- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Live Cell Imaging to Monitor Axonal Pruning in Drosophila Motor Neurons
(*contributed equally to this work) Published: Vol 15, Iss 13, Jul 5, 2025 DOI: 10.21769/BioProtoc.5367 Views: 2027
Reviewed by: Oneil Girish BhalalaRupkatha BanerjeeAmr Galal Abdelraheem IbrahimAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Lipid-Mediated Sequential Recruitment of Proteins Via Dual SLIPT and Dual SLIPTNVOC in Live Cells
Kristina V. Bayer and Richard Wombacher
Nov 5, 2025 1597 Views

Imaging the Entire Sexual Life Cycle of the Budding Yeast Saccharomyces cerevisiae Using a Microfluidic Platform
Taylor Kennedy [...] Orlando Argüello-Miranda
Dec 5, 2025 1466 Views
A Simple Protocol for Periodic Live Cell Observation of Flagellate Stages in the Lichen Alga Trebouxia
Enrico Boccato [...] Mauro Tretiach
Jan 20, 2026 172 Views
Abstract
Over the lifespan of an individual, brain function requires adjustments in response to environmental changes and learning experiences. During early development, neurons overproduce neurite branches, and neuronal pruning removes the unnecessary neurite branches to make a more accurate neural circuit. Drosophila motoneurons prune their intermediate axon bundles rather than the terminal neuromuscular junction (NMJ) by degeneration, which provides a unique advantage for studying axon pruning. The pruning process of motor axon bundles can be directly analyzed by real-time imaging, and this protocol provides a straightforward method for monitoring the developmental process of Drosophila motor neurons using live cell imaging.
Key features
• Long-range projecting axon bundles of Drosophila motor neurons extending from soma on the ventral nerve cord (VNC) undergo degeneration rather than retraction during metamorphosis.
• The pruning process of motor axon bundles can be directly observed by real-time live-cell imaging.
• The complete clearance of axon bundles occurs approximately 22 h after pupal formation (22 h APF).
• Mushroom body (MB) γ neuron axon pruning regulatory genes are conserved for motor neurons.
Keywords: NeurodevelopmentGraphical overview
Background
During the early stages of nervous system development, nerve branches and connections are excessively generated. This includes a number of branches of axons and dendrites far greater than what is needed in an adult individual. Neural pruning is the process by which unnecessary synaptic connections between neural cells are selectively eliminated during the development of the nervous system [1,2]. This process occurs primarily in childhood and adolescence and is an essential mechanism for the brain to efficiently and optimally function as it matures. It is worth noting that numerous neurodevelopmental disorders, like autism spectrum disorder (ASD) and schizophrenia, are considered to be correlated with aberrant neural pruning [3–7]. In autistic individuals, it was discovered that insufficient pruning of neuronal connections in certain areas of the brain may lead to abnormal functioning of neural circuits. Conversely, excessive pruning is a possible cause of schizophrenia [8]. By investigating the mechanisms of abnormal neural pruning, we can deeply explore the roots of the pathogenesis of these disorders.
During metamorphosis in the holometabolous insect Drosophila, early neurons undergo stereotypic pruning to re-establish adult-specific neural circuits. One widely recognized type IV dendritic arborization (C4da) sensory neuron that perceives pain in Drosophila undergoes dendrite-specific pruning during development [9–12]. In addition, the dendrites and axons of the mushroom body (MB) γ neuron in the central nervous system (CNS) of Drosophila are also known to undergo pruning [13–15]. Although MB γ neurons provide fundamental insights into the mechanisms of axonal pruning, they require fixed tissue analysis and present imaging challenges in dense central brains. Here, we describe a novel method to monitor the process of axonal pruning in Drosophila motor neurons. Using live-cell imaging, we found that axons in the middle part of Drosophila motoneurons complete their pruning by degradation during development, while their terminal neuromuscular junctions are pruned by retraction [16]. Thus, motor-neuron axon bundles project peripherally in a stereotyped array, allowing for real-time live imaging, providing characteristic temporal windows and mechanistic properties (fragmentation or degeneration), and supporting systematic genetic screening to find new regulators. On the other hand, the development of Drosophila motor neurons as a model for axon pruning complements the MB γ neuron system by offering a distinct neuronal context, technical advantages, and functional readouts. Overall, the establishment of this method provides an ideal platform for future investigations into the regulatory mechanisms of axon pruning.
Materials and reagents
Reagents
1. 20× PBS (Sangon Biotech, catalog number: B548117-0500)
Note: Prior to observation, pupae are washed repeatedly 3–4 times with PBS [500 mL of 20× PBS stock solution is diluted to 10 L with H2O to become 1× PBS; storage conditions: 25 °C (room temperature)] buffer to remove surface debris such as adherent food.
2. Glycerol, 90% (Sangon Biotech, catalog number: 56-81-5); store at room temperature
3. Silicone resin (DOW CORNING-High vacuum grease); store at room temperature
Note: An appropriate amount of silicone resin is squeezed around the perimeter of the slide with the sample in the center to maintain a flexible gap between the slide and the coverslip to prevent the sample from being pressed and damaged.
