- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Virus Isolation and Rice Protoplast Infection
Published: Vol 15, Iss 14, Jul 20, 2025 DOI: 10.21769/BioProtoc.5383 Views: 2523
Reviewed by: Tasleem JavaidAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Workflow for a Functional Assay of Candidate Effectors From Phytopathogens Using a TMV-GFP-based System
Peng Cao [...] Yuyan An
Apr 20, 2025 1706 Views
Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth
Zeinab Fotoohiyan and Ali Salehi Sardoei
Oct 5, 2025 1340 Views
Reproducible Emu-Based Workflow for High-Fidelity Soil and Plant Microbiome Profiling on HPC Clusters
Henrique M. Dias [...] Christopher Graham
Jan 20, 2026 370 Views
Abstract
Rice (Oryza sativa), a staple crop sustaining half of humanity’s caloric intake, is threatened by numerous insect-vector-transmitted diseases, such as rice stripe disease, caused by the rice stripe virus (RSV). Most genetic studies on plant antiviral defense mechanisms rely on natural or artificial infection and transgenic approaches, which require months of plant transformation. Here, we present a streamlined protocol that enables rapid analysis of RSV–host interactions within three days. The method encompasses three key phases: (1) polyethylene glycol (PEG)-based precipitation of RSV virions from infected plant tissues, (2) sequential purification through differential ultracentrifugation with glycerol cushion optimization, and (3) high-efficiency transfection of purified virions into rice protoplasts via PEG-mediated delivery. Viral replication is quantitatively assessed using RT-qPCR targeting viral RNA and immunoblotting with RSV nucleocapsid protein-specific monoclonal antibodies. This approach eliminates dependency on stable transgenic lines, allowing the simultaneous introduction of exogenous plasmids for functional studies. Compared with conventional methods requiring several months for transgenic plant generation, our protocol delivers analyzable results within three days, significantly accelerating the exploration of antiviral mechanisms and resistance gene screening.
Key features
• The protocol purifies virus particles of RSV with strong infection capacity, which can directly infect rice protoplasts when co-transfected with exogenous plasmids for functional studies.
• This transgene-independent approach accelerates antiviral determinant profiling from several months to three days.
Keywords: Rice stripe virusBackground
Rice stripe virus (RSV), a negative-stranded RNA virus of the genus Tenuivirus (order Bunyavirales), is a devastating pathogen transmitted by the small brown planthopper (Laodelphax striatellus Fallén) that causes severe yield losses in East Asian rice production [1–4]. Unlike other Bunyavirales members, RSV possesses filamentous, non-enveloped virions containing RNA-dependent RNA polymerase (RdRp), essential for replication in both plant hosts and insect vectors [5,6]. Current RSV management primarily relies on pesticide application to control the insect vector, though this approach risks inducing pesticide resistance and environmental contamination. Developing virus-resistant rice cultivars through molecular breeding offers a more sustainable solution but requires a more profound understanding of rice–RSV interactions [7,8].
Conventional plant antiviral studies predominantly employ natural or artificial infection methods, including viruliferous insect feeding, viral RNA/virion inoculation, or Agrobacterium-mediated delivery of viral DNA [9–11]. These approaches necessitate stable transgenic plant lines that require months to develop and validate, significantly delaying resistance gene characterization and antiviral pathway elucidation [12]. While plant protoplast systems bypass the need for stable transformation by enabling transient gene expression, their application in RSV research has been constrained by inefficient virion purification methods [13–15].
Our optimized protocol synergizes PEG-based virion precipitation with glycerol-cushion ultracentrifugation to obtain strong infectious RSV particles with preserved RdRp activity. When combined with PEG-mediated protoplast transfection, this system enables rapid investigation of antiviral mechanisms and high-throughput resistance gene screening within 3 days.
