- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluation of In Vitro Cytotoxic Activity of CAR-T Cells Using Patient-Derived Organoids
Published: Vol 15, Iss 13, Jul 5, 2025 DOI: 10.21769/BioProtoc.5369 Views: 2657
Reviewed by: Dipak Kumar PoriaMartin V KolevSamuele Tardito

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Isolation and Co-culture of Paneth Cells and Intestinal Stem Cells
Ryosuke Isotani [...] Toshimasa Yamauchi
Sep 20, 2025 3452 Views
Standardized Culture of Skin Fibroblasts From Punch Biopsies for Germline DNA Isolation in Myeloid Malignancies: A Practical Bedside-to-Laboratory Approach
Parampreet Kour [...] Pulkit Rastogi
Oct 5, 2025 1410 Views
Combining Microwave Ablation With CAR-T-Cell Therapy in Tumor-Bearing Mouse Models
Bihui Cao [...] Jia Shen
Oct 20, 2025 2319 Views
Abstract
Adoptive immune cell therapy, especially chimeric antigen receptor T (CAR-T) cells, has emerged as a promising strategy in solid tumor treatment, owing to its unique ability to specifically recognize and effectively eliminate tumor cells. Patient-derived organoids (PDOs) offer a robust and physiologically relevant platform for assessing the safety and efficacy of CAR-T-cell-based therapies. We now describe a detailed protocol for an in vitro evaluation system based on the co-culture of PDOs and CAR-T cells. This system encompasses the establishment of tumor organoids from patient tumor specimens, the isolation of T cells from matched peripheral blood mononuclear cells (PBMCs), and the generation of antigen-specific CAR-T cells. Through the use of fluorescent labeling to visualize different cells and apoptosis-related events post-interaction, along with quantitative analyses of T-cell proliferation, tumor organoid apoptosis, and the secretion of immune effector molecules, this system enables a robust and multifaceted evaluation of CAR-T cell cytotoxicity in vitro. Collectively, this co-culture system provides a systematic and reproducible in vitro platform for evaluating the functional activity of CAR-T cells and advancing research in tumor immunology and immunotherapy.
Keywords: Patient-derived organoidsGraphical overview
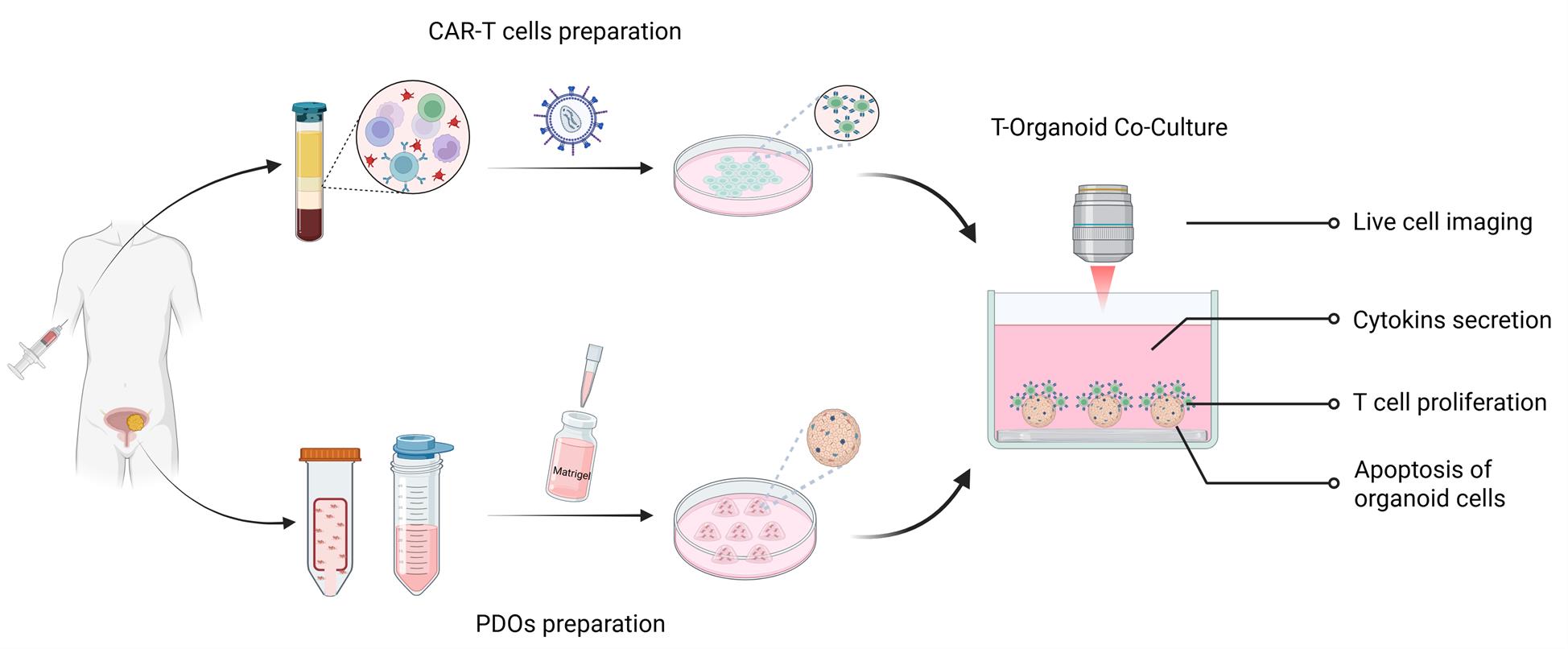
Background
Patient-derived organoids (PDOs) not only preserve the heterogeneity of the original tumor cells but also retain a diverse range of oncological characteristics. As a result, they have become a powerful model for accurately predicting clinical drug sensitivity and providing a reliable platform for preclinical drug development and validation [1]. However, existing tumor organoid culture systems have inherent limitations. The absence of an immune system poses significant challenges in evaluating immune-based therapies. To address this, immune cell–tumor organoid co-culture models have emerged as a valuable complementary approach. These models offer a high-fidelity platform for investigating cell–cell interactions within the tumor microenvironment and advancing the development of novel cancer immunotherapies [2].