4. OK6-Gal4 (Bloomington Stock Center, catalog number: BSC64199)
5. EcR-RNAi (Bloomington Stock Center, catalog number: BSC9327)
6. UAS-EcR-B1DN (Bloomington Stock Center, catalog number: BSC9452)
5. UAS-Uba1-RNAi (Tsinghua RNAi Stock Center, catalog number: THU2127)
6. UAS-mCD8-GFP (Su Wang; Southeast University, China)
Laboratory supplies
1. 200 and 1,250 μL pipette tips (Biosharp, catalog number: BS-RT)
2. 10 μL pipette tips (A-gen, catalog number: T-10-RS)
3. Glass culture dish (Normax, catalog number: 5058541)
4. 1.5 mL microcentrifuge tubes (Sangon Biotech, catalog number: F601620-0010)
5. Microscope slides (CITOTEST, catalog number: 1A5105)
6. Microscope cover glass (CITOTEST, catalog number: 10212432c)
Equipment
1. Pipettes (Eppendorf, model: Research® plus, catalog number: 3120000062) are used to clean the surface debris of Drosophila pupae
2. Stereomicroscope (Motic, model: SMZ168) is used to observe, dissect, and isolate samples from the period after pupal formation (APF)
3. Confocal laser scanning microscope (Carl Zeiss, model: LSM900) is used to image axon bundles in motor neurons
4. Anatomical forceps (Dumont, catalog number: 0209-5-PO) are used to dissect and isolate samples from the APF period
5. Anatomical scissors (RWD, catalog number: S11037-08) are used to assist in dissecting samples from the APF period
Software and datasets
1. Image J (Fiji; https://imagej.net/software/fiji/)
2. Prism 9 (GraphPad Prism 9.0)
3. ZEN (2012 blue edition)
4. Adobe Photoshop (2018)
5. Adobe Illustrator (2024)
Procedure
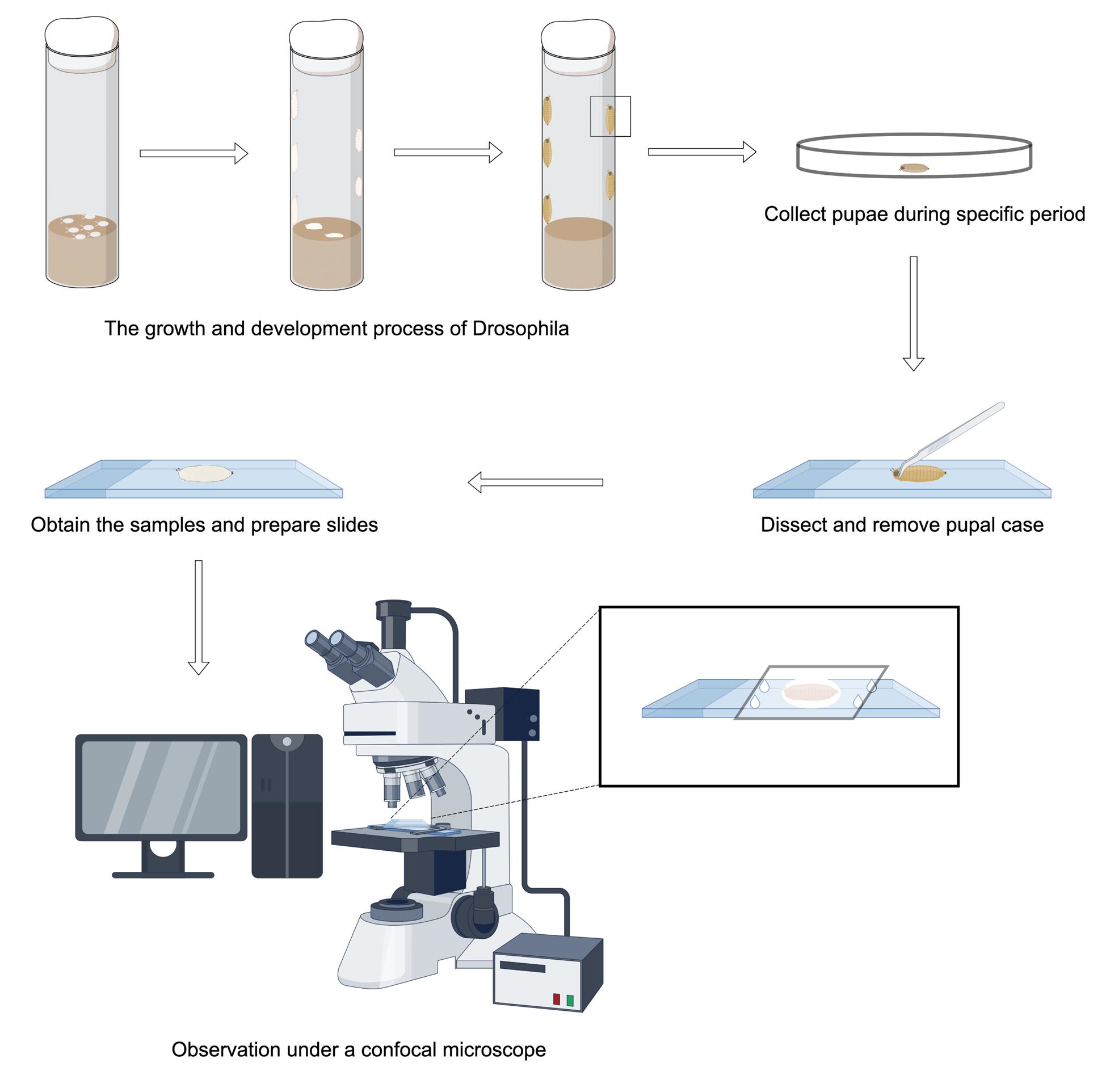
Construction of fluorescently labeled motor neurons: With a P-element positioned upstream of the rapgap1 gene, OK6-Gal4 is a Gal4 driver line that primarily drives transcription in Drosophila motor neurons [17]. By fusing the mouse CD8 transmembrane domain to GFP and putting it under UAS control, the transgenic responder construct known as UAS-mCD8-GFP allows membrane-localized GFP expression anywhere Gal4 is present. We genomically integrate mCD8-GFP and OK6-Gal4 in Drosophila by crossing them to consistently drive motor neuron-specific expression of membrane-tethered GFP. The pruning process of motor neuron axons is specifically labeled and observed in real time at different stages of Drosophila development using this method.
A. Collect embryos
1. At 25 , place approximately 10 male and 20 female Drosophila together in a transparent polystyrene (PS) fly tube containing food at the bottom. The food should occupy approximately one-quarter of the tube's volume. Following mating, the female flies deposit eggs within the fly tube.
2. After approximately 30 min, transfer adult fruit flies to a new transparent PS tube filled with food, keeping the original mating tube. This means that embryos are collected within 30 min (Figure 1).