Materials and reagents
Biological materials
1. Seeds of Oryza sativa L. japonica. cv. Nipponbare (preserved by our laboratory)
2. RSV-infected rice materials (preserved by our laboratory)
Reagents
1. Phosphate buffer saline (PBS), 0.5 M, pH 7.5 (Macklin, catalog number: S885311)
2. 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
3. Triton X-100 (Sigma-Aldrich, catalog number: T8787)
4. Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884)
5. Tirchloromethane/chloroform (Beijing Tong Guang Fine Chemicals Company, catalog number: 112050)
6. Polyethylene glycol (PEG) 6000 (Sigma-Aldrich, catalog number: 528877)
7. PEG 4000 (Sigma-Aldrich, catalog number: 95904)
8. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S5886)
9. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541)
10. Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C3306)
11. Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 484016)
12. Hydrochloric acid (HCl) (Xilong Scientific, catalog number: SQC5791)
13. Glycerol (Sigma-Aldrich, catalog number: G5516)
14. 2-(N-Morpholino) ethanesulfonic acid, 4-Morpholineethanesulfonic acid (MES) (Sigma-Aldrich, catalog number: M3671)
15. D-Mannitol (Sangon Biotech, catalog number: A600335)
16. Bovine serum albumin (BSA) (Beyotime Biotechnology, catalog number: ST023)
17. Mouse anti-RSV nucleocapsid monoclonal antibody (provided by Dr. Jianxiang Wu from Zhejiang University and Dr. Feng Cui from the Institute of Zoology, Chinese Academy of Sciences)
18. Anti-Mouse IgG(H+L), HRP conjugate (Promega, catalog number: W4021)
19. Rapid Silver Staining kit (Sangon Biotech, catalog number: C500021)
20. MeilunGel precast PAGE gel (10%, Bis-tris, 1.0 mm) (MeilunBio, catalog number: MA0465-1)
21. PageRuler prestained protein ladder (10–180 kDa) (Thermo Scientific, catalog number: 26616)
22. TRIzol reagent (Thermo Scientific, catalog number: 15596018)
23. Diethyl pyrocarbonate (DEPC) (Sigma-Aldrich, catalog number: D5758)
24. RQ1 DNase I (Promega, catalog number: M198A)
25. M-MLV reverse transcriptase (Invitrogen, catalog number: M1701)
26. Recombinant RNasin ribonuclease inhibitor (Promega, catalog number: N2515)
27. 2× M5 HiPer Real-time PCR Super mix with High Rox (SYBR green, with anti-Taq) (Mei5 Biotech, catalog number: MF013-01)
28. Cellulase “ONOZUKA” R-10 (Yalult, catalog number: L0012)
29. Macerozyme R-10 (Yalult, catalog number: L0021)
30. Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266)
31. Tris (VWR Life Science, catalog number: 0497)
32. Tween-20 (Xilong Scientific, catalog number: 12900102)
33. Non-fat dry milk powder (Mei5 Biotech, catalog number: MF33421)
34. Sodium hypochlorite solution (Xilong Scientific, catalog number: 17702246)
35. Murashige and Skoog basal medium (Sigma-Aldrich, catalog number: M5519)
Solutions
1. PBS-EDTA solution (see Recipes)
2. W1 solution (see Recipes)
3. W5 solution (see Recipes)
4. PEG-CaCl2 solution (see Recipes)
5. Enzyme solution (see Recipes)
6. MMG solution (see Recipes)
7. Wash buffer (see Recipes)
8. Blocking buffer/antibody dilution buffer (see Recipes)
Recipes
1. PBS-EDTA solution
To prepare EDTA 1 M, pH 8.0 solution, dissolve 14.612 g of EDTA in ultrapure water, adjust pH to 8.0 with KOH, bring the final volume to 50 mL, sterilize using a 0.22 μm filter, and store at 4 °C. Prepare PBS-EDTA solution fresh on the day of use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| PBS (0.5 M, pH 7.5) | 0.1 M | 10 mL |
| 2-Mercaptoethanol | 0.1% | 50 μL |
| Triton X-100 | 1% | 500 μL |
| EDTA (1 M, pH 8.0) | 0.01 M | 500 μL |
| Ultrapure water | n/a | 38.95 mL |
2. W1 solution
To prepare D-mannitol 1 M solution, dissolve 9.109 g of D-mannitol in 50 mL of ultrapure water and sterilize using a 0.22 μm filter. To prepare MES-KOH 100 mM, pH 5.7 solution, dissolve 0.9762 g of MES in ultrapure water, adjust pH to 5.7 with KOH, bring the final volume to 50 mL, and sterilize with a 0.22 μm filter. To prepare KCl 1 M solution, dissolve 3.728 g of KCl in 50 mL of ultrapure water and sterilize with a 0.22 μm filter. Prepare W1 solution in accordance with the following table, sterilize with a 0.22 μm filter, and store at 4 °C for no more than 3 months.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| D-mannitol (1 M) | 0.5 M | 25 mL |
| MES-KOH (100 mM, pH 5.7) | 4 mM | 2 mL |
| KCl (1 M) | 20 mM | 1 mL |
| Ultrapure water | n/a | 22 mL |
3. W5 solution
To prepare NaCl 5 M solution, dissolve 14.61 g of NaCl in 50 mL of ultrapure water and sterilize with a 0.22 μm filter. To prepare CaCl2 1 M solution, dissolve 7.3505 g of CaCl2·2H2O in 50 mL of ultrapure water and sterilize by 0.22 μm filter. Prepare W5 solution in accordance with the following table, sterilize with a 0.22 μm filter, and store at 4 °C for no more than 3 months.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| NaCl (5 M) | 154 mM | 1.54 mL |
| CaCl2 (1 M) | 125 mM | 6.25 mL |
| KCl (1 M) | 5 mM | 250 μL |
| MES-KOH (100 mM, pH 5.7) | 2 mM | 1 mL |
| Ultrapure water | n/a | 40.96 mL |
4. PEG-CaCl2 solution
Prepare PEG-CaCl2 solution fresh on the day of use.
