streptomyces sp. VITGV01 against selected human pathogens. Res J Biotechnol. 17(12): 16–28. https://doi.org/10.25303/1712rjbt16028
- Meier-Kolthoff, J. P. and Göker, M. (2019). TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun. 10(1): 1–10. https://doi.org/10.1038/s41467-019-10210-3
- Patel, J. K., Madaan, S. and Archana, G. (2018). Antibiotic producing endophytic Streptomyces spp. colonize above-ground plant parts and promote shoot growth in multiple healthy and pathogen-challenged cereal crops. Microbiol Res. 215: 36–45. https://doi.org/10.1016/j.micres.2018.06.003
Article Information
Publication history
Received: Apr 11, 2025
Accepted: Jun 15, 2025
Available online: Jul 4, 2025
Published: Jul 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
How to cite
Pattapulavar, V., Ramanujam, S., Muthusamy, S., Panchal, S. and Christopher, J. G. (2025). Metabolite Production and Extraction of Indole Compound From the Tomato Endophyte Streptomyces sp. VITGV100. Bio-protocol 15(14): e5386. DOI: 10.21769/BioProtoc.5386.
Category
Microbiology > Microbial genetics > Whole genome sequencing
Biochemistry > Other compound > Small molecular
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