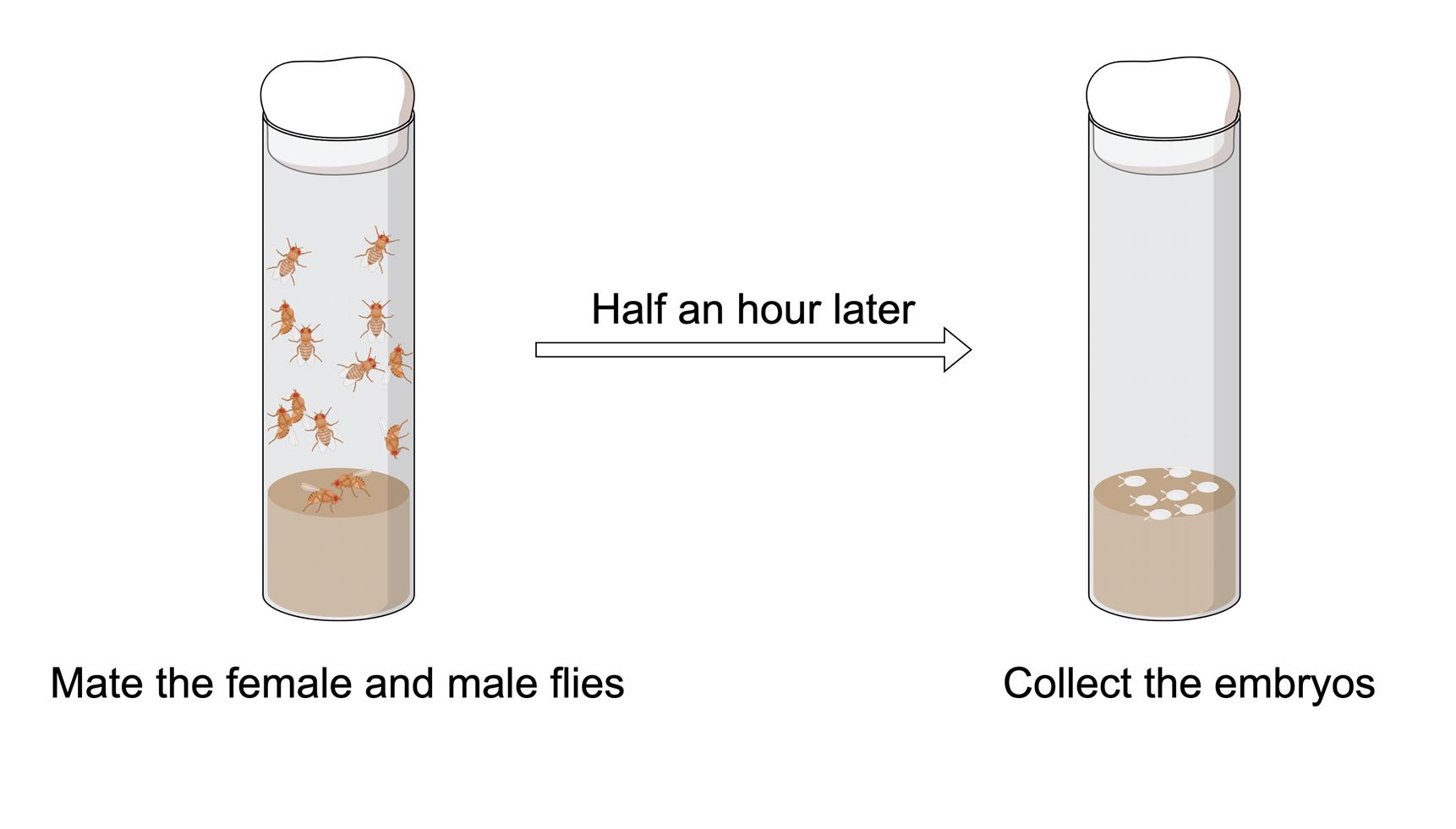
Figure 1. Process of embryo collection
B. Collect Drosophila samples
1. Collect Drosophila at the white pupa (WP), 14 h after pupal formation (APF), 16 h APF, 18 h APF, and 22 h APF developmental stages separately.
2. Wash samples with 1× PBS buffer 3–4 times and wipe clean with paper towels to remove debris like food that adheres to the surface of the samples.
C. Sample processing at the WP phase
1. Mount the WP with 90% glycerol in the center of the slide, surrounded by four small drops of silicone resin and covered with a coverslip. This provides support between the coverslip and the slide to prevent damage to the sample (Figure 2).
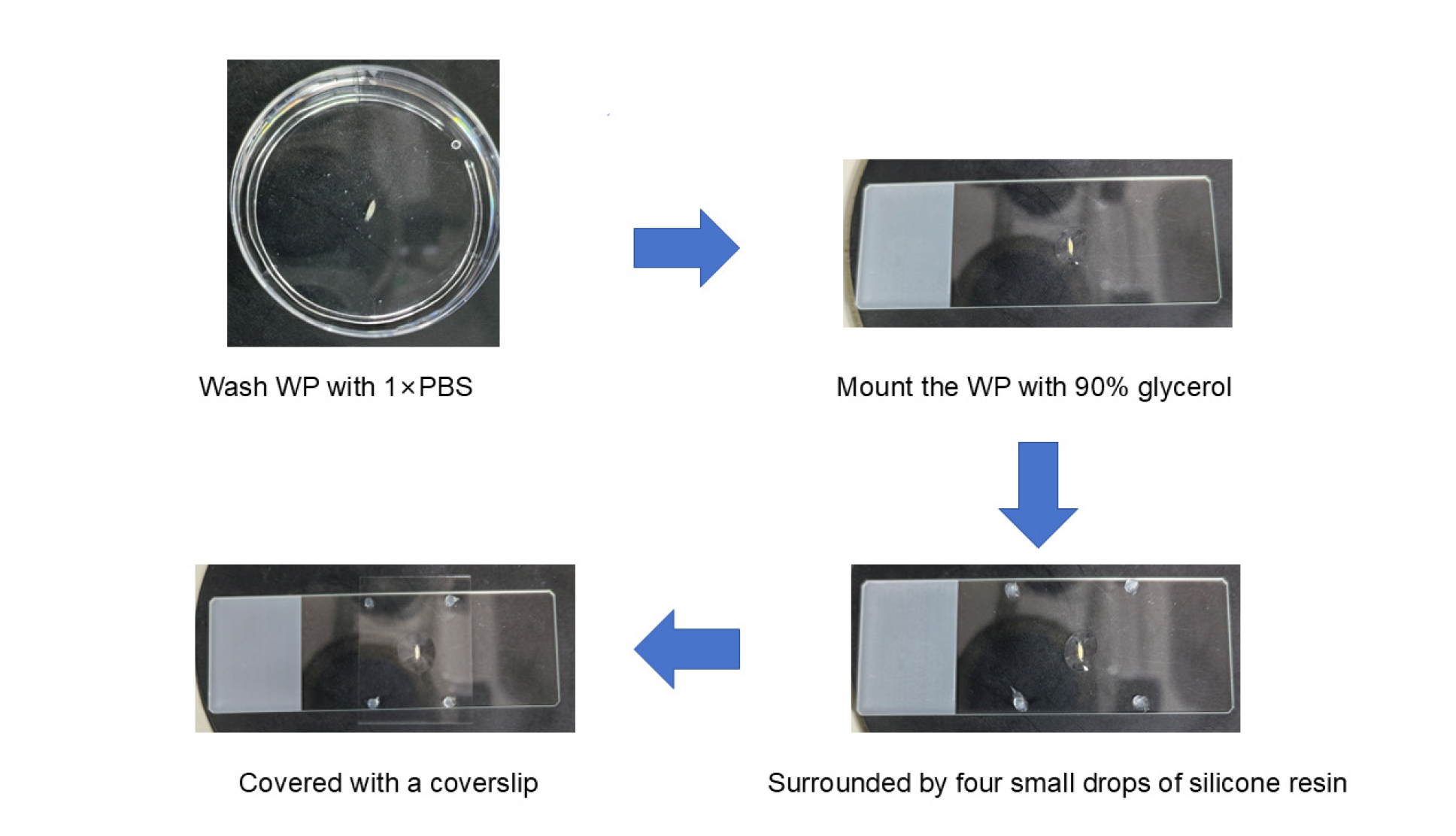
Figure 2. Process of sample processing at the white pupa (WP) phase
D. Sample processing at the APF stage
Note: The handling of samples during the APF period is more complicated than the WP stage due to the requirement of removing the pupal cases from their surfaces as well as taking the easily breakable samples out from their interiors.
1. First, wash the APF pupae and dry with a paper towel. Adhere the double-sided tape on the slide and place APF pupae on the double-sided tape with the dorsal surface facing upward.