| Reagent | Final concentration | Volume (for 20 mL) |
|---|---|---|
| D-mannitol (1 M) | 0.2 M | 4 mL |
| CaCl2 (1 M) | 100 mM | 2 mL |
| PEG 4000 | 40% | 8 g |
| Ultrapure water | n/a | bring the final volume to 20 mL |
5. Enzyme solution
Prepare enzyme solution in accordance with the following table and add the reagents in the following sequence. After cellulase and macerozyme are thoroughly mixed and dissolved, incubate the mixture in a 55 °C water bath for 10 min, cool naturally, then add CaCl2 and BSA. Finally, sterilize the solution with a 0.22 μm filter and store at -80 °C for no more than 3 months.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| D-mannitol (1 M) | 0.4 M | 20 mL |
| MES-KOH (100 mM, pH 5.7) | 20 mM | 10 mL |
| KCl (1 M) | 20 mM | 1 mL |
| Cellulase “ONOZUKA” R-10 | 1.5% (w/v) | 0.75 g |
| Macerozyme R-10 | 0.7% (w/v) | 0.35 g |
| CaCl2 (1 M) | 10 mM | 0.5 mL |
| BSA (5% w/v) | 0.1% (w/v) | 1 mL |
| Ultrapure water | n/a | bring the final volume to 50 mL |
6. MMG solution
To prepare MgCl2 1 M solution, dissolve 4.7605 g of MgCl2 in 50 mL of ultrapure water and sterilize with a 0.22 μm filter. Prepare MMG solution in accordance with the following table, sterilize with a 0.22 μm filter, and store at 4 °C for no more than 3 months.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| D-mannitol (1 M) | 0.4 M | 20 mL |
| MgCl2 (1 M) | 15 mM | 0.75 mL |
| MES-KOH (100 mM, pH 5.7) | 4 mM | 2 mL |
| Ultrapure water | n/a | bring the final volume to 50 mL |
7. Wash buffer
To prepare Tris-HCl 1 M, pH 8.0 solution, dissolve 121.14 g of Tris in ultrapure water, adjust pH to 8.0 with HCl, bring the final volume to 1,000 mL, sterilize by autoclaving, and store at room temperature. Prepare wash buffer in accordance with the following table and store at 4 °C for no more than 3 months.
| Reagent | Final concentration | Volume (for 1,000 mL) |
|---|---|---|
| Tris-HCl (1 M, pH 8.0) | 0.2 M | 200 mL |
| NaCl | 1.5 M | 87.75 g |
| Tween-20 | 1‰ | 1 mL |
| Ultrapure water | n/a | bring the final volume to 1,000 mL |
8. Blocking buffer/Antibody dilution buffer
Dissolve 5% (v/v) non-fat dry milk in wash buffer and prepare fresh on the day of use.
Laboratory supplies
1. 10 μL pipette tips (Axygen, catalog number: AXYTF300RS)
2. 200 μL pipette tips (Axygen, catalog number: AXYTF200RS)
3. 1,000 μL pipette tips (Axygen, catalog number: AXYTF1000RS)
4. Sterile syringe filters (Millipore, catalog number: SLGV033RB)
5. Miracloth (Millipore, catalog number: 475855)
6. Sterile 50 mL conical tubes (Corning, catalog number: 430828)
7. Sterile 1.5 mL microtubes (Axygen, catalog number: AXYMCT150CS)
8. 2 mL microtubes, round bottom, snap-cap (Biosharp, catalog number: BS-20-M)
9. 3.5 mL open-top thick-wall polycarbonate tube (Beckman Coulter, catalog number: 349622)
10. Sterile 12-well cell culture plate (NEST Biotechnology, catalog number: 712001)
11. Nitrocellulose blotting membrane (GE Healthcare Life Science, catalog number: 10600003)
12. Sterile cell strainer (40 μm nylon mesh) (ThermoFisher Scientific, catalog number: 22363547)
Equipment
1. Refrigerated ultracentrifuge (Beckman Coulter, model: Optima MAX-XP)
Procedure
A. PEG-based precipitation of RSV virions from infected plant tissues
1. Inoculate 2-week-old rice seedlings with RSV-carrying small brown planthopper (Laodelphax striatellus Fallén) for 2 days. After the insects are removed, return the rice plants to the greenhouse at 32 °C in light (16 h), 28 °C in dark (8 h), and at a relative humidity of 60% ± 5%. Collect aboveground parts of RSV-infected rice plants with typical disease symptoms (Figure 1A) 4 weeks post-infection and grind them into fine powder using liquid nitrogen.