CAR-T cell therapy leverages genetic engineering to modify T cells, enabling them to specifically recognize tumor antigens and precisely target cancer cells. This approach offers new hope to patients with solid tumors that are unresponsive to conventional treatments [3]. The development of every effective CAR-T cell therapy requires extensive preclinical evaluation and validation. Organoids preserve key characteristics such as tumor cell heterogeneity, making them a more accurate model of in vivo tumor conditions compared to traditional tumor cell lines [4].
In our previous investigations, we demonstrated that PDOs reliably recapitulate the antigen expression profiles of their corresponding primary tumor tissues. Owing to this, PDOs represent a valuable platform for the preclinical in vitro evaluation of chimeric antigen receptor T (CAR-T) cell therapies [5,6]. In the present study, we summarize and refine a streamlined in vitro co-culture system for assessing CAR-T cell function. This work aims to advance the broader application of organoid models in diverse tumor immunology and immunotherapy research contexts.
Materials and reagents
Reagents
1. DPBS, no calcium, no magnesium (Gibco, catalog number: 14190144)
2. AlbumiNZTM bovine albumin low IgG (BSA) (MP Biomedicals, catalog number: 02199897-CF)
3. Advanced DMEM/F-12 (Gibco, catalog number: 12634010)
4. Antibiotic-Antimycotic (Gibco, catalog number: 15240062)
5. GlutaMAX supplement (Gibco, catalog number: 35050061)
6. HEPES (1 M) (Thermo Fisher, catalog number: 15630080)
7. B-27 Supplement (50×), serum free (Gibco, catalog number: 17504044)
8. Fetal bovine serum (FBS) (Gibco, catalog number: 10091148)
9. Recombinant human R-spondin 1 protein (R&D Systems, catalog number: 4645-RS-250)
10. Recombinant human Noggin protein (Peprotech, catalog number: 120-10C-1000)
11. Recombinant human EGF protein (Peprotech, catalog number: 100-15)
12. Recombinant human basic FGF protein (Peprotech, catalog number: 100-18B)
13. Recombinant human FGF-10 protein (Peprotech, catalog number: 100-26)
14. Y-27632 (Abmole, catalog number: M1817)
15. N-Acetylcysteine (Sigma, catalog number: A9165)
16. Nicotinamide (Sigma, catalog number: N0636)
17. SB202190 monohydrochloride hydrate (Sigma, catalog number: S7076)
18. A83-01 (Sigma, catalog number: SML0788)
19. Matrigel® growth factor reduced (GFR) basement membrane matrix, LDEV free (Corning, catalog number: 356231)
20. Collagenase, type II, powder (Gibco, catalog number: 17101015)
21. DNase I (Roche, catalog number: 11284932001)
22. TrypLE Express (Gibco, catalog number: 12605036)
23. CD3 microbeads (Miltenyi Biotec, catalog number: 130-097-043)
24. OctoMACSTM separator (Miltenyi Biotec, catalog number: 130-042-109)
25. Dynabeads human T-activator CD3/CD28 for T-cell expansion and activation (Gibco, catalog number: 11131D)
26. Recombinant human IL-2 protein (Peprotech, catalog number: 200-02)
27. Recombinant human IL-15 protein (Peprotech, catalog number: 200-15)
28. X-VIVO 15 serum-free medium (LONZA, catalog number: 04-418Q)
29. Propidium iodide (Solarbio, catalog number: C0080)
30. Hoechst 33342 (Solarbio, catalog number: C0031)
31. CFSE (Invitrogen, catalog number: 65085084)
32. 0.5 M EDTA (pH 8.0) (Solarbio, catalog number: E1170)
33. Penicillin–streptomycin (Gibco, catalog number: 15070063)
34. Ficoll-PaqueTM PLUS density gradient media (Cytiva, catalog number: 17144002)
35. Authentikine TNF-alpha ELISA kit (Proteintech, catalog number: KE00154)
36. OptEIA Human IFN-γ ELISA kit (BD Pharmingen, catalog number: 550612)
37. Cleaved Caspase-3 (Asp175) ELISA kit (Abcam, catalog number: ab220655)
38. Anti-MUC1 antibody (Cell Signaling Technology, catalog number: 4358S)
39. Goat anti-mouse IgG (H+L) highly cross-absorbed secondary antibody, Alexa Fluor 555 (Thermo Fisher, catalog number: A21422)
40. G4S linker (E702V) rabbit mAb, Alexa Fluor 488 conjugate (Cell Signaling Technology, catalog number: 50515)
41. Recovery cell culture freezing medium (Gibco, catalog number: 12648010)
42. Lentiviral vector encoding a CAR sequence composed of MUC1 scFV (HMFG2), an IgD hinge, CD28 transmembrane domain, 4-1BB, and CD3ξ signaling domains, synthesized by Hanbio, China. The corresponding lentivirus, with a titer exceeding 1 × 108 TU/mL, was also produced by the vendor. Viral aliquots were stored at -80 until use.