2. Open the pupal case of the head by making an incision with pointed forceps. Insert the tip of the forceps into the incision and carefully cut along the body axis laterally up to about one-third of the entire body with an anatomical scissor. This process needs to be carefully affixed to the pupal case manipulation in order to prevent the dissecting tool from touching the internal samples. Unfold the incised case and adhere it to the double-sided tape in order to expose the cream-colored APF-stage Drosophila inside.
3. Switching to blunt-tipped forceps, carefully clamp the internal APF-stage Drosophila. At this time, attention needs to be paid to the strength to prevent squeezing the sample out.
4. Place the APF-stage Drosophila on a slide and process it following the steps mentioned in Section C. The entire experimental flow of the APF phase is shown in Figure 3.
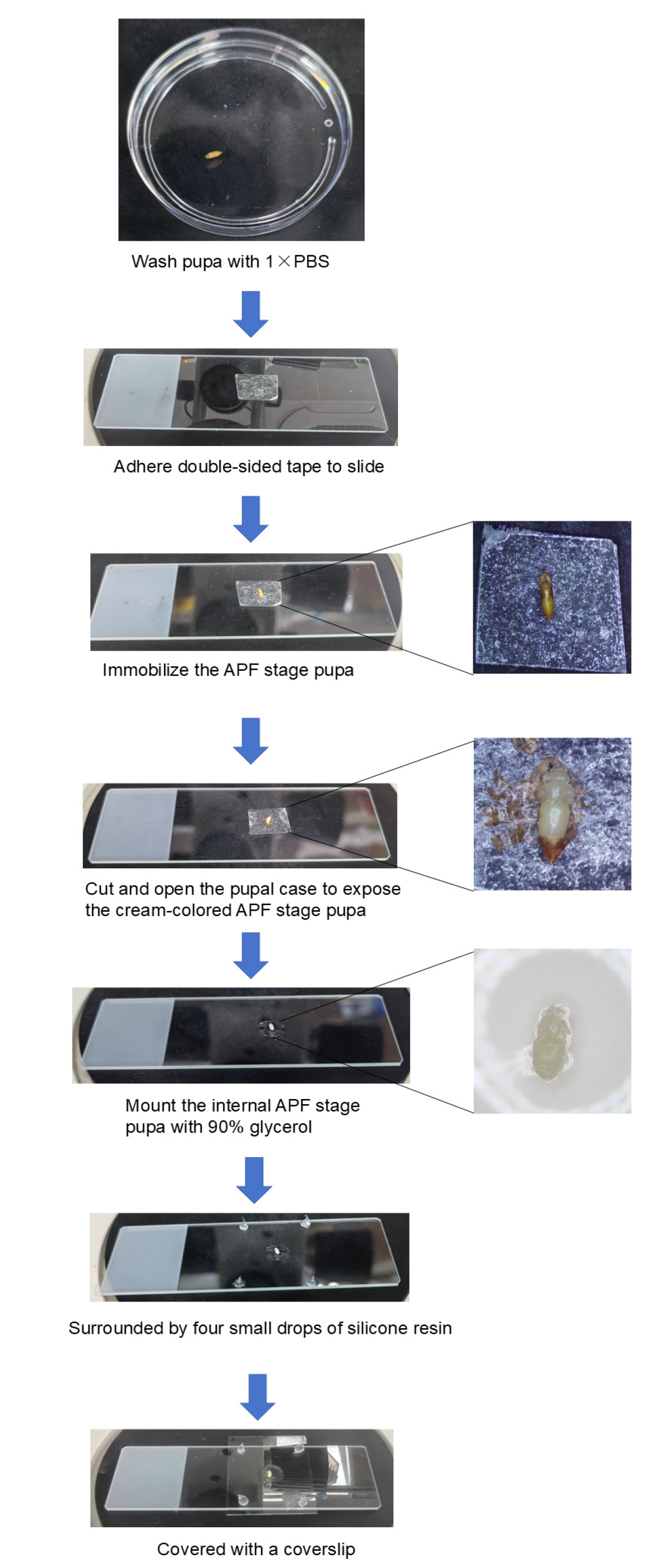
Figure 3. Process of sample processing at the after-pupal-formation (APF) phase
E. Live-cell imaging
1. Live imaging of different developmental stages of Drosophila is performed with a laser-scanning confocal microscope.
2. GFP-labeled motor neurons can be found in the field of view. The dendrites and somas of motor neurons are situated in the ventral nerve cord (VNC), and the long axonal bundles extend from the somas in a lateral direction, finally arriving at the surface of the muscle to form the terminal neuromuscular junction.