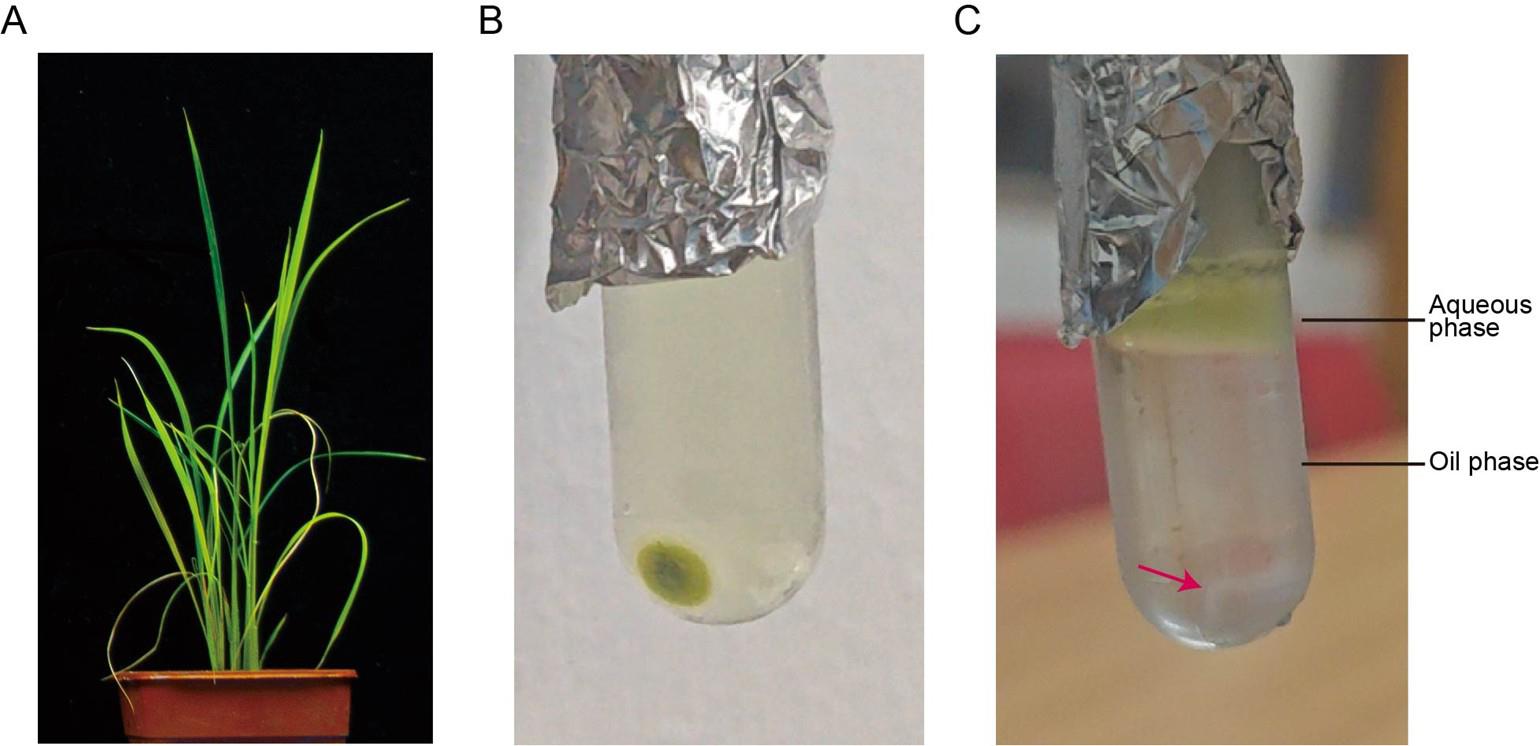
Figure 1. Purification of rice stripe virus (RSV) from RSV-infected rice plants via ultracentrifugation. (A) Typical disease symptoms in RSV-infected rice samples. (B) Virion pelleting by ultracentrifugation. Dark green pellets containing RSV virions are visible at the bottom of the tube. (C) Purifying RSV virions through 20% glycerol cushion ultracentrifugation. The red arrow indicates white RSV virion-containing pellets at the tube bottom.
