- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Derivation and Culture of Enriched Phrenic-Like Motor Neurons From Human iPSCs
Published: Vol 15, Iss 13, Jul 5, 2025 DOI: 10.21769/BioProtoc.5363 Views: 2277
Reviewed by: Philipp WörsdörferAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation, Culture, and Identification of Primary Müller Cells from Human Retina
Yingying Chen [...] Ling Zhu
Oct 5, 2021 3912 Views
Isolation and Culture of Cranial Neural Crest Cells from the First Branchial Arch of Mice
Hiroki Ueharu [...] Yuji Mishina
Apr 5, 2022 3071 Views
Isolation and Culture of Neural Stem/Progenitor Cells from the Hippocampal Dentate Gyrus of Young Adult and Aged Rats
Mina Afhami [...] Koorosh Shahpasand
Oct 5, 2023 2267 Views
Abstract
The fatal motor neuron (MN) disease amyotrophic lateral sclerosis (ALS) is characterized by progressive degeneration of the phrenic MNs (phMNs) controlling the activity of the diaphragm, leading to death by respiratory failure. Human experimental models to study phMNs are lacking, hindering the understanding of the mechanisms of phMN degeneration in ALS. Here, we describe a protocol to derive phrenic-like MNs from human induced pluripotent stem cells (hiPSC-phMNs) within 30 days. During spinal cord development, phMNs emerge from specific MN progenitors located in the dorsalmost MN progenitor (pMN) domain at cervical levels, under the control of a ventral-to-dorsal gradient of Sonic hedgehog (SHH) signaling and a rostro-caudal gradient of retinoic acid (RA). The method presented here uses optimized concentrations of RA and the SHH agonist purmorphamine, followed by fluorescence-activated cell sorting (FACS) of the resulting MN progenitor cells (MNPCs) based on a cell-surface protein (IGDCC3) enriched in hiPSC-phMNs. The resulting cultures are highly enriched in MNs expressing typical phMN markers. This protocol enables the generation of hiPSC-phMNs and is highly reproducible using several hiPSC lines, offering a disease-relevant system to study mechanisms of respiratory MN dysfunction. While the protocol has been validated in the context of ALS research, it can be adopted to study human phrenic MNs in other research fields where these neurons are of interest.
Key features
• This protocol generates enriched hiPSC-derived phrenic motor neuron cultures.
• The protocol can be used to develop models to study human respiratory motor neuron disease.
• The protocol allows the generation of phrenic motor neuron preparations with potential for motor neuron replacement strategies.
• The protocol requires experience in hiPSC culturing and FACS-based cell sorting for a successful outcome.
Keywords: Phrenic motor neuronsGraphical overview
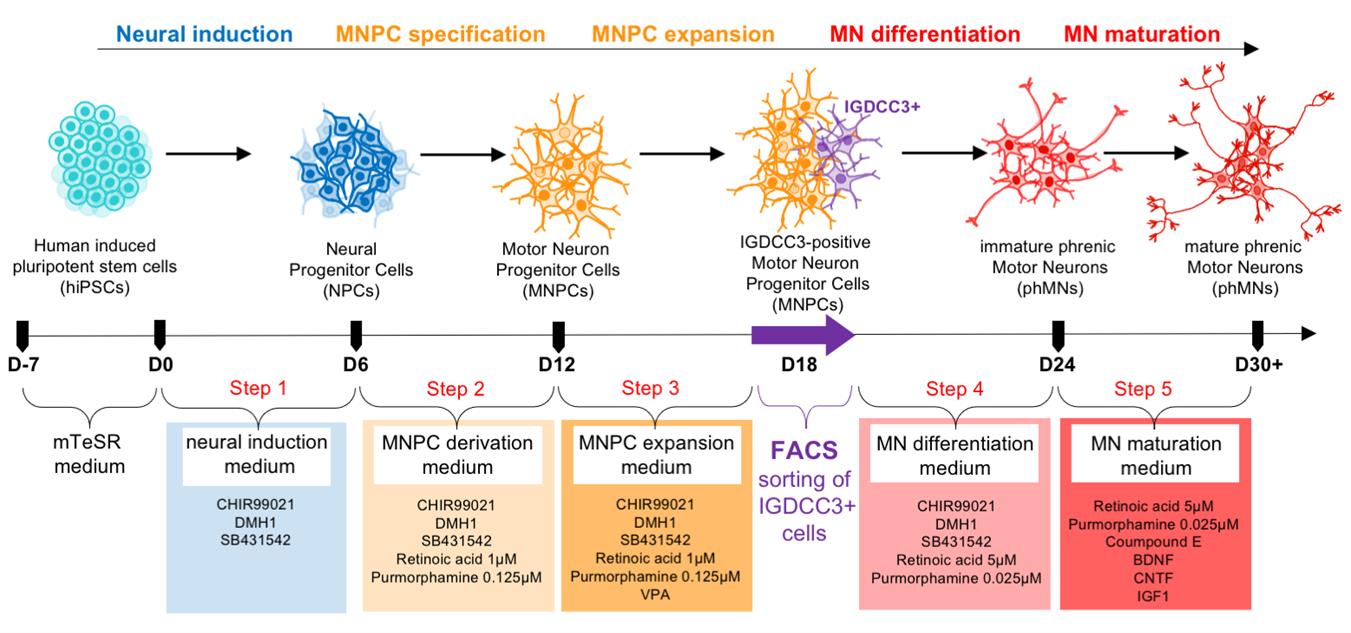
Representation of the experimental design to generate human induced pluripotent stem cell (hiPSC)-derived cultures enriched in phrenic motor neurons using the indicated combination of small molecules and FACS-based cell sorting.