3. Select the axon bundles between the soma and the neuromuscular junction to be photographed and record the pruning of the axons at different developmental stages (Figure 4).
4. Observe the process of axon degradation at different stages to identify the key time points at which axons begin to prune and when they are fully pruned.
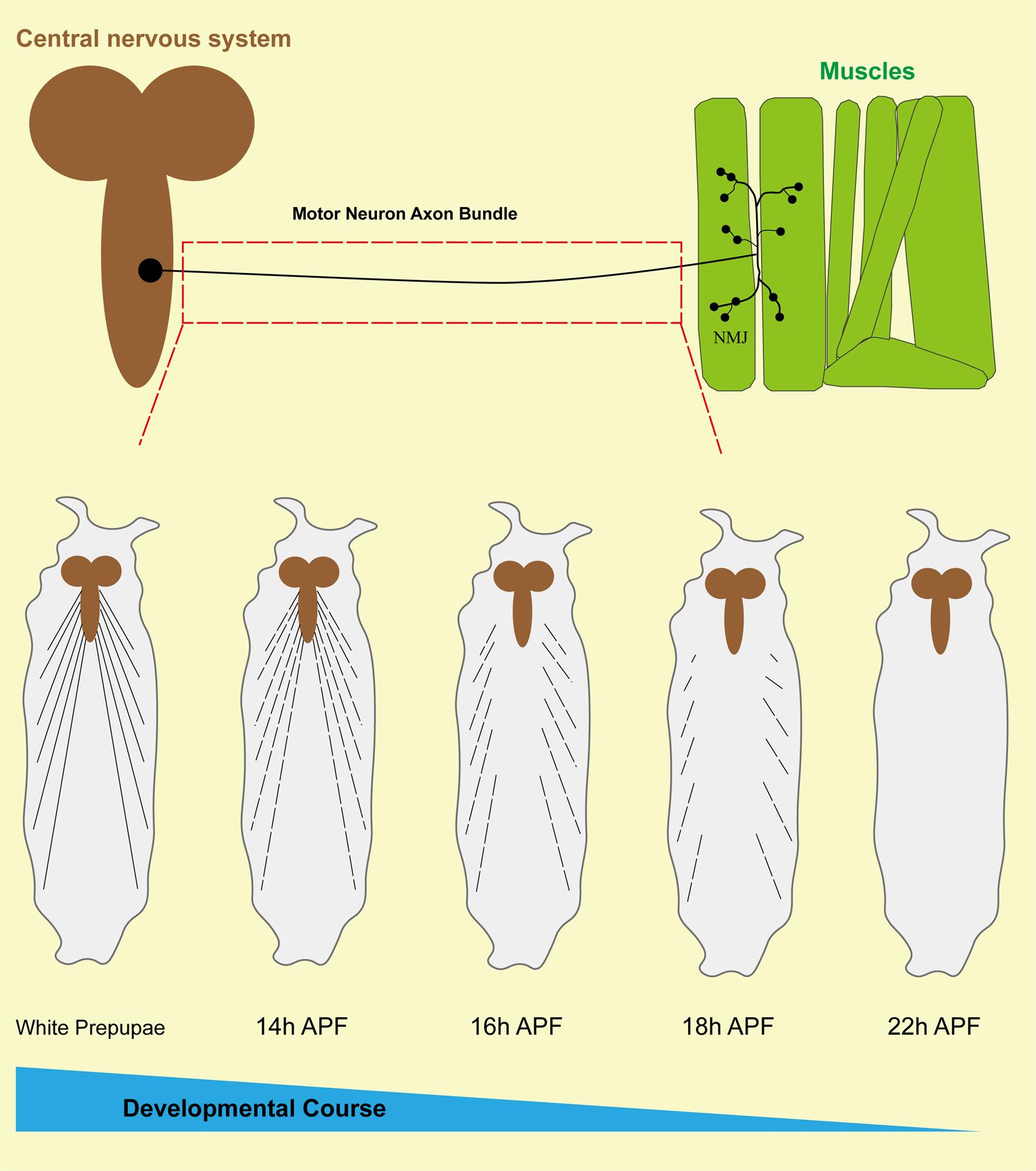
Figure 4. Schematic diagram of axon bundle pruning in Drosophila motor neurons. The upper part of this figure reveals the morphology and distribution of a Drosophila motoneuron, with the soma situated at the surface of the ventral nerve cord (VNC), extending long axon bundles, and terminating in contact with the muscle to form the neuromuscular junction (NMJ). The red dotted box reveals the region we examined. The bottom portion of this figure shows that the axon bundles of motor neurons undergo pruning at different stages of development in Drosophila.
Data analysis
The results of the pruning of intermediate axons of motor neurons at different developmental stages show that at approximately 14 h APF, the axons start to show discontinuous segmental structures, followed by an almost complete axonal clearance at approximately 22 h APF (Figure 5). Remarkably, 22 h APF can be used as a key time point for subsequent observation and screening of regulators of axonal pruning, similar to 16 h APF, which is commonly utilized as a time point for identifying the regulatory mechanisms of dendritic pruning in C4da neurons [18]. Also, using this model, we found that knockdown of Uba1 and EcR-B1, as well as overexpression of dominant-negative EcR-B1DN (two classical modulators of neural pruning), in motor neurons, result in consistent axonal pruning defects (Figure 6).