43. NH4Cl (Aladdin, catalog number: A116373)
44. KHCO3 (Aladdin, catalog number: P639954)
45. Antifade solution (Sigma, catalog number: S7114)
46. Neutral buffered formalin (Solarbio, catalog number: G2161)
47. TBST buffer (Solarbio, catalog number: T1085)
Solutions
1. Organoid culture basal medium (see Recipes)
2. Bladder cancer organoid medium (see Recipes)
3. CAR-T cell culture medium (see Recipes)
4. MACS buffer (see Recipes)
5. Organoid recovery solution (see Recipes)
6. Tissue digestion solution (see Recipes)
7. RBC lysis buffer (see Recipes)
Recipes
1. Organoid culture basal medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Advanced DMEM/F-12 | n/a | top up to 500 mL |
| HEPES | 1% | 5 mL |
| GlutaMAX | 1% | 5 mL |
| Antibiotic-Antimycotic | 1% | 5 mL |
| Total | n/a | 500 mL |
2. Bladder cancer organoid medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Organoid culture basal medium | n/a | top up to 100 mL |
| B-27 supplement | 1× | 5 mL |
| N-acetylcysteine | 1.25 mM | 250 μL |
| Nicotinamide | 10 mM | 1 mL |
| SB202190 | 10 μM | 33.4 μL |
| A83-01 | 500 nM | 5 μL |
| Recombinant human R-spondin 1 | 500 ng/mL | 500 μL |
| Recombinant human noggin | 100 ng/mL | 100 μL |
| Recombinant human FGF-10 | 20 ng/mL | 20 μL |
| Recombinant human FGF-2 | 5 ng/mL | 5 μL |
| Recombinant human EGF | 50 ng/mL | 50 μL |
| Y-27632* | 10 μM | 5 μL |
| Total | n/a | 100 mL |
*Only required for initial derivation of organoids (first passage)
3. CAR-T cell culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI 1640 | n/a | top up to 100 mL |
| FBS | 10% | 10 mL |
| Penicillin-streptomycin | 1% | 5 mL |
| Recombinant human IL-2 | 50 IU/mL | 5 μL |
| Recombinant human IL-15 | 1 ng/mL | 1 μL |
| Total | n/a | 100 mL |
4. MACS buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DPBS | n/a | top up to 100 mL |
| BSA | 0.5% | 0.5 g |
| EDTA (pH 8.0) | 2 mM | 400 μL |
| Total | n/a | 100 mL |
5. Organoid recovery solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DPBS | n/a | top up to 100 mL |
| EDTA (pH 8.0) | 10 mM | 2 mL |
| Y27632 | 10 μM | 10 μL |
| Total | n/a | 10 mL |
6. Tissue digestion solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Advanced DMEM/F-12 | n/a | top up to 50 mL |
| Collagenase II | 5 mg/mL | 1 mL |
| DNAse I | 100 μg/mL | 0.5 mL |
| Y27632 | 10 μM | 5 μL |
| Total | n/a | 50 mL |
7. RBC lysis buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NH4Cl | 150 mM | 1.208 g |
| KHCO3 | 10 mM | 0.1 g |
| EDTA (500 mM pH 8.0) | 100 μM | 20 μL |
| Total | n/a | 100 mL |
Laboratory supplies
1. Costar 6-well clear TC-treated multiple well plates (Corning, catalog number: 3516)
2. Costar 24-well clear TC-treated multiple well plates (Corning, catalog number: 3738)
3. Costar 48-well clear TC-treated multiple well plates (Corning, catalog number: 3548)
4. 10/200/1,000 μL universal pipette tips (Axygen, catalog number: T-300, T-200-C-R-S, T-1000-B-R-S)
5. 15 mL PP centrifuge tubes (Corning, catalog number: 340052)
6. 50 mL PP centrifuge tubes (Corning, catalog number: 430290)
7. 70 μm cell strainer (Corning, catalog number: 352350)
8. 100 mm TC-treated culture dish (Corning, catalog number: 430167)
9. Falcon® round-bottom polystyrene tubes (Corning, catalog number: 352235)
10. Cryogenic vials (Corning, catalog number: A38053)
Equipment
1. Biological safety cabinet (Heal Force, catalog number: HFsafe-1200LC)
2. Centrifuge (Eppendorf, catalog number: 5702)3. Inverted microscope (Olympus, catalog number: CKX53)
4. Constant temperature water bath (Shanghai Yiheng, catalog number: HWS-24)
5. Carbon dioxide incubator (Thermo Fisher, catalog number: 3110)
6. Ice maker (Xuehua, catalog number: IMS-40)
7. Shaking incubator (Shanghai Shiping, catalog number: SPH-211B)
8. Cell counter (Thermo Fisher, catalog number: Countess IIFL)
9. Multifunctional microplate reader (PerkinElmer, catalog number: VICTOR Nivo)
10. Confocal microscope (Nikon, catalog number: AX)11. Flow cytometer (BD Biosciences, catalog number: FACS ARIA )
Procedure
A. Establishment of tumor organoids from resection tissue
1. Under aseptic conditions, wash the tissue sample twice on a Petri dish with cold DPBS and mince it into small fragments that can pass through a 1 mL tip.
2. Transfer tissue fragments to a 15 mL Falcon tube and add 5 mL of tumor tissue digestion solution. Incubate the mixture at 37 °C for 30–60 min. Mix 5–10 times every 10 min and check an aliquot of the solution for the presence of single cells under the microscope.
3. To terminate the digestion, add an equal volume of DMEM/F-12 supplemented with 20% FBS once the suspension contains more than 70% single cells.
4. Pass the mixture through a 70 μm filter and pellet cells by centrifuging at 300× g for 4 min. Discard the supernatant.
5. Wash the cell pellet with 10 mL of prewarmed DPBS and centrifuge the tube at 300× g for 4 min. Discard the supernatant.
6. If the pellet appears red, lyse erythrocytes by incubating for 5 min in 5 mL of RBC lysis buffer at room temperature. After lysis, add 10 mL of DPBS and centrifuge at 300× g for 4 min.
7. Resuspend the pellet with 1 mL of basal medium and take a 10 μL aliquot for cell counting. Add an additional 9 mL of the basal medium to the cell suspension and centrifuge the mixture at 300× g for 4 min.
8. Resuspend the final pellet in 30 μL of ice-cold organoid medium and add 270 μL of cold Matrigel.
Note: The recommended seeding density is 1–3 × 104 cells per 10 μL of Matrigel mixture.
Critical: Matrigel should be thawed on ice at 4 °C overnight. It is essential to keep it cold and work quickly throughout the process.