Background
Respiratory failure is the primary cause of death in amyotrophic lateral sclerosis (ALS) patients [1,2] and is mainly the result of the degeneration of phrenic motor neurons (phMNs) that control the major inspiratory muscle, the diaphragm [3]. Compared to other spinal MNs, phMNs present distinct developmental origin, topology, and electrophysiological properties [4–7]. In ALS animal models, phMN progressive loss begins before symptom onset [8] and is more pronounced than other spinal respiratory MNs [9,10], suggesting selective cell death mechanisms among different MN subtypes [3,11–14]. Consequently, model systems based on other MN subtypes cannot account for the specialized biology of phMNs and their vulnerability to degeneration in ALS. The study of motor neuron pathophysiology in ALS and other MN diseases has been significantly facilitated by the advent of hiPSC-based approaches, enabling robust and reliable generation of human MNs. To date, the best-characterized and most commonly used hiPSC-based MN derivation protocols generate heterogeneous cultures containing mainly MNs of the lateral (LMC) and median (MMC) motor columns [15–19], with only 10% of MNs expressing the phMN marker gene, SCIP [20]. The intrinsic heterogeneity of currently available hiPSC-derived MN preparations and the scarcity of phMNs in these cultures make them inadequate to study phMN pathophysiology, including mechanisms of phMN degeneration in ALS or other respiratory MN diseases. We therefore sought to develop methods to generate phMN-enriched cultures from hiPSCs.
Materials and reagents
Biological materials
1. Human induced pluripotent cell (iPSC) line NCRM-1 (National Institutes of Health Stem Cell Resource, Bethesda, MD, USA, identification number: ND50028)
2. Human iPSC line CS29iALS-C9nxx (hereafter CS29-ALS for brevity) and corrected human iPSC line CS29iALS-C9n1.ISOnxx (CS29 isogenic) (Cedars-Sinai, Los Angeles, CA, USA, identification number: CVCL_W559)
3. Human iPSC line CS52iALS-C9nxx (CS52-ALS) and corrected human iPSC line CS52iALS-C9n6.ISOnxx (CS52 isogenic) (Cedars-Sinai, Los Angeles, CA, USA, identification number: CVCL_JC27)
Reagents
1. mTeSR1 medium (400 mL mTeSR1 basal medium bottle + mTeSR1 5× supplement to combine before use) (STEMCELL Technologies, catalog number: 85850)
2. CorningTM MatrigelTM hESC-qualified matrix (Fisher Scientific, catalog number: 08-774-552)
3. Gentle cell dissociation reagent (STEMCELL Technologies, catalog number: 07174)
4. Poly-L-ornithine hydrobromide (PLO) (Sigma-Aldrich, catalog number: P3655)
5. Mouse laminin (Sigma-Aldrich, catalog number: L2020)
6. DMEM/F12 (Thermo Fisher Scientific, catalog number: 11320033)
7. GlutaMAX (Thermo Fisher Scientific, catalog number: 35050-061)
8. Neurobasal medium (Thermo Fisher Scientific, catalog number: 21103-049)
9. N2 supplement 100× (Thermo Fisher Scientific, catalog number: 17502-048)
10. B27 supplement 50×, serum free (Thermo Fisher Scientific, catalog number: 1704-044)
11. L-Ascorbic acid (Sigma-Aldrich, catalog number: A5960)
12. Antibiotic–antimycotic 100× (Thermo Fisher Scientific, catalog number: 15240-062)
13. CHIR99021 (STEMCELL Technologies, catalog number: 72054)
14. DMH1 (Sigma-Aldrich, catalog number: D8946)
15. SB431542 (Tocris Bioscience, catalog number: 1614)
16. Retinoic acid (RA) (Sigma-Aldrich, catalog number: R2625)
17. Purmorphamine (Sigma-Aldrich, catalog number: SML-0868)
18. Valproic acid sodium salt (VPA) (Sigma-Aldrich, catalog number: P4543)
19. Compound E (Calbiochem, catalog number: 565790)
20. Recombinant human insulin-like growth factor 1 (IGF-1) (R&D Systems, catalog number: 291-G1-200)
21. Human brain–derived neurotrophic factor recombinant protein (BDNF) (Thermo Fisher Scientific, catalog number: PHC7074)
22. Recombinant human ciliary neurotrophic factor (CNTF) (R&D Systems, catalog number: 257-NT-050)
23. Dendritic polyglycerol amine (dPGA) (kindly provided by Dr. T. E. Kennedy, MNI, McGill University, Montreal, Canada)
24. ROCK inhibitor (Y-27632 2HCl) (Tocris Bioscience, catalog number: 1254)
25. Trypan blue solution, 0.4% (Thermo Fisher Scientific, Gibco, catalog number: 15250061)
26. Paraformaldehyde (PFA) (EMD, Sigma Millipore, catalog number: 8187150100)
27. Glycerol, 99+%, extra pure (Thermo Scientific Chemicals, Fisher Scientific, catalog number: AC158920025)
28. Normal donkey serum (NDS) (Jackson ImmunoResearch, catalog number: 017-000-121)
29. Normal goat serum (NGS) (Jackson ImmunoResearch, catalog number: 005-000-121)
30. BSA (Sigma Millipore, catalog number: A7906)
31. Triton, 100× (Sigma Millipore, catalog number: T8787)
32. Goat anti-SRY-BOX 1 (SOX1), 1/500 (R&D Systems, catalog number: AF3369)
33. Rabbit anti-HOMEOBOX C4 (HOXC4), 1/150; kindly provided by Dr. Jeremy Dasen, New York University School of Medicine, New York, NY)
34. Rabbit anti-PAX6, 1/500 (Covance, catalog number: PRB-278P, can now be found on the BioLegend website, under the catalog number 901302)
35. Mouse anti-PAX6 clone AD1.5, 1/150 (Sigma Millipore Corp., catalog number: MAB5554)
36. Rat panTLE monoclonal antibody supernatant, 1/10 (originally developed by Stifani and colleagues21)
37. Mouse anti-OLIG2, 1/100 (Sigma Millipore Corp., catalog number: MABN50)
38. Goat anti-OLIG2, 1/200 (R&D Systems, catalog number: AF2418)
39. Rabbit anti-LIMB HOMEOBOX CONTAINING 3 (LHX3), 1/100 (Abcam, catalog number: ab14555, now discontinued)