2. Weigh 10 g of the above powder and blend the powder in 40 mL of pre-chilled PBS-EDTA solution in sterile 50 mL conical tubes.
3. Filter the homogenate through two layers of Miracloth to remove large debris.
4. Add pure chloroform into the filtrate until a concentration of 20% and stir for 15 min at room temperature using a glass rod.
5. Centrifuge the stirred liquids at 5,000× g for 20 min at 4 °C using a fixed-angle rotor in a refrigerated centrifuge, for clarification.
6. During centrifugation, grind PEG 6000 into fine powder.
7. Transfer the supernatant into a new sterile 50 mL conical tube and measure the volume of the supernatant.
8. Slowly add PEG 6000 powder and NaCl into the supernatant until a final concentration of 6% PEG 6000 (w/v) and 0.1 M NaCl. Stir the mixture until PEG 6000 and NaCl are fully dissolved.
9. Keep the mixture in an ice bath for 4 h and then rotate at 4 °C overnight.
10. Centrifuge the mixture at 5,000× g for 20 min at 4 °C using a fixed-angle rotor in a refrigerated centrifuge to pellet RSV virion.
B. Sequential purification of viral particles through differential ultracentrifugation with glycerol cushion optimization
1. Resuspend the pellets collected before in 1 mL of pre-chilled 0.01 M PBS (pH 7.5) and transfer the homogenate into a new sterile pre-chilled 1.5 mL microtube.
2. Centrifuge the homogenate at 5,000× g for 10 min at 4 °C using a fixed-angle rotor in a refrigerated centrifuge.
3. Transfer the supernatant into a sterile pre-chilled 3.5 mL open-top thick-wall polycarbonate tube and centrifuge the mixture at 100,000× g for 2 h at 4 °C using a fixed-angle rotor (TLA-100.3) in a refrigerated ultracentrifuge.
4. After centrifugation (Figure 1B), discard the supernatant and resuspend the pellets in 300 μL of pre-chilled 0.01 M PBS (pH 7.5) through repeated pipetting.
5. Carefully lay the resuspended solution on top of a pre-chilled 20% glycerol cushion inside a new sterile pre-chilled 3.5 mL open-top thick-wall polycarbonate tube and centrifuge at 100,000× g for 2 h at 4 °C using a fixed-angle rotor (TLA-100.3) in a refrigerated ultracentrifuge.
6. After centrifugation (Figure 1C), discard the supernatant, resuspend the pellets in 300 μL of pre-chilled 0.01 M PBS (pH 7.5) through repeated pipetting, and transfer the resuspended solution (containing purified RSV virion) into a new sterile pre-chilled 1.5 mL microtube.
7. Analyze the RSV virion by silver staining (Figure 2A) and immunoblotting (Figure 2B). Determine the virus concentration using the Bradford assay with BSA as a standard.
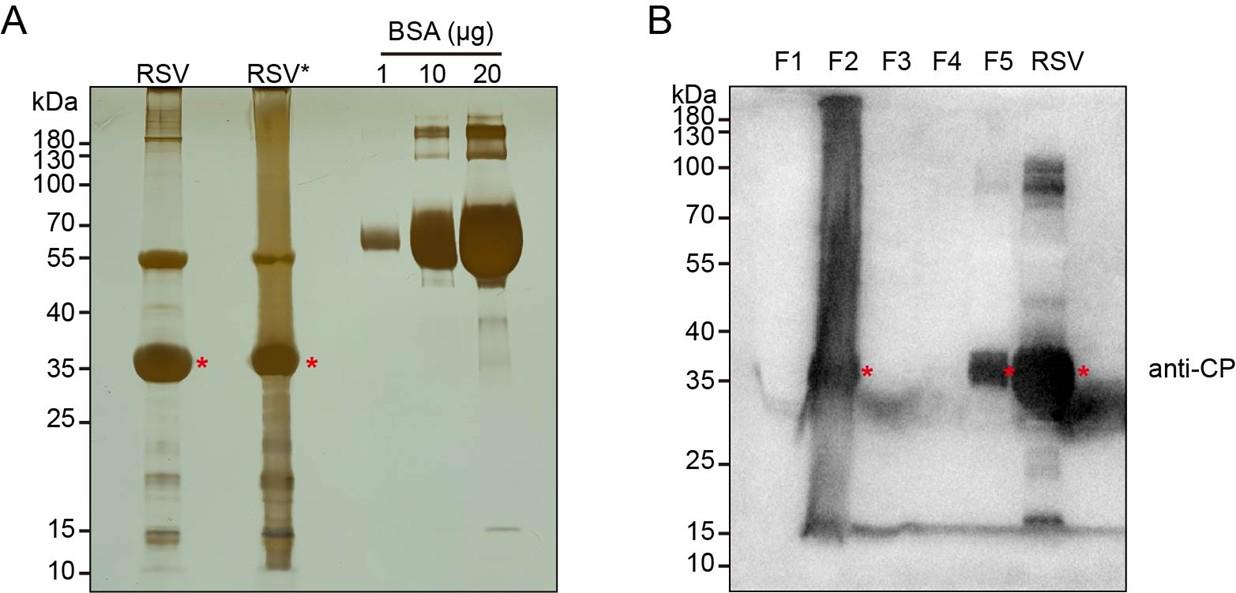
Figure 2. Analysis of purified rice stripe virus (RSV) virions by silver staining and immunoblotting. (A) Silver staining of purified RSV virion. RSV*: Pellets from step B2 undergoing an identical subsequent purification procedure as RSV virions. 1, 10, and 20 μg of BSA are subjected to silver staining simultaneously as reference standards. (B) Immunoblotting analysis of the purified RSV virions using a monoclonal antibody against RSV nucleocapsid protein (CP). Fraction designation: F1 (step A10 supernatant); F2 (step B2 pellets); F3 (step B3 supernatant); F4/F5 (aqueous/oil phases in step B5). Asterisks denote the bands corresponding to RSV CP at the predicted molecular weight.