9. Plate the cells by dispensing 30 μL droplets into a 6-well culture plate. Invert the plate and incubate at 37 °C for 15 min to allow the Matrigel to solidify.
10. Once solidified, return the plate to its upright position and overlay with organoid medium. Fill unused wells with sterile DPBS to minimize evaporation. Incubate the plate under standard culture conditions (37 °C, 5% CO2).
11. Replace the culture medium with fresh organoid medium twice a week until passaging is required.
Note: Organoid formation is typically visible within 1-week post-incubation (Figure 1).
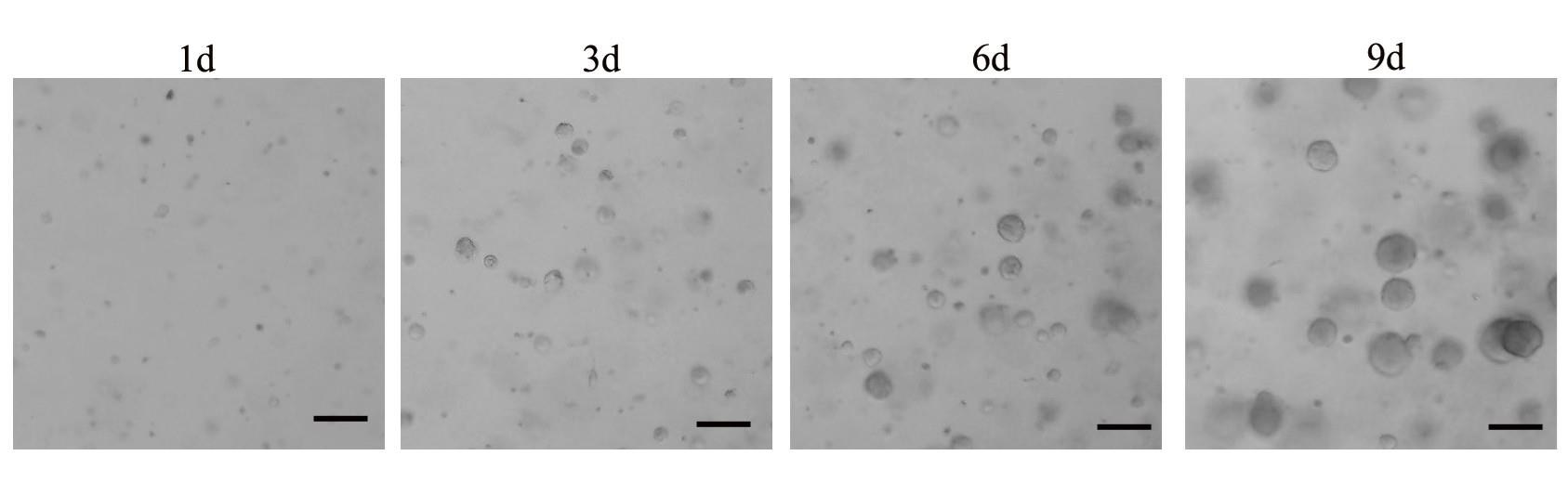
Figure 1. Representative images of organoid cultures during the initial days following cell plating. Small spherical cell clusters were observed as early as Day 3, progressively increasing in size and reaching an average diameter of approximately 100μm by Day 9. Scale bar, 100 μm.
B. Passaging organoids for maintenance
1. Scrap the Matrigel domes containing organoids using a P1000 pipette tip and transfer the suspension to a 15 mL tube. Centrifuge at 300× g for 4 min.
Note: To prevent Matrigel/organoid adhesion to the pipette tips, pre-rinse the tips several times with sterile PBS + 0.1% BSA.
2. Discard the supernatant and resuspend organoid-Matrigel drops in 5 mL of prewarmed TrypLE Express. Incubate at 37 °C for 5–10 min. Gently shake and mix evenly every 2 min.
Note: Avoid over-trypsinization; check under a microscope to ensure that >70% of organoids are digested into single cells.
3. Stop the digestion by adding twice the volume of organoid culture basal medium. Centrifuge at 300× g for 4 min and discard the supernatant.
4. Wash the pellet with 10 mL of DPBS and centrifuge again at 300× g for 4 min. Discard the supernatant.
5. Repeat steps A8–11.
C. Cryopreservation of organoids
1. Collect organoids using a P1000 pipette tip and transfer the suspension to a 15 mL tube. Centrifuge at 300× g for 4 min.Note: Cryopreservation is most effective when organoids are frozen 3–5 days after passaging.
2. Discard the supernatant and resuspend the organoid–Matrigel pellet in 5 mL of prewarmed TrypLE Express. Incubate at 37 °C for 3 min.
3. Stop the digestion by adding twice the volume of organoid culture basal medium. Centrifuge at 300× g for 4 min and discard the supernatant.
4. Resuspend the pellet with recovery cell culture freezing medium. Aliquot 1 mL of the suspension per cryovial and quickly place the cryovials into a freezing container. Store at -80 °C overnight.
5. Transfer cryovials to liquid nitrogen for long-term storage.
D. Thawing organoids
1. Quickly thaw cryovials containing organoids in a 37 °C water bath.
2. Transfer the entire contents of the cryovial to a 15 mL centrifuge tube and add 5 mL of organoid culture basal medium dropwise into the cryovial.
3. Centrifuge the mixture at 300× g for 4 min and discard the supernatant.
4. Plate the organoids as described in steps A8–11.
E. Surface antigen expression
1. Remove organoid medium and add 3 mL of ice-cold organoid recovery solution.
2. Scrap the Matrigel domes containing organoids with a P1000 pipette tip and transfer the suspension to a 15 mL tube.
3. Incubate the suspension on ice for 20–30 min on a shaking platform set to 60 rpm.
4. Centrifuge at 300× g for 4 min. Gently aspirate the supernatant and remove the remaining Matrigel layer.
5. Add 1 mL of 10% neutral buffered formalin and fix the organoids at 4 °C for 24 h.
6. Perform paraffin embedding and sectioning of the fixed organoids, as well as deparaffinization and rehydration of sections, as described in [7].