40. Rabbit anti-FORKHEAD BOX PROTEIN 1 (FOXP1), 1/350 (Abcam, catalog number: ab16645)
41. Rabbit anti-HOMEOBOX A5 (HOXA5), 1/150 (kindly provided by Dr. Jeremy Dasen, New York University School of Medicine, New York, NY)
42. Mouse anti-HOMEOBOX PROTEIN HB9 (HB9), 1/30 (DSHB, catalog number: 81.5C10-c)
43. Mouse anti-ISLET1 (ISL1), 1/30 (DSHB, catalog number: 39.4D5-c)
44. Guinea pig anti-SCIP, 1/16,000 (kindly provided by Dr. Jeremy Dasen, New York University School of Medicine, New York, NY)
45. Goat anti-ChAT, 1/100 (Sigma Millipore, catalog number: AB144P)
46. Chicken anti-ChAT, 1/1000 (Aves Labs, catalog number: CAT)
47. Donkey anti-goat secondary antibody Alexa FluorTM 488, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-11055)
48. Donkey anti-rabbit secondary antibody Alexa FluorTM 647, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-31573)
49. Donkey anti-mouse secondary antibody Alexa FluorTM 555, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-31570)
50. Donkey anti-rat secondary antibody Alexa FluorTM 488, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-21208)
51. Goat anti-mouse secondary antibody Alexa FluorTM 647, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-21235)
52. Goat anti-rabbit secondary antibody Alexa FluorTM 488, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-11008)
53. Goat anti-guinea pig secondary antibody Alexa FluorTM 555, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-21435)
54. Goat anti-chicken secondary antibody Alexa FluorTM 647, 1/1,000 (Thermo Fisher Scientific, Invitrogen, catalog number: A-21449)
55. Fluoromount G® mounting medium (Southern Biotech. catalog number: 0100-01)
56. Hoechst 33258 solution (Millipore Sigma, catalog number: 94403)
57. Dulbecco’s PBS, no calcium, no magnesium (D-PBS) (Thermo Fisher Scientific, catalog number: 14190144)
58. Live/DeadTM Fixable Aqua (Thermo Fisher Scientific, catalog number: L34957)
59. Fetal bovine serum, qualified, heat-inactivated (FBS) (Gibco, catalog number: 12484-028)
60. Sodium azide (NaN3) (Millipore Sigma, catalog number: S2002-5G)
61. Alexa Fluor® 647-conjugated human IGDCC3 extracellular antibody, 1:20 (R&D Systems, catalog number: FAB8559R)
62. SpectraComp® Unmixing Controls beads (Slingshot Bio, catalog number: SSB-05-B)
63. ViaComp® beads (Slingshot Bio, catalog number: SSB-07-A)
Solutions
1. Poly-L-ornithine stock solution (see Recipes)
2. Laminin stock solution (see Recipes)
3. Matrigel stock solution (see Recipes)
4. Rock inhibitor stock solution (see Recipes)
5. CHIR99021 stock solution (see Recipes)
6. DMH1 stock solution (see Recipes)
7. SB431542 stock solution (see Recipes)
8. Ascorbic acid stock solution (see Recipes)
9. Purmorphamine stock solution (see Recipes)
10. Retinoic acid stock solution (see Recipes)
11. Valproic acid stock solution (see Recipes)
12. Compound E stock solution (see Recipes)
13. BDNF stock solution (see Recipes)
14. CNTF stock solution (see Recipes)
15. IGF-1 stock solution (see Recipes)
16. Neural induction medium (Step 1) (see Recipes)
17. Motor neuron progenitor cell derivation medium (Step 2) (see Recipes)
18. Motor neuron progenitor cell expansion medium (Step 3) (see Recipes)
19. Motor neuron differentiation medium (Step 4) (see Recipes)
20. Motor neuron maturation medium (Step 5) (see Recipes)
21. FACS buffer (see Recipes)
22. Blocking solution (see Recipes)
Recipes
1. Poly-L-ornithine stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Poly-L-ornithine (PLO) | 1 mg/mL | 100 mg |
| PBS | n/a | 100 mL |
Dissolve PLO in PBS, aliquot, and store at -80 °C as a stock solution.
2. Laminin stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Laminin | 1 mg/mL | 100 μL |
| DMEM/F12 | n/a | 20 mL |
Prepare 100 μL aliquots of laminin to be stored at -80 °C. For use, dilute 100 μL of Laminin into 20 mL of DMEM/F12 media to a final concentration of 5 µg/mL and store at -20 °C (see step B1c). Once thawed on ice, diluted laminin can be stored at 4 °C, in the dark, for 1–2 weeks.
3. Matrigel stock solution
Prepare 100 μL aliquots of Matrigel in microtubes covered with aluminum foil (to hide from light, see General note 6) and store at -80 °C as a stock solution. For use, thaw aliquoted Matrigel on ice 1 h before adding it to cold DMEM/F12 at a final concentration of 1/100 (see step A1a–b). Freeze-thaw cycles should be minimized by aliquoting into one-time-use aliquots.
4. Rock inhibitor stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Rock inhibitor | 10 mM | 50 mg |
| DMSO | n/a | 15.612 mL |
Dissolve Rock inhibitor in DMSO, aliquot, and store at -80 °C as a stock solution. Once thawed, Rock inhibitor aliquots can be kept for 1–4 weeks at 4 °C. Rock inhibitor is used at a final concentration of 10 μM.
5. CHIR99021 stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| CHIR99021 | 3 mM | 10 mg |
| DMSO | n/a | 7.168 mL |
Dissolve CHIR99021 in DMSO, aliquot, and store at -80 °C as a stock solution.
6. DMH1 stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| DMH1 | 4 mM | 5 mg |
| DMSO | n/a | 3.2856 mL |
Dissolve DMH1 in DMSO, aliquot, and store at -80 °C as a stock solution.
7. SB431542 stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| SB431542 | 10 mM | 10 mg |
| DMSO | n/a | 2.604 mL |
Dissolve SB431542 in DMSO, aliquot, and store at -80 °C as a stock solution.