8. Store the sample at 4 °C for short-term use (≤14 days; no significant infectivity loss) or -80 °C until further use (≤3 months; no significant infectivity loss). Avoid repeated freeze-thaw cycles to prevent loss in infectivity.
C. Rice protoplast preparation
1. Wash the dehusked mature Nipponbare seeds with 75% ethanol for 1 min and then sterilize twice with 20% (v/v) sodium hypochlorite solution for 15 min each.
2. Wash twice with sterilized ddH2O for 10 min each and air dry for 10 min on sterilized filter paper.
3. Put the embryo upside and plug the seeds into the 1/2 MS medium (pH 5.8).
4. Put the seeds at 28 °C on dark for 10~14 days.
5. Thaw the enzyme solution naturally at room temperature and ensure the buffer is totally mixed.
6. Cut the leaf sheaths of the 10~14-day-old Nipponbare (150 plants) into 0.5 mm pieces with sharp razor blades and then submerge in enzyme solution (20 mL).
7. Vacuum-infiltrate the mixture under -0.05~-0.07 MPa pressure for 30 min.
8. Incubate the mixture at 28 °C in the dark for 7 h with gentle shaking (40 rpm).
9. Filter the mixture through a 40 μm nylon mesh sterile cell strainer and collect the flow-through samples.
10. Resuspend the pieces in 20 mL of W5 solution gently and subsequently filter through another 40 μm nylon mesh sterile cell strainer to collect the flow-through samples.
11. Mix the flow-through samples from step C10 and C11 and centrifuge at 100× g for 3 min at room temperature using a swing-bucket rotor to pellet the protoplasts.
12. Carefully remove the supernatant using a pipette, add 2 mL of W5 solution to resuspend the pellet by gently tapping the centrifuge tube, and add 8 mL of W5 solution to the protoplast suspension. Mix it by gently shaking and incubate on ice for 30 min.
13. Centrifuge the mixture at 100× g for 3 min at room temperature using a swing-bucket rotor to pellet the protoplasts.
14. Resuspend the protoplast at 3 × 105 cells per mL in MMG solution for the transfection procedure below.
D. Co-transfection of purified virions with exogenous plasmids into rice protoplasts
1. When conducting rice protoplast infection, gently mix 1 μg of RSV virion, 10 μg of corresponding plasmid (e.g., a plasmid expressing a candidate gene for studying its role in antiviral defense, the corresponding empty vector, or a plasmid encoding a non-relevant gene as controls), 100 μL of freshly prepared rice protoplast (~3 × 104 cells), and 110 μL of PEG-CaCl2 solution in a sterile 2 mL microtube.
2. Incubate the mixture for 15 min in the dark at room temperature.
3. Add 440 μL of W5 solution to the mixture and stop the transfection by gentle inversion until totally mixed.
4. Centrifuge the mixture at 100× g for 3 min at room temperature using a swing-bucket rotor.
5. Carefully discard the supernatant and add 500 μL of W1 solution to resuspend the protoplast by gentle inversion.
6. Transfer the protoplast suspension into a 12-well cell culture plate, seal the plate with parafilm, and incubate overnight in the dark at room temperature.