7. Dilute the primary antibody in 5% BSA according to the recommended ratio and prepare for use.
8. Encircle the organoid section with a PAP pen, apply the diluted antibody, and incubate in a humidified chamber at 4 °C for 12 h.
9. Discard the antibody solution and wash the slides three times for 5 min each in 100 mL of TBST buffer with gentle shaking (60 rpm).
10. Incubate the sections in fluorescent dye-conjugated secondary antibodies diluted in 5% BSA for 1 h at room temperature.
11. Wash the slides three times for 5 min each in 100 mL of TBST with gentle shaking (60 rpm). Mount coverslip using antifade solution and seal the coverslip with nail polish.
12. Capture images under a confocal microscope.
F. Isolation of human PBMCs
1. Transfer 10 mL of fresh peripheral blood into a 50 mL tube and add an equal volume of DPBS. Mix gently to suspend.
2. Add 10 mL of Ficoll-paqueTM PLUS density gradient media into another 50 mL centrifuge tube. Slowly add the diluted blood sample onto the surface of the Ficoll-Paque PLUS to form a distinct interface.
Critical: Perform this step gently and slowly to keep the liquid surface stable and ensure clear stratification.
3. Carefully place the centrifuge tube into a horizontal rotor centrifuge. Balance the tubes and centrifuge at 1,200× g for 20 min at room temperature, with acceleration and deceleration set to 0.
Note: After centrifugation, four distinct layers can be observed in the tube. The top layer is the plasma, the middle layer is the PBMC, the transparent liquid layer below is the Ficoll-Paque PLUS, and the bottom layer is the sediment of red blood cells and granulocytes.
4. Carefully aspirate the PBMC layer and transfer it to a new sterile 50 mL tube.
5. Add DPBS to bring the volume to 50 mL. Centrifuge at 1,000× g for 10 min and discard the supernatant.
6. Repeat step F5 to ensure thorough washing.
7. If the pellet appears red, resuspend it in 5 mL of RBC lysis buffer and incubate at room temperature for 5 min. After lysis, wash the cells with DPBS and centrifuge at 300× g for 10 min.
8. Count the cells using an automated cell counter. Resuspend the PBMCs in MACS buffer for subsequent T-cell isolation.
G. Isolation and activation of T cells
1. Centrifuge the cell suspension at 300× g for 10 min. Aspirate supernatant completely.
2. Resuspend the cell pellet in 80 µL of MACS buffer per 10 total cells.
3. According to the cell suspension, add one-fourth of the volume of CD3 microbeads. Mix thoroughly and incubate for 15 min at 4 °C.
4. Wash the cells by adding 10 mL of MACS buffer, and centrifuge at 300× g for 10 min. Aspirate the supernatant completely.
5. Resuspend up to 108 cells in 500 μL of MACS buffer for magnetic separation.
6. Place the MACS column into the magnetic field of a suitable MACS separator. Prepare the column by rinsing with 5 mL of MACS buffer.
7. Apply the cell suspension onto the column. Collect the flowthrough containing unlabeled cells.
8. Wash the column with 500 µL of MACS buffer. Collect additional unlabeled cells that pass through.
9. Remove the column from the MACS separator and place it in a suitable collection tube. Add 500 µL of MACS buffer to the column and immediately flush out the magnetically labeled CD3+ T cells by firmly pushing the plunger into the column.
10. Centrifuge the eluted cell suspension at 300× g for 5 min. Aspirate the supernatant.
11. Resuspend the cell pellet with CAR-T cell medium and seed the isolated CD3+T cells into a 24-well plate at a density of 1 × 106 cells per 2 mL.
Note: Typically, 5 × 106 to 15 × 106 CD3+T cells can be obtained from 10 mL of peripheral blood following Ficoll separation and magnetic bead sorting.
12. Transfer the appropriate volume of CD3/CD28 beads into a 1.5 mL centrifuge tube. Add 1 mL of MACS buffer, vortex for 5 s, then place the tube on a magnetic stand for 1 min. Carefully aspirate and discard the supernatant.
13. Resuspend the beads with CAR-T cell medium at a ratio of 25 μL per 1 × 106 CD3+ T cells and add them to each well to initiate T-cell activation.
Note: Clusters of proliferating T-cell clones are typically visible under a microscope following activation with CD3/CD28 beads (Figure 2).
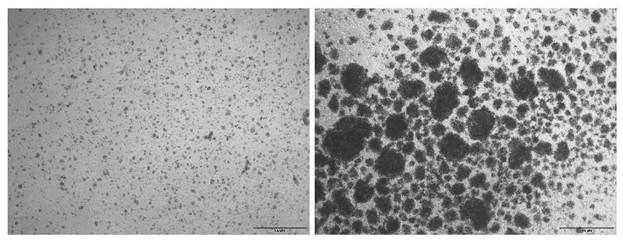
Figure 2. Representative brightfield images of sorted CD3 T cells before and after activation with CD3/CD28 beads. The left panel shows isolated T cells prior to stimulation, while the right panel depicts cells 48 h after activation with CD3/CD28 beads. Scale bar, 100 μm.
H. Lentiviral infection of T cells
Note: Take out the lentivirus from the -80 °C freezer and thaw it on ice prior to use.
1. After 2 days of activation, carefully discard 1.4 mL of the culture medium and gently resuspend the remaining cell suspension. Count cells using an automated cell counter.
2. Seed cells at a density of 1 × 10 cells per 500 μL in a 24-well plate and incubate at 37 °C for 1 h.
3. Calculate the required volume of the lentivirus based on a multiplicity of infection (MOI) of 10 using the following formula:
Volume of virus (μL) = MOI × number of cells/viral titer (TU/mL) × 1,000
4. Add polybrene to a final concentration of 5 µg/mL and IL-2 to a concentration of 50 U/mL.
5. Seal the 24-well plate with parafilm and centrifuge at 800× g for 30 min at 32 °C, using an acceleration setting of 5 and a deceleration setting of 0.