8. Ascorbic acid stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Ascorbic acid | 200 mM | 176.12 mg |
| H2O | n/a | 5 mL |
Dissolve ascorbic acid in H2O, aliquot in microtubes covered with aluminum foil (to hide from light), and store at -80 °C as a stock solution.
9. Purmorphamine stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Purmorphamine | 2 mM | 5 mg |
| DMSO | n/a | 4.802 mL |
Dissolve purmorphamine in DMSO, aliquot, and store at -80 °C as a stock solution.
10. Retinoic acid stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Retinoic acid | 10 mM | 30 mg |
| DMSO | n/a | 10 mL |
Dissolve retinoic acid in DMSO, aliquot in microtubes covered with aluminum foil (to hide from light, see General note 4), and store at -80 °C as a stock solution.
11. Valproic acid stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Valproic acid | 0.5 M | 831 mg |
| H2O | n/a | 10 mL |
Dissolve valproic acid in H2O, aliquot, and store at -80 °C as a stock solution.
12. Compound E stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Compound E | 0.1 mM | 1 mg |
| DMSO | n/a | 20.388 mL |
Dissolve compound E in DMSO, aliquot, and store at -80 °C as stock solution.
13. BDNF stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| BDNF | 10 µg/mL | 10 µg |
| Distilled water | n/a | 1 mL |
Dissolve BDNF in distilled water, aliquot, and store at -80 °C as a stock solution.
14. CNTF stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| CNTF | 10 µg/mL | 10 µg |
| Distilled water | n/a | 1 mL |
Dissolve CNTF in distilled water, aliquot, and store at -80 °C as a stock solution.
15. IGF-1 stock solution
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| IGF-1 | 10 µg/mL | 10 µg |
| Distilled water | n/a | 1 mL |
Dissolve IGF-1 in distilled water, aliquot, and store at -80 °C as stock solution.
16. Neural induction medium (Step 1)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM/F12 | n/a | 14.4 mL |
| Neurobasal medium | 1/1 | 14.4 mL |
| N2 (200×) | 0.5× | 150 μL |
| B27 (100×) | 0.5× | 300 μL |
| L-GlutaMAX (200×) | 0.5× | 150 μL |
| Antibiotic-antimycotic (100×) | 1× | 300 μL |
| Ascorbic acid (200 mM) | 100 μM | 15 μL |
| CHIR99021 (3 mM) | 3 μM | 30 μL |
| DMH1 (4 mM) | 2 μM | 15 μL |
| SB (10 mM) | 2 μM | 6 μL |
| Total | n/a | 30 mL |
17. Motor neuron progenitor cell derivation medium (Step 2)
| Reagent (stock concentration) | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM/F12 | n/a | 14.4 mL |
| Neurobasal medium | 1/1 | 14.4 mL |
| N2 (200×) | 0.5× | 150 μL |
| B27 (100×) | 0.5× | 300 μL |
| L-GlutaMAX (200×) | 0.5× | 150 μL |
| Antibiotic-antimycotic (100×) | 1× | 300 μL |
| Ascorbic acid (200 mM) | 100 μM | 15 μL |
| CHIR99021 (3 mM) | 1 μM | 10 μL |
| DMH1 (4 mM) | 2 μM | 15 μL |
| SB (10 mM) | 2 μM | 6 μL |
| Retinoic acid (10 mM) | 1 μM | 3 μL |
| Purmorphamine (2 mM) | 0.125 μM | 1.875 μL |
| Total | n/a | 30 mL |
18. Motor neuron progenitor cell expansion medium (Step 3)
| Reagent (stock concentration) | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM/F12 | n/a | 14.4 mL |
| Neurobasal medium | 1/1 | 14.4 mL |
| N2 (200×) | 0.5× | 150 μL |
| B27 (100×) | 0.5× | 300 μL |
| L-GlutaMAX (200×) | 0.5× | 150 μL |
| Antibiotic-antimycotic (100×) | 1× | 300 μL |
| Ascorbic acid (200 mM) | 100 μM | 15 μL |
| CHIR99021 (3 mM) | 3 μM | 30 μL |
| DMH1 (4 mM) | 2 μM | 15 μL |
| SB (10 mM) | 2 μM | 6 μL |
| Retinoic acid (10 mM) | 1 μM | 3 μL |
| Purmorphamine (2 mM) | 0.125 μM | 1.875 μL |
| Valproic acid (0.5 M) | 500 μM | 30 μL |
| Total | n/a | 30 mL |
19. Motor neuron differentiation medium (Step 4)
| Reagent (stock concentration) | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM/F12 | n/a | 14.4 mL |
| Neurobasal medium | 1/1 | 14.4 mL |
| N2 (200×) | 0.5× | 150 μL |
| B27 (100×) | 0.5× | 300 μL |
| L-GlutaMAX (200×) | 0.5× | 150 μL |
| Antibiotic-antimycotic (100×) | 1× | 300 μL |
| Ascorbic acid (200 mM) | 100 μM | 15 μL |
| CHIR99021 (3 mM) | 3 μM | 30 μL |
| DMH1 (4 mM) | 2 μM | 15 μL |
| SB (10 mM) | 2 μM | 6 μL |
| Retinoic acid (10 mM) | 5 μM | 15 μL |
| Purmorphamine (2 mM) | 0.025 μM | 0.375 μL |
| Total | n/a | 30 mL |
20. Motor neuron maturation medium (Step 5)
| Reagent (stock concentration) | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM/F12 | n/a | 14.4 mL |
| Neurobasal medium | 1/1 | 14.4 mL |
| N2 (200×) | 0.5× | 150 μL |
| B27 (100×) | 0.5× | 300 μL |
| L-GlutaMAX (200×) | 0.5× | 150 μL |
| Antibiotic-antimycotic (100×) | 1× | 300 μL |
| Ascorbic acid (200 mM) | 100 μM | 15 μL |
| Retinoic acid (10 mM) | 5 μM | 15 μL |
| Purmorphamine (2 mM) | 0.025 μM | 0.375 μL |
| Compound E (0.1 mM) | 0.1 μM | 30 μL |
| BDNF (10 µg/mL) | 10 ng/mL | 30 μL |
| CNTF (10 µg/mL) | 10 ng/mL | 30 μL |