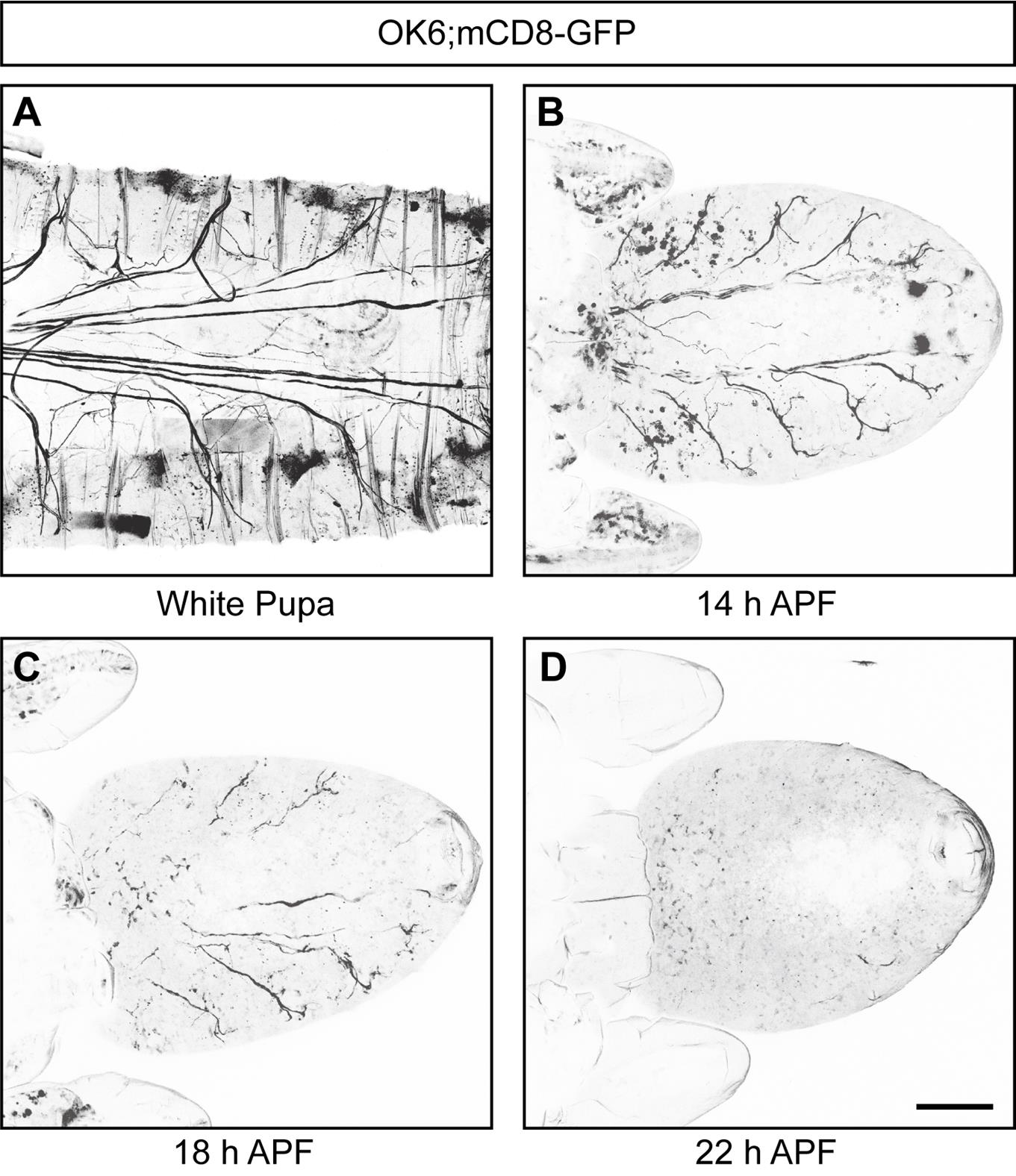
Figure 5. Drosophila motor neuron axon bundles undergo pruning during development. (A–D) Representative confocal live images of the development of mCD8-GFP-labeled motor neurons at WP (A), 14 h APF (B), 18 h APF (C), and 22 h APF (D), showing that Drosophila motor neuron axon bundles experience pruning processes during development. Scale bar, 200 μm.