6. Transfer the plate to a 37 °C cell incubator and incubate for 4 h.
7. Add 2 mL of prewarmed CAR-T cell medium to each well and continue culturing for 48 h.
8. Replace the medium every 2–3 days, maintaining a cell concentration of 1 × 106 cells/mL throughout the culture period.
9. At 48 h post-infection, collect 200 μL of the cell suspension and centrifuge at 300× g for 3 min.
10. Resuspend the cell pellet in 100 μL of DPBS and add 2 μL of anti-G4S linker antibody(Alexa Fluor 488 conjugate). Incubate at room temperature for 30 min in the dark.
11. Wash the stained cells twice with 1 mL of DPBS containing 1% BSA. Centrifuge each time at 300× g for 3 min.
12. Resuspend the cells with 200 μL of DPBS and analyze by flow cytometry using a BD FACSAria II.
I. Tumor organoid–CAR-T cell co-culture system
I1. Preparation of tumor organoids (Day 1)
1. Mix Matrigel with cold DPBS at a 1:1 ratio. Add 600 µL of the mixture to each well of a 24-well plate. Immediately transfer the plate to a 37 °C incubator for 1 h to solidify the coating.
2. Retrieve the plate containing passaged organoids from Section B or thawed organoids from Section D and transfer it to the biological safety cabinet.
3. Scrap the Matrigel domes containing organoids with a P1000 pipette tip and transfer the suspension to a 15 mL tube. Centrifuge at 300× g for 4 min.
4. Discard the supernatant and resuspend the organoid–Matrigel pellet in 5 mL of cold organoid recovery solution. Incubate on ice for 20–30 min on a shaking platform at 60 rpm.
5. Centrifuge at 300× g for 4 min. A clear stratification will be visible between the cell pellet and the transparent Matrigel. Carefully aspirate and discard the supernatant and the Matrigel layer.
6. Resuspend the cell pellet in 10 mL of cold DPBS and centrifuge at 300× g for 4 min. Discard the supernatant.
Note: A small aliquot of the resuspension can be taken for microscopic counting of organoid spheres.
7. Stain the organoids by adding Hoechst 33342 dye solution at a final concentration of 10 μg/mL. Incubate at room temperature for 30 min.
8. Wash the organoids with 10 mL of DPBS and centrifuge the tube at 300× g for 4 min. Discard the supernatant.
9. Resuspend the stained organoids in VIVO-15 to a final density of 2 × 105/mL and seed 500 μL of the suspension into the Matrigel-coated wells prepared earlier.
10. Place the plate in a cell incubator and culture overnight.
I2. Preparation of CAR-T cells (Day 2)
1. Collect the engineered CAR-T cells from Section H into a 15 mL centrifuge tube. Centrifuge at 300× g for 4 min and discard the supernatant.
2. Resuspend the cell pellet in 1 μM of CFSE solution and incubate at room temperature for 30 min.
3. Wash the stained cells with 10 mL of DPBS and centrifuge at 300× g for 4 min. Discard the supernatant.
Note: A portion of the cell suspension can be used for counting using an automated cell counter.
4. Resuspend the CAR-T cells in VIVO-15 to a final concentration of 4 × 106 cells/mL. Set aside for subsequent use.
I3. Co-culture (Day 2)
1. Transfer the plate used for pre-seeding organoids from Section I1 to the biological safety cabinet for co-culture setup.
2. Aspire 500 μL of CAR-T cells prepared in Section I2 into each well containing pre-seeded organoids, maintaining an organoid-to-T-cell ratio of 1:20. Fill any unused wells with sterile DPBS to prevent evaporation.
3. Incubate the plate under standard culture conditions (37 °C, 5% CO2) for 72 h.
I4. Downstream assays (Day 5)
1. After 72 h of co-culture, add propidium iodide (PI) dye into each well at a final concentration of 10 μg/mL, followed by an additional 30-min incubation.
2. Upon completion of staining, gently transfer the plate to an inverted confocal microscope for image acquisition (Figure 3). Fluorescence channel configuration is as shown inTable 1.
Table 1. Fluorescent dyes, corresponding detection channels, and laser/filter settings for imaging
| Dye | Channel | Excitation laser (nm) | Emission filter (nm) |
| Hoechst 33342 | Blue | 405 | 420–480 |
| CFSE | Green | 488 | 500–550 |
| PI | TRITC | 561 | 570–650 |
3. The acquisition mode settings are as follows:
Scan mode: Frame
Frame size: 1,024 × 1,024
Averaging: 2
Bit depth: 8-bit
4. After imaging, carefully aspirate the supernatant from each well and detect the contents of IFN-γ and TNF-α according to the manufacturer’s instructions for the ELISA kit.
5. Aspirate a portion of the cell suspension and pass it through a flow cytometry tube equipped with a filter. The filtered cells are used for flow cytometric analysis of CFSE fluorescence intensity.
6. Use the remaining portion of the cell suspension to detect cleaved Caspase-3 (Asp175) according to the manufacturer’s instructions for the ELISA kit.