| IGF-1 (10 µg/mL) | 10 ng/mL | 30 μL |
| Total | n/a | 30 mL |
21. FACS buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| FBS | 5% | 20 mL |
| NaN3 | 0.1% w/v | 2 mL (stock: 20% w/v) |
| D-PBS | n/a | 378 mL |
| Total | n/a | 400 mL |
22. Blocking solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NDS or NGS | 5% | 500 μL |
| BSA | 0.6% | 60 μL |
| Triton 100× | 0.5% | 50 μL |
| PBS 1× | n/a | 9.4 mL |
| Total | n/a | 10 mL |
Laboratory supplies
1. 6-cm BioLiteTM cell culture treated dishes (Thermo Fisher Scientific, catalog number: 130181)
2. 25 cm2 BioLiteTM cell culture treated flasks (T25 flasks) (Fisher Scientific, catalog number: 12-556-009)
3. 24-well BioLiteTM microwell plates (Thermo Fisher Scientific, catalog number: 130186)
4.Serological pipettes, disposable, sterile (5 mL) (Thermo Fisher Scientific, catalog number: 170366N)
5. Serological pipettes, disposable, sterile (10 mL) (Thermo Fisher Scientific, catalog number: 170367N)
6. Conical tubes (15 mL) (Thermo Fisher Scientific, catalog number: 339650)
7. Conical tubes (50 mL) (Thermo Fisher Scientific, catalog number: 339652)
8. Coverslips (Thermo Fisher Scientific, catalog number: 12-545-80)
9. Kimtech ScienceTM KimwipesTM delicate task wipes (Kimberly-Clark Professional, catalog number: 34120)
10. MACS® SmartStrainer (30 μm) (Miltenyi Biotec, catalog number: 130-098-458)
11. FisherbrandTM frosted microscope slides (Thermo Fisher Scientific, catalog number: 12-550-343)
12. Falcon® 5 mL round bottom polystyrene test tube (Corning, catalog number: 352008)
Equipment
1. Laboratory centrifuge with rotors for 15 and 50 mL conical tubes (Eppendorf, catalog number: 5702)
2. FisherbrandTM IsotempTM general purpose deluxe water baths (Fisher Scientific, model: Isotemp GPD 05, catalog number: FSGPD05)
3. Inverted phase contrast microscope (Zeiss, model: Axiovert 40C, catalog number: Z-AXIO40C)
4. LUNA-IITM automated cell counter (Logos Biosystems Inc.; model: LUNA-II, catalog number: L40001)
5. FACSAria Fusion (Becton-Dickinson Biosciences, catalog number: 656700G5)
6. BD FACSAriaTM fusion temperature control option (TCO) fusion (Becton-Dickinson Biosciences, catalog number: 643154)
Software and datasets
1. BD FACSDiva Software (Becton-Dickinson Biosciences, Version: 8.0.1)
Procedure
A. Culture of human iPSCs
1. Preparation of Matrigel-coated dishes
a. Thaw aliquoted Matrigel on ice 1 h before use and prepare one conical tube of cold DMEM/F12 (keep on ice).
b. Add thawed Matrigel to the cold DMEM/F12 medium and mix well to reach the final concentration according to batch instructions (1/100, 100 μL of Matrigel for 1 mL of DMEM/F12).
c. Immediately add 2 mL of the cold diluted Matrigel per 6-cm culture dish. Swirl the dish to spread the Matrigel solution evenly across the surface and incubate at room temperature for at least 2 h.
d. Before use, gently tilt the dish to allow the excess Matrigel solution to collect on one side of the dish and aspirate without scratching the coated surface.
2. Thawing and culture of human iPSCs
a. To start the cell culture, thaw human iPSCs at low passage number in a water bath (37 °C, maximum 1 min).
Note: For the purpose of our experimental design [22], we thawed iPSCs from the following five lines: NCRM1, CS29-isogenic, CS29-ALS, CS52-isogenic, and CS52-ALS.
b. Add thawed iPSCs to a 15 mL conical tube with 9 mL of DMEM/F12.
b. Centrifuge cells at 1,300× g for 3 min at room temperature.
c. Discard supernatant and resuspend cells in 1 mL of mTeSR medium.
d. Count (see General notes) and seed cells at a concentration of 0.3 × 106 cells/mL on 6-cm culture dishes coated with Matrigel.
e. For each culture dish, add 3 mL of mTeSR medium and 3 μL of ROCK inhibitor.
f. Swirl flask to spread cells and medium evenly across the surface.
g. Incubate cells at 37 °C and 5% CO2 for 6–7 days, until they reach 70%–80% confluence.
h. Change medium (3 mL/dish) and check cell proliferation under microscope daily until ready for the next step (approximately 6 days).
B. Induction of human iPSCs into neural progenitor cells (NPCs): Days 0–6
1. Preparation of coated flasks and medium (Day -1)
a. One day before starting the protocol (Day -1), prepare PLO-coated flasks by adding 2.5 mL of PLO (10 μg/mL, diluted in PBS, see Recipes) per T25 flask.
b. Swirl flask to spread PLO solution evenly across the surface and incubate at 37 °C and 5% CO2 for at least 2 h.
c. Aspirate and rinse 3 times with D-PBS before adding 2.5 mL of mouse laminin (5 µg/mL in DMEM/F12, see Recipes).
d. Swirl flask to spread laminin evenly across the surface and incubate at 37 °C and 5% CO2 until ready for use the next day.
e. The next day, aspirate laminin before use.
f. Prepare neural induction medium (Step 1, see Recipes).