7. Transfer the protoplast into a sterile 2 mL microtube and centrifuge at 100× g for 3 min at room temperature using a swing-bucket rotor to collect the infected protoplast after 18 h incubation. Analyze the RSV RNAs and RSV nucleocapsid protein accumulation using RT-qPCR and immunoblotting. Primers used in RT-qPCR are RNA3-F (5’-AACGTGCACTAAGAGGTGGT-3’) and RNA3-R (5’-CTGTCACTCTAGGCTCCTTC-3’). The cycling conditions used in RT-qPCR are as follows:
Step 1: 95 °C for 1 min
Step 2: 95 °C for 15 s
Step 3: 55 °C for 20 s
Step 4: 72 °C for 30 s
Plate read
Go to step 2, 39 cycles
Step 5: Melt curve 65 °C to 95 °C, increment 0.5 °C, for 5 s
Step 6: End
Data analysis
1. Silver staining
Purified RSV virions are analyzed by silver staining (Figure 2A). Briefly, 10 μL of purified RSV virions are mixed with 2.5 μL of 5× loading buffer and boiled at 95 °C for 5 min. The sample is electrophoresed in a 10% SDS-PAGE gel at 120 V for 50 min, followed by silver staining using a Sangon Biotech Rapid Silver Staining kit according to the manufacturer’s instructions.
2. Immunoblotting
Immunoblotting confirmation is performed as shown in Figure 2B. Following electrophoresis on 10% SDS-PAGE, separated proteins are transferred onto a nitrocellulose membrane. After blocking for 1 h at room temperature, the membrane is probed with mouse anti-RSV nucleocapsid monoclonal antibody (1:5,000 dilution) for 2 h at room temperature, and then incubated with HRP-conjugated anti-mouse IgG(H+L) secondary antibody (1:10,000 dilution) for 1 h at room temperature. Following incubation with primary or secondary antibody, the nitrocellulose membrane is washed four times with wash buffer for 5 min each. Images from immunoblotting are collected using the Molecular lmager ChemiDoc XRS+ (Bio-Rad) system.
Validation of protocol
This protocol has been used and validated in the following research article:
Huang et al. [16]. Perception of viral infections and initiation of antiviral defence in rice. Nature (Extended Data Figure 4d).
General notes and troubleshooting
Problem 1: Low protoplast viability.
Solutions:
a. Optimize plant growth conditions as they directly influence the viability of isolated protoplasts.
b. Minimize exposure time between cutting rice leaf sheaths and submersion in enzyme solution to prevent tissue desiccation, which reduces protoplast viability.
Problem 2: Low transfection efficiency.
Solutions:
a. Increase the virus dosage when conducting rice protoplast infection.
b. Extend the incubation time in step D2.
Quality control metrics:
a. Approximately 750 μg of RSV virion can generally be extracted from 10 g of RSV-infected rice materials.
b. Successful transfection was confirmed by a ≥1.5-fold increase in viral accumulation via RT-qPCR and immunoblot analysis at 18 h post-transfection, relative to the input virus.
Acknowledgments
Conceptualization, Y.L.; Investigation, Y.H.; Writing—Original Draft, Y.H.; Writing—Review & Editing, Z.Y. and Y.L.; Funding acquisition, Y.L.; Supervision, Y.L. Funding sources that supported this work were provided by the Ministry of Science and Technology (2021YFA1300702) and the Natural Science Foundation of China (31530062 and 32090010). The protocol was described and validated in Huang et al. [16], which was modified from Wang et al. [10] and Lu et al. [17].
Competing interests
The authors declare no conflicts of interest.