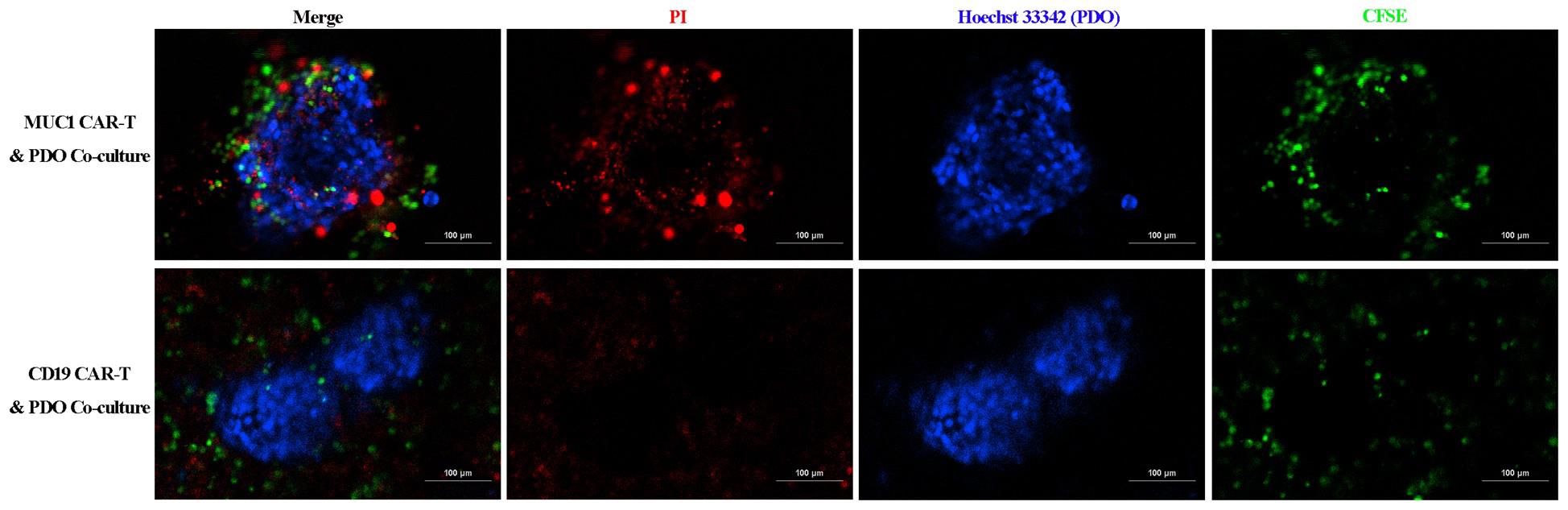
Figure 3. Confocal images of the co-culture of patient-derived organoids (PDOs) and CAR-T cells after 72h of incubation. In this example, the PDOs stained with Hoechst 33324 are surrounded by the MUC1 CAR-T cells (visualized in green), exhibiting a PI-positive apoptotic signal that progressively intensifies from the periphery toward the center of the organoids. In contrast, this apoptotic pattern is absent in organoids co-cultured with control CD19 CAR-T cells. Scale bar, 100 μm.
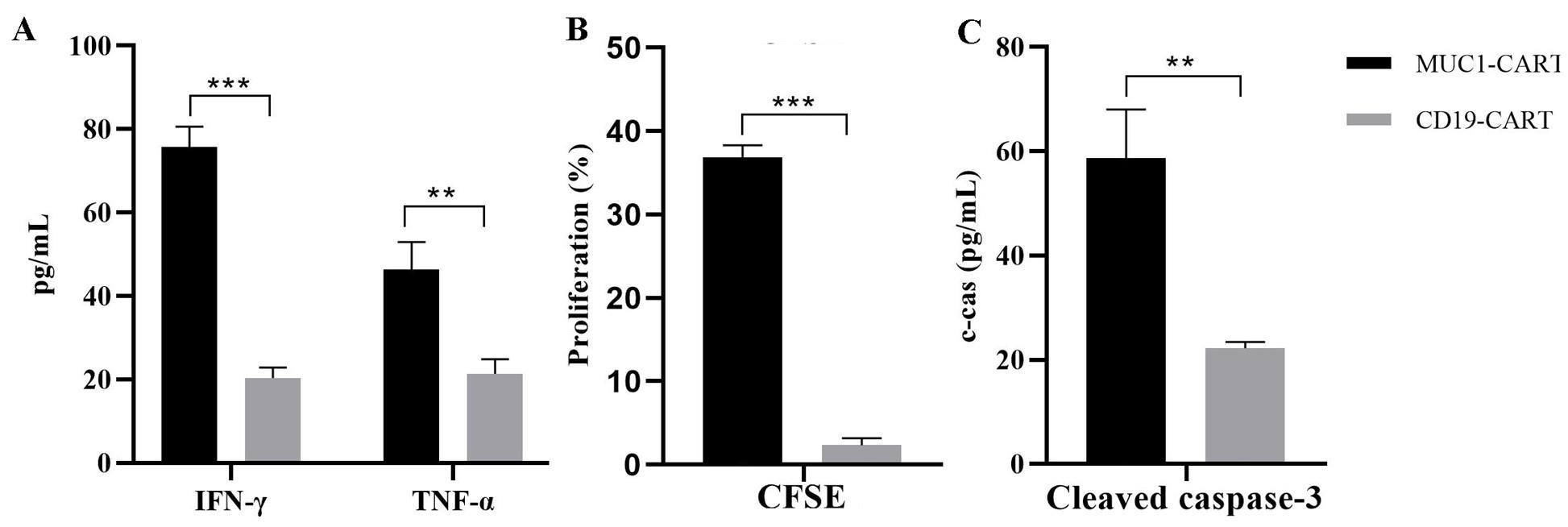
Figure 4. Quantification analysis of immune-mediated killing in the co-culture of patient-derived organoid (PDO) and CAR-T cells. (A) Quantification of the production of tumor necrosis factor (TNF)-α and interferon (IFN)-γ by ELISA after 72 h of organoid co-culture with MUC1 CAR-T cells or CD19 CAR-T cells. (B) Level of cleaved caspase-3 in organoids after co-culture with MUC1 CAR-T cells or CD19 CAR-T cells. (C) Quantification of the percentage of carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells after incubation with organoids for 72 h. Values represent mean ± standard error of the mean (SEM) (n = 3). **p < 0.01; ***p < 0.001 by two-tailed, unpaired t-test.