2. Passaging of human iPSCs (Day 0)
a. On Day 0, aspirate mTeSR medium and rinse with D-PBS (10 mL/dish).
b. Aspirate D-PBS, add 5 mL of gentle cell dissociation reagent per culture dish, and incubate for 5 min at 37 °C and 5% CO2.
c. Detach cells by pipetting with a 1,000 μL pipette.
d. Collect cells and dissociation solution into a 15 mL conical tube and add 10 mL of DMEM/F12 to stop the reaction.
e. Centrifuge cells at 1,300× g for 3 min at room temperature. Discard supernatant and resuspend cells in 1 mL of DMEM/F12.
g. Count and seed cells at a concentration of 1 × 106 cells/mL on T25 flasks coated with PLO and laminin. For each flask, add 5 mL of neural induction medium (Step 1) and 5 μL of ROCK inhibitor.
g. Swirl flask to spread cells and medium evenly across the surface.
h. Incubate flasks at 37 °C and 5% CO2 for 6 days.
i. On Day 1, observe cultures under the microscope: most cells should have attached to the bottom of the flask.
j. Aspirate and change neural induction medium (Step 1) to eliminate debris and dying cells that have not attached.
k. Change medium (Step 1) every other day until ready for the next step (on Day 6).
C. Derivation of NPCs into phrenic-like motor neuron progenitor cells (MNPCs): Days 6–12
1. Preparation of medium, coated flasks, and coverslips (Day 5)
a. On Day 5, prepare motor neuron progenitor cell derivation medium (Step 2, see Recipes) and PLO/laminin-coated flasks (as described in step B1a–e).
b. On the same day, prepare 24-well plates with PLO/laminin-coated coverslips to use for quality control immunocytochemical characterization of the NPCs.
c. Add 300 μL of PLO (10 µg/mL) per well.
d. Swirl plate to spread PLO solution evenly across the coverslip surface and incubate at 37 °C and 5% CO2 for at least 2 h.
e. Aspirate and rinse 3 times with PBS before adding 300 μL of laminin (5 µg/mL in DMEM/F12).
f. Swirl plate to spread laminin evenly across the coverslip surface and incubate.
g. The next day, aspirate laminin before use.
2. Passaging of NPCs (Day 6)
a. On Day 6, aspirate Step 1 medium and rinse with D-PBS (10 mL/dish).
b. Aspirate D-PBS, add 5 mL of gentle cell dissociation reagent per culture dish, and incubate for 5 min at 37 °C and 5% CO2.
c. Detach cells by pipetting up/down with a 1,000 μL pipette.
d. Collect cells and dissociation solution into a 15 mL conical tube and add 10 mL of DMEM/F12 to stop the reaction.
e. Centrifuge cells at 1,300× g for 3 min at room temperature. Discard supernatant and resuspend cells in 1 mL of DMEM/F12.
f. Count and seed cells at a concentration of 1 × 106 cells/mL on T25 flasks coated with PLO and laminin.
g. Keep a minimum of 150,000 cells per iPSC line to be plated on coverslips for immunocytochemical characterization (see step C3).
h. For each flask, add 5 mL of motor neuron progenitor cell derivation medium (Step 2) and 5 μL of ROCK inhibitor.
i. Swirl flask to spread cells and medium evenly across the surface.
j. Incubate flasks at 37 °C and 5% CO2 for 6 days.
k. The next day (Day 7), observe cultures under the microscope: most cells should have attached to the bottom of the flask.
l. Aspirate and change motor neuron progenitor cell derivation medium (Step 2) to eliminate debris and dying cells that have not attached.
m. Change medium (Step 2) every other day until ready for the next step (on Day 12).
3. NPCs plating for immunocytochemical characterization (Day 6)
a. Seed cells at 30,000 cells/mL on coverslips in a 24-well plate.
b. Add 300 μL of neural induction medium (Step 1) and 0.3 μL of ROCK inhibitor per well.
c. Swirl plate to spread cells and medium evenly across the coverslip surface.
d. Incubate plate(s) at 37 °C and 5% CO2 for 1 day, before fixing cells (as described in step C4).
4. NPC fixation (Day 7)
a. On Day 7, wash cells in 24-well plates with D-PBS 1×.
b. Aspirate and add 500 μL of 4% PFA per well, leaving it for 15 min at room temperature.
c. Aspirate and rinse twice with D-PBS 1×.
d. Aspirate and add 1 mL of 50% glycerol (diluted in PBS 1×) per well, before storing the plate in the dark at 4 °C until ready to start immunocytochemistry (as described in step C5).
5. Immunocytochemical characterization of NPCs
a. Wash coverslips 2 × 5 min in PBS 1× (500 μL per well) at room temperature.
b. Remove coverslips from the wells and place them on a clean surface in a box that can be placed in the dark.
c. Permeabilization: aspirate PBS and add blocking solution (50 μL per coverslip), leaving it for 10 min at room temperature.
Note: For permeabilization, blocking, and washing steps, do not aspirate excess solution but rather absorb it by gently tapping the side of the coverslip on a KimwipeTM (see Troubleshooting, Problem 2).
d. Blocking: aspirate and replace with fresh blocking solution (50 μL per coverslip), leaving it for 1 h at room temperature.
e. Prepare primary antibody solutions (here: goat anti-SOX1, rabbit anti-HOXC4, mouse anti-PAX6, diluted in blocking solution) and add 50 μL per coverslip, leaving it for 2 h at room temperature.
f. Wash coverslips 5 × 5 min in blocking solution (50 μL per coverslip) at room temperature.
g. Prepare secondary antibody solutions (diluted in blocking solution) and add 50 μL per coverslip, leaving it for 1 h at room temperature (or 4 h at 4 °C) in the dark.
h. Wash coverslips 2 × 5 min in blocking solution (50 μL per coverslip) at room temperature in the dark.
i. Wash coverslips 2 × 5 min in PBS 1× (50 μL per coverslip) at room temperature in the dark.
j. Perform DNA staining with Hoechst (1:5,000 in PBS 1×, 50 μL per coverslip) for 3 min at room temperature in the dark.
k. Wash coverslips 3 × 2 min in PBS 1× (50 μL per coverslip) at room temperature in the dark.
l. Mount coverslips with Fluoromount G® mounting medium on microscope slides. Prepare microscope slides one by one by adding one drop of mounting medium per coverslip (for a maximum of 4 coverslips per microscope slide). Remove excess PBS from the coverslip with a KimwipeTM and gently place the coverslip (cells facing the microscope slide) over the drop of mounting medium on the microscope slide. Store slides at 4 °C in the dark until ready to acquire images.