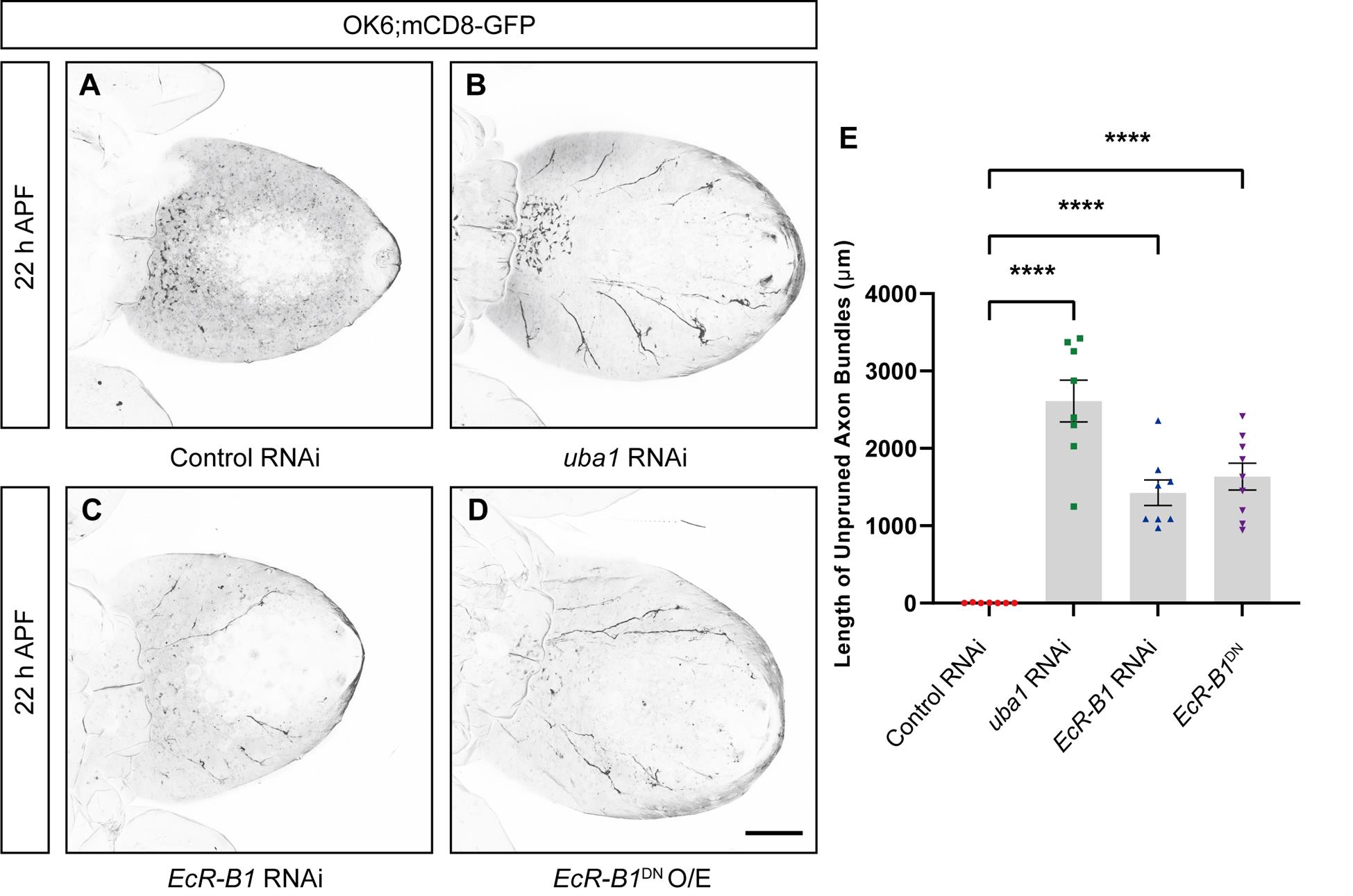
Figure 6. Uba1 and EcR-B1 are involved in regulating axon bundle pruning in motor neurons. (A–D) Representative confocal live images of the development of mCD8-GFP-labeled motor neurons of control RNAi (A), uba1 RNAi (B), EcR-B1 RNAi (C), and O/E EcR-B1DN (D) at 22 h APF. (E) Quantification of total length of unpruned motor axon bundles at 22 h APF. O/E represent as overexpress. Data are presented as mean ± SEM. One-way ANOVA with Bonferroni’s test was applied to determine statistical significance. ****p < 0.0001. Scale bars, 200 μm.
Quantification and analysis of axon pruning
After capturing the axon bundles via confocal laser scanning microscopy, images of intermediate axon bundles of motor neurons at different developmental stages and of various genotypes are exported to TIF format using Zen software. The TIF images are then converted to black and white using Adobe Photoshop to bring out the morphology of the axons; then, processed images are finalized in Adobe Illustrator for publication. For statistical analysis, we can use Image J (Fiji) software to open the TIF image, select Set measurements in the menu bar under Analyze, and check the box Bounding rectangle. Next, go back to the open image, use Segmented line to draw the lines along the unpruned axons, and calculate the length of the corresponding axon using the shortcut Ctrl+M. One-way ANOVA with Bonferroni test is applied to determine significance when multiple groups are present. Error bars in all graphs represent SEM. Statistical significance is defined as ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, and ns, not significant. Importantly, we can utilize this platform to screen genes and environmental factors involved in axon pruning in different genetic and toxicological contexts.
Validation of protocol
This protocol has been used and validated in the following research article:
Xu et al. [16]. Establishment of a novel axon pruning model of Drosophila motor neuron. Biol Open (Figures 2 and 5).
By using confocal microscopy to image motor neurons marked with mCD8-GFP driven by OK6-Gal4, the authors observed axon bundle degeneration in Drosophila motor neurons during metamorphosis. This study first shows that axon pruning occurs through degeneration, not retraction (see Figure 2 from [16]).
Through the application of RNA interference (RNAi) and dominant-negative techniques to selectively suppress the expression or function of Babo (TGF-β receptor), EcR-B1 (ecdysone receptor B1 subtype), Bsk (JNK homolog), and Uba1 (Ubiquitin-activating enzyme 1) in motor neurons, the authors observed defects in axon pruning. These findings suggest that TGF-β signaling, ecdysone signaling, JNK signaling, and the ubiquitin-proteasome system are critical for motor axon pruning in motor neurons (see Figure 5 from [16]).
General notes and troubleshooting
1. Prior to live-cell imaging of motor neurons, the debris adhering to the pupal surface needs to be washed off with PBS to prevent these substances from appearing as autofluorescence that affects image quality during imaging.
2. When dissecting samples from the AFP period, it is necessary to make an incision in the head with pointed forceps and then use pointed forceps assisted with anatomical scissors to cut along the body axis from the lateral side of the body. This process needs to be carefully affixed to the pupal case manipulation in order to prevent the dissecting tool from touching the internal samples. After completing the incision of the pupal case, the internal APF-stage Drosophila needs to be taken out by switching to blunt-tipped forceps.
3. After dissecting and taking out the APF-stage Drosophila to the middle of the slide, the sample should be surrounded by drops of silicone resin. This step adds a flexible gap between the coverslip and slide to prevent damage to the sample due to pressure.
4. A small amount of 90% glycerol is added to the middle of the coverslip, in contact with the sample, and spread over a small area before adding the coverslip. Due to the small amount of glycerol added and the spreading, air bubbles will not be generated.
5. When taking live images of the WP stage, do not select Drosophila that have just pupated. They can, however, be within half an hour of pupation, to prevent interference with image quality due to movement of the organism during laser confocal photography.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32100784), the Natural Science Foundation of Jiangsu Province (BK20221458), and the Fundamental Research Funds for the Central Universities (2242024RCB0031), also known as the Southeast University Zhishan Young Scholars Program. Some of the components of the Graphical Overview are provided by Figdraw (Graphical overview, Copyright Code: PUASSb4360; Figure 1, Copyright Code: UAUIU6655a). This protocol was described and validated in Xu et al [16].