Data analysis
Statistical analyses were performed using GraphPad Prism 7. Unless otherwise specified, all summary data are presented as mean ± standard error of the mean. Student’s t-test was adopted to compare the differences between the two groups. All flow cytometric analyses of cells were performed using a FACSAria flow cytometer, and data were analyzed using the FlowJo software v8.8.7. Fluorescence images were obtained using a Nikon AX confocal microscope and processed using the corresponding software.
General notes and troubleshooting
General notes
1. All procedures are conducted in a biosafety cabinet.
2. All operations involving Matrigel should be carried out on ice, including thawing it on ice.
3. Before constructing CAR-T cells, it is necessary to clearly determine in advance which antigen is highly expressed on the surface of organoid cells.
4. According to cell status and experiment progress, the organoids or CAR-T cells can be cryopreserved. When all the experimental conditions are met, they can be thawed for co-culture and subsequent detection.
5. Before co-culture, organoids and T cells should be pre-stained with different tracers to facilitate the observation of their interaction. According to the laboratory's equipment conditions, one can choose to take timed fluorescent photos or directly use a live cell workstation for real-time monitoring to obtain the experimental results.
Troubleshooting
Problem 1: Primary cells seeded from tumor tissue fail to form round organoids after 2 weeks in culture.
Possible cause: Poor quality of initial tissue sample or reduced cell viability due to overly aggressive enzymatic digestion during tissue dissociation.
Solution: Optimize the tissue dissociation protocol to minimize cell damage and preserve progenitor cell populations. Additionally, consider testing samples from multiple donors to rule out sample-specific variability.
Problem 2: PDOs do not recover well after thawing.
Possible cause: Suboptimal freezing conditions or insufficient recovery support in early culture.
Solution: Ensure organoids are frozen in an optimized cryopreservation medium (e.g., 10% DMSO and 90% fetal bovine serum, or a specialized organoid freezing solution). During thawing, rapidly transfer the organoids into prewarmed culture medium supplemented with a ROCK inhibitor (e.g., Y-27632) to enhance post-thaw recovery and viability.
Problem 3: Low yield of CAR-T cells.
Possible cause: The efficiency of viral infection is low.
Solution: Repackage the virus or increase the MOI during the infection process.
Problem 4: Too few organoid cells recorded.
Possible cause: Significant cell loss during the organoid recovery process and failure of some cells to reform spheres after plating.
Solution: Increase the number of cells plated while maintaining the desired T-cell-to-tumor cell ratio. Additionally, extend the incubation time to allow sufficient recovery and spheroid reformation.
Problem 5: Fluorescent signal is too weak to detect cells after 72 h.
Possible cause: Fluorescent dye decays significantly during co-culture, resulting in diminished signal over time.
Solution: Use more stable or longer-lasting cell dyes. Alternatively, label organoids and CAR-T cells by transducing them with lentiviral vectors encoding enhanced green fluorescent protein (EGFP) or red fluorescent protein (RFP) for sustained fluorescence throughout the assay.
Acknowledgments
This work was supported by funding from the Guangdong Basic and Applied Basic Research Foundation (grant no. 2022A1515110675). This protocol was adapted from our previous publications (Yu et al., 2021;Li et al., 2022). Figure 1 in this protocol was created with BioRender. Yu, L. (2025) https://BioRender.com/atzzatc.
Competing interests
The author declares no conflicts of interest.
Ethical considerations
This study was approved by the Research Ethics Committee of Shenzhen Second Peoples’ Hospital and was conducted according to the guidelines of the local law. All patients provided informed written consent for sample use.
References
- Yan, H. H., Chan, A. S., Lai, F. L. and Leung, S. Y. (2023). Organoid cultures for cancer modeling. Cell Stem Cell. 30(7): 917–937. https://doi.org/10.1016/j.stem.2023.05.012
- Gu, Z., Wu, Q., Shang, B., Zhang, K. and Zhang, W. (2023). Organoid co‐culture models of the tumor microenvironment promote precision medicine. Cancer Innov. 3(1): e101. https://doi.org/10.1002/cai2.101
- Almåsbak, H., Aarvak, T. and Vemuri, M. C. (2016). CAR T Cell Therapy: A Game Changer in Cancer Treatment. J Immunol Res. 2016: 1–10. https://doi.org/10.1155/2016/5474602
- Liu, L., Yu, L., Li, Z., Li, W. and Huang, W. (2021). Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J Transl Med. 19(1): 40. https://doi.org/10.1186/s12967-020-02677-2
- Yu, L., Li, Z., Mei, H., Li, W., Chen, D., Liu, L., Zhang, Z., Sun, Y., Song, F., Chen, W., et al. (2021). Patient‐derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR‐T cells in vitro. Clin Transl Immunol. 10(2): e1248. https://doi.org/10.1002/cti2.1248
- Li, Z., Xu, H., Yu, L., Wang, J., Meng, Q., Mei, H., Cai, Z., Chen, W. and Huang, W. (2022). Patient‐derived renal cell carcinoma organoids for personalized cancer therapy. Clin Transl Med. 12(7): e970. https://doi.org/10.1002/ctm2.970
- Li, Z., Yu, L., Chen, D., Meng, Z., Chen, W. and Huang, W. (2021). Protocol for generation of lung adenocarcinoma organoids from clinical samples. STAR Protoc. 2(1): 100239. https://doi.org/10.1016/j.xpro.2020.100239
Article Information
Publication history
Received: Apr 7, 2025
Accepted: Jun 3, 2025
Available online: Jul 2, 2025
Published: Jul 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Yu, L. (2025). Evaluation of In Vitro Cytotoxic Activity of CAR-T Cells Using Patient-Derived Organoids. Bio-protocol 15(13): e5369. DOI: 10.21769/BioProtoc.5369.
Category
Immunology > Immunotherapy > CAR-T
Cancer Biology > Tumor immunology > Cancer therapy
Cell Biology > Cell isolation and culture > Co-culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link