D. Expansion of MNPCs: Days 12–18
1. Preparation of medium, coated flasks, and coverslips (Day 11)
a. On Day 11, prepare PLO/laminin-coated flasks (as described in step B1a–e) and motor neuron progenitor cell expansion medium (Step 3, see Recipes).
b. On the same day, prepare 24-well plates with PLO/laminin-coated coverslips (as described in step C1c–g) to use for immunocytochemical characterization of the MNPCs.
2. Passaging of MNPCs (Day 12)
a. On Day 12, aspirate Step 2 medium and rinse with D-PBS (10 mL/ dish).
b. Aspirate D-PBS, add 5 mL of gentle cell dissociation reagent per culture dish, and incubate for 5 min at 37 °C and 5% CO2.
c. Detach cells by pipetting up/down with a 1,000 μL pipette.
d. Collect cells and dissociation solution into a 15 mL conical tube and add 10 mL of DMEM/F12 to stop the reaction.
e. Centrifuge cells at 1,300× g for 3 min at room temperature. Discard supernatant and resuspend cells in 1 mL of DMEM/F12.
f. Count and seed cells at a concentration of 1 × 106 cells/mL on T25 flasks coated with PLO and laminin.
g. Keep a minimum of 150,000 cells per iPSC line to be plated on coverslips for immunocytochemical characterization (see step D3).
h. For each flask, add 5 mL of motor neuron progenitor cell expansion medium (Step 3) and 5 μL of ROCK inhibitor.
i. Swirl flask to spread cells and medium evenly across the surface.
j. Incubate flasks at 37 °C and 5% CO2 for 6 days.
k. The next day (Day 13), observe cultures under the microscope: most cells should have attached to the bottom of the flask.
l. Aspirate and change motor neuron progenitor cell expansion medium (Step 3) to eliminate debris and dying cells that have not attached.
m. Change medium (Step 3) every other day until ready for the next step (on Day 18).
3. MNPCs plating for immunocytochemical characterization (Day 12)
a. Seed cells at 30,000 cells/mL on coverslips in a 24-well plate.
b. Add 300 μL of motor neuron progenitor cell induction medium (Step 2) and 0.3 μL of ROCK inhibitor per well.
c. Swirl plate to spread cells and medium evenly across the coverslip surface.
d. Incubate plate(s) at 37 °C and 5% CO2 for 1 day, before fixing cells (as described in step C4).
4. Immunocytochemical characterization of MNPCs
For quality control characterization of the MNPCs by immunochemistry, follow the instructions provided in step C5 with the following specific antibodies: mouse anti-OLIG2 together with rat anti-TLE and rabbit anti-PAX6; and mouse anti-OLIG2 together with rabbit anti-HOXA5.
Note: See an example of the resulting MNPCs in Figure 1.
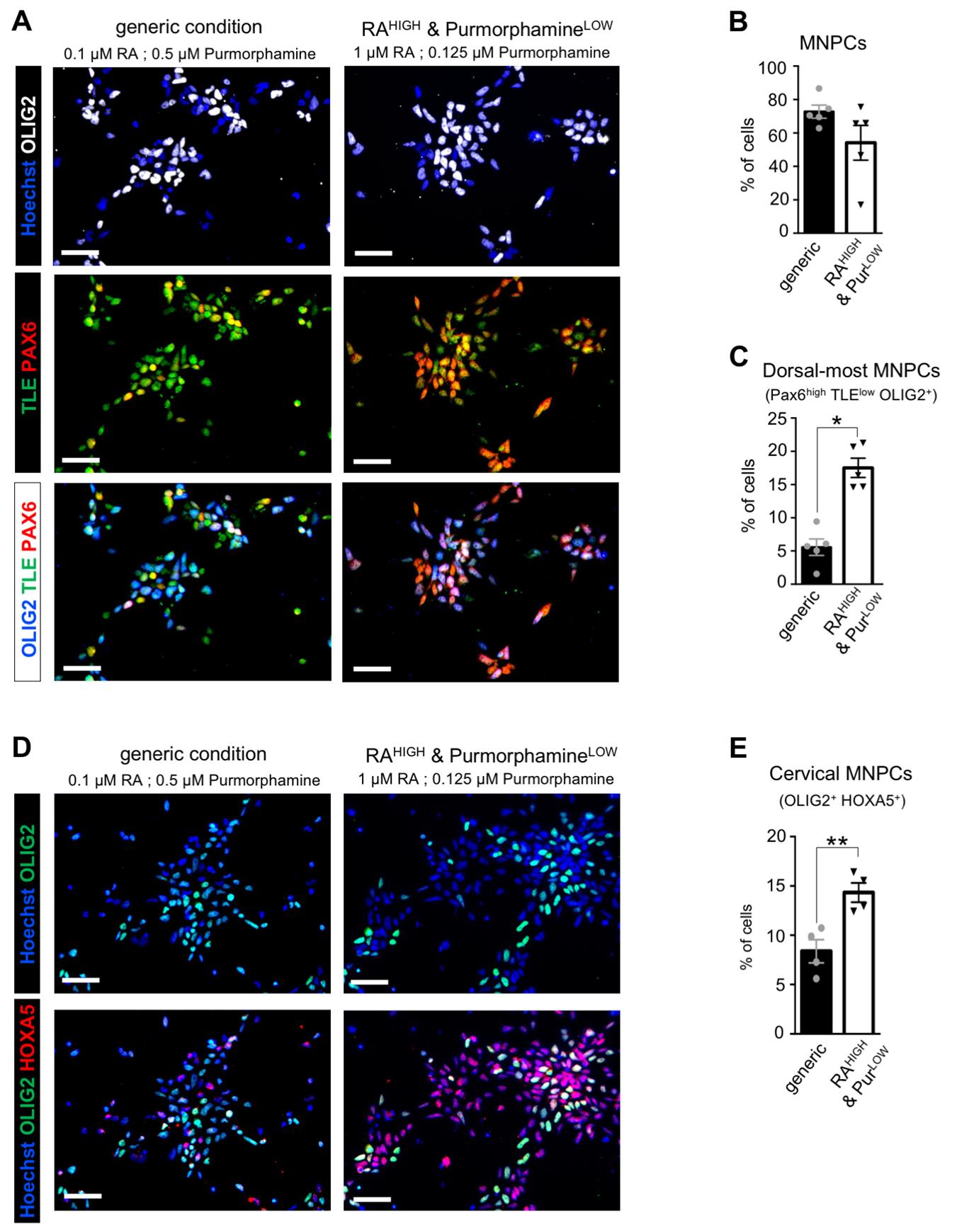
Figure 1. Characterization of human induced pluripotent stem cell (hiPSC)-derived motor neuron progenitor cells (MNPCs): proportion of cervical dorsalmost MNPCs. (A) Representative images of MNPCs stained with anti-OLIG2 (white), anti-TLE (green), and anti-PAX6 (red) antibodies after 12 days of culture in the generic or RAHIGH & PurmorphamineLOW conditions. Comparison with generic MNPCs (derived with 0.5 μM Purmorphamine and 0.1 μM retinoic acid (RA), as described previously [20,22]) allows for analysis of changes in the proportion of dorsalmost cervical MNPCs. (B) Quantification of MNPCs (OLIG2+ cells) as percentages of the total number of cells. (C) Quantification of the dorsalmost MNPCs (PAX6high/TLElow OLIG2+ cells) as percentages of the total number of cells. (D) Representative images of MNPCs stained with anti-OLIG2 (green) and anti-HOXA5 (red) antibodies after 12 days of differentiation in the generic or RAHIGH & PurmorphamineLOW conditions. (E) Quantification of cervical MNPCs (HOXA5+/OLIG2+ cells) as percentages of the total number of cells. Scale bars = 50 μm. One-way ANOVA and Holm Sidak’s post-hoc multiple comparisons test; *p < 0.05; **p < 0.005; n = 4–5 cultures per condition (with >500 cells in 3 random fields for each culture). Adapted from Thiry et al. Communications Biology [22], DOI: 10.1038/s42003-024-05925-z (Figure 1).