References
- Wu, J. G., Yang, G. Y., Zhao, S. S., Zhang, S., Qin, B. X., Zhu, Y. S., Xie, H. T., Chang, Q., Wang, L., Hu, J., et al. (2022). Current rice production is highly vulnerable to insect-borne viral diseases. Natl Sci Rev. 9(9): e1093/nsr/nwac131. https://doi.org/10.1093/nsr/nwac131
- Yao, S., Kang, J., Guo, G., Yang, Z., Huang, Y., Lan, Y., Zhou, T., Wang, L., Wei, C., Xu, Z., et al. (2022). The key micronutrient copper orchestrates broad-spectrum virus resistance in rice. Sci Adv. 8(26): eabm0660. https://doi.org/10.1126/sciadv.abm0660
- Wu, J., Yang, Z., Wang, Y., Zheng, L., Ye, R., Ji, Y., Zhao, S., Ji, S., Liu, R., Xu, L., et al. (2015). Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. eLife. 4: e05733. https://doi.org/10.7554/elife.05733
- Wu, J., Yang, R., Yang, Z., Yao, S., Zhao, S., Wang, Y., Li, P., Song, X., Jin, L., Zhou, T., et al. (2017). ROS accumulation and antiviral defence control by microRNA528 in rice. Nat Plants. 3(1): e203. https://doi.org/10.1038/nplants.2016.203
- Lu, G., Li, S., Zhou, C., Qian, X., Xiang, Q., Yang, T., Wu, J., Zhou, X., Zhou, Y., Ding, X. S., et al. (2019). Tenuivirus utilizes its glycoprotein as a helper component to overcome insect midgut barriers for its circulative and propagative transmission. PLoS Pathog. 15(3): e1007655. https://doi.org/10.1371/journal.ppat.1007655
- Toriyama, S. (1986). An RNA-dependent RNA polymerase associated with the filamentous nucleoproteins of rice stripe virus. J Gen Virol. 67(7): 1247–1255. https://doi.org/10.1099/0022-1317-67-7-1247
- Nicaise, V. (2014). Crop immunity against viruses: outcomes and future challenges. Front Plant Sci. 5: e00660. https://doi.org/10.3389/fpls.2014.00660
- Kang, B. C., Yeam, I. and Jahn, M. M. (2005). Genetics of plant virus resistance. Annu Rev Phytopathol 43(1): 581–621. https://doi.org/10.1146/annurev.phyto.43.011205.141140
- Yang, Z., Huang, Y., Yang, J., Yao, S., Zhao, K., Wang, D., Qin, Q., Bian, Z., Li, Y., Lan, Y., et al. (2020). Jasmonate signaling enhances RNA silencing and antiviral defense in rice. Cell Host Microbe 28(1): 89–103.e8. https://doi.org/10.1016/j.chom.2020.05.001
- Wang, Q., Liu, Y., He, J., Zheng, X., Hu, J., Liu, Y., Dai, H., Zhang, Y., Wang, B., Wu, W., et al. (2014). STV11 encodes a sulphotransferase and confers durable resistance to rice stripe virus. Nat Commun. 5(1): 4768. https://doi.org/10.1038/ncomms5768
- Wang, Y., Gong, Q., Wu, Y., Huang, F., Ismayil, A., Zhang, D., Li, H., Gu, H., Ludman, M., Fátyol, K., et al. (2021). A calmodulin-binding transcription factor links calcium signaling to antiviral RNAi defense in plants. Cell Host Microbe 29(9): 1393–1406.e7. https://doi.org/10.1016/j.chom.2021.07.003
- Nishimura, A., Aichi, I. and Matsuoka, M. (2006). A protocol for agrobacterium-mediated transformation in rice. Nat Protoc. 1(6): 2796–2802. https://doi.org/10.1038/nprot.2006.469
- Chen, S., Tao, L., Zeng, L., Vega‐Sanchez, M. E., Umemura, K. and Wang, G. L. (2006). A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Mol Plant Pathol. 7(5): 417–427. https://doi.org/10.1111/j.1364-3703.2006.00346.x
- Zhang, Y., Su, J., Duan, S., Ao, Y., Dai, J., Liu, J., Wang, P., Li, Y., Liu, B., Feng, D., et al. (2011). A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods. 7(1): 30. https://doi.org/10.1186/1746-4811-7-30
- He, F., Chen, S., Ning, Y. and Wang, G. (2016). Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr Protoc plant Biol. 1(2): 373–383. https://doi.org/10.1002/cppb.20026
- Huang, Y., Yang, J., Sun, X., Li, J., Cao, X., Yao, S., Han, Y., Chen, C., Du, L., Li, S., et al. (2025). Perception of viral infections and initiation of antiviral defence in rice. Nature. 641(8061): 173–181. https://doi.org/10.1038/s41586-025-08706-8
- Lu, G., Yao, M., Zhou, Y. and Tao, X. (2020). Purification of rice stripe virus. Bio Protoc. 10(6): e3565. https://doi.org/10.21769/bioprotoc.3565
Article Information
Publication history
Received: May 8, 2025
Accepted: Jun 16, 2025
Available online: Jul 3, 2025
Published: Jul 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Huang, Y., Yang, Z. and Li, Y. (2025). Virus Isolation and Rice Protoplast Infection. Bio-protocol 15(14): e5383. DOI: 10.21769/BioProtoc.5383.
Category
Plant Science > Plant physiology > Biotic stress
Plant Science > Plant immunity > Host-microbe interactions
Microbiology > Microbe-host interactions > Virus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