Author Contributions: Conceptualization: M.R.; Methodology: M.R.; K.L.; W.X.; S.W.; Software: M.R.; K.L.; W.X.; S.W.; Validation: M.R.; K.L.; W.X.; S.W.; Formal analysis: M.R.; K.L.; W.X.; Investigation: M.R.; K.L.; W.X.; Resources: M.R.; S.W.; Data curation: M.R.; W.X.; Writing – Original Draft: M.R.; K.L.; Writing – Review & Editing: M.R.; K.L.; X.M.; Funding Acquisition: M.R.
Competing interests
The authors declare no competing interests.
References
- Luo, L. and O'Leary, D. D. (2005). Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 28(1): 127–156. https://doi.org/10.1146/annurev.neuro.28.061604.135632
- Williams, D. W. and Truman, J. W. (2005). Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 132(16): 3631–3642. https://doi.org/10.1242/dev.01928
- Tsai, N. P., Wilkerson, J. R., Guo, W., Maksimova, M. A., DeMartino, G. N., Cowan, C. W. and Huber, K. M. (2012). Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95. Cell. 151(7): 1581–1594. https://doi.org/10.1016/j.cell.2012.11.040
- Tang, G., Gudsnuk, K., Kuo, S. H., Cotrina, M. L., Rosoklija, G., Sosunov, A., Sonders, M. S., Kanter, E., Castagna, C., Yamamoto, A., et al. (2014). Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron. 83(5): 1131–1143. https://doi.org/10.1016/j.neuron.2014.07.040
- Cardozo, P. L., de Lima, I. B. Q., Maciel, E. M., Silva, N. C., Dobransky, T. and Ribeiro, F. M. (2019). Synaptic Elimination in Neurological Disorders. Curr Neuropharmacol. 17(11): 1071–1095. https://doi.org/10.2174/1570159x17666190603170511
- Sellgren, C. M., Gracias, J., Watmuff, B., Biag, J. D., Thanos, J. M., Whittredge, P. B., Fu, T., Worringer, K., Brown, H. E., Wang, J., et al. (2019). Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 22(3): 374–385. https://doi.org/10.1038/s41593-018-0334-7
- Germann, M., Brederoo, S. G. and Sommer, I. E. (2021). Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders. Curr Opin Psychiatry. 34(3): 222–227. https://doi.org/10.1097/yco.0000000000000696
- Penzes, P., Cahill, M. E., Jones, K. A., VanLeeuwen, J. E. and Woolfrey, K. M. (2011). Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 14(3): 285–293. https://doi.org/10.1038/nn.2741
- Rui, M., Bu, S., Chew, L. Y., Wang, Q. and Yu, F. (2020). The membrane protein Raw regulates dendrite pruning via the secretory pathway. Development. e191155. https://doi.org/10.1242/dev.191155
- Rui, M., Ng, K. S., Tang, Q., Bu, S. and Yu, F. (2020). Protein phosphatase PP2A regulates microtubule orientation and dendrite pruning in Drosophila. EMBO Rep. 21(5): e201948843. https://doi.org/10.15252/embr.201948843
- Rui, M., Kong, W., Wang, W., Zheng, T., Wang, S. and Xie, W. (2023). Droj2 Facilitates Somatosensory Neurite Sculpting via GTP-Binding Protein Arf102F in Drosophila. Int J Mol Sci. 24(17): 13213. https://doi.org/10.3390/ijms241713213
- Rui, M. (2024). Recent progress in dendritic pruning of Drosophila C4da sensory neurons. Open Biol. 14(7): e240059. https://doi.org/10.1098/rsob.240059
- Lee, T., Marticke, S., Sung, C., Robinow, S. and Luo, L. (2000). Cell-Autonomous Requirement of the USP/EcR-B Ecdysone Receptor for Mushroom Body Neuronal Remodeling in Drosophila. Neuron. 28(3): 807–818. https://doi.org/10.1016/s0896-6273(00)00155-0
- Watts, R. J., Hoopfer, E. D. and Luo, L. (2003). Axon Pruning during Drosophila Metamorphosis. Neuron. 38(6): 871–885. https://doi.org/10.1016/s0896-6273(03)00295-2
- Zheng, X., Wang, J., Haerry, T. E., Wu, A. H., Martin, J., O'Connor, M. B., Lee, C. H. and Lee, T. (2003). TGF-β Signaling Activates Steroid Hormone Receptor Expression during Neuronal Remodeling in the Drosophila Brain. Cell. 112(3): 303–315. https://doi.org/10.1016/s0092-8674(03)00072-2
- Xu, W., Kong, W., Gao, Z., Huang, E., Xie, W., Wang, S. and Rui, M. (2023). Establishment of a novel axon pruning model of Drosophila motor neuron. Biol Open. 12(1): e059535. https://doi.org/10.1242/bio.059535
- Sanyal, S. (2009). Genomic mapping and expression patterns of C380, OK6 and D42 enhancer trap lines in the larval nervous system of Drosophila. Gene Expression Patterns. 9(5): 371–380. https://doi.org/10.1016/j.gep.2009.01.002
- Zheng, T., Long, K., Wang, S. and Rui, M. (2025). Glial-derived TNF/Eiger signaling promotes somatosensory neurite sculpting. Cell Mol Life Sci. 82(1): 47. https://doi.org/10.1007/s00018-024-05560-1
Article Information
Publication history
Received: Mar 2, 2025
Accepted: Jun 3, 2025
Available online: Jun 19, 2025
Published: Jul 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Long, K., Xu, W., Miao, X., Wang, S. and Rui, M. (2025). Live Cell Imaging to Monitor Axonal Pruning in Drosophila Motor Neurons. Bio-protocol 15(13): e5367. DOI: 10.21769/BioProtoc.5367.
Category
Neuroscience > Development > Neuron
Neuroscience > Cellular mechanisms > Neuronal fate
Cell Biology > Cell imaging > Live-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link