E. FACS-based sorting of phrenic-like MNPCs: Day 18
1. Dissociation of the cells for FACS-based sorting (Day 18)
a. On Day 18, aspirate medium and rinse with D-PBS (10 mL/dish).
b. Aspirate D-PBS, add 5 mL of gentle cell dissociation reagent per culture dish, and incubate for 5 min at 37 °C and 5% CO2.
c. Detach cells by pipetting up/down with a 1,000 μL pipette.
d. Collect cells and dissociation solution into a 15 mL conical tube and add 10 mL of DMEM/F12 to stop the reaction.
e. Centrifuge cells at 1,300× g for 3 min at room temperature. Discard supernatant and resuspend cells in 1 mL of DMEM/F12.
f. Filter the cells with a 30 µm cell strainer to obtain single cells in suspension in 1 mL of DMEM/F12 in a 15 mL conical tube.
g. Add 1 μL of ROCK inhibitor and place the tube on ice until ready for the next step.
2. Washing of the cells with D-PBS twice (to remove any trace of media)
a. Place the cells in a 5 mL FACS tube.
b. Add 2 mL of D-PBS to the 5 mL FACS tube.
c. Centrifuge the cells at 300× g for 5 min with low braking and discard supernatant.
d. Add 2 mL of D-PBS to the 5 mL FACS tube.
e. Centrifuge the cells at 300× g for 5 min with low braking and discard the supernatant.
f. Add D-PBS to make a final volume of 200 μL.
g. Count cells in suspension using a hematocytometer.
3. Cell suspension concentration adjustment
a. Adjust the concentration of the initial cell suspension to 1 million cells per milliliter per tube using D-PBS.
a. Create multiple tubes if more than 1 million cells are counted.
b. Keep 100,000 cells aside for a no-antibody or dye control (only FACS buffer).
4. Viability staining using Thermo-Fisher’s Live/Dead Fixable Aqua reagent
a. Add 1 µL of Live/Dead Fixable Aqua Dye per tube.
Note: The Live/Dead Fixable reagent is reconstituted by adding 50 μL of DMSO to the lyophilized tube. Mix well by pipetting up/down.
b. Mix well by vortexing the FACS tube and incubate the cells at room temperature for 30 min in the dark.
c. Add 2 mL of D-PBS to the 5 mL FACS tube.
d. Centrifuge the cells at 300× g for 5 min with low braking and discard the supernatant.
e. Add 2 mL of D-PBS to the 5 mL FACS tube.
f. Centrifuge the cells at 300× g for 5 min with low braking and completely discard the supernatant.
5. Extracellular staining of IGDCC3
a. For each FACS tube, create a mix of antibodies in FACS buffer (see Recipes). The IGDCC3 antibody dilution used is 1:20. The final stain volume is 100 μL.
Note: In the no-antibody or dye control, add only 100 μL of FACS buffer to the FACS tube.
b. Incubate the cells at room temperature for 30 min in the dark.
c. Add 2 mL of D-PBS to the 5 mL FACS tube.
d. Centrifuge the cells at 300× g for 5 min with low braking and discard the supernatant.
e. Add 2 mL of D-PBS to the 5 mL FACS tube.
f. Centrifuge the cells at 300× g for 5 min with low braking and completely discard the supernatant.
g. Add 500 μL of FACS buffer and place the FACS tube at 4 °C until ready to be acquired by FACS.
6. Compensation control for FACS
Note: This step can be performed in parallel with cell staining (step E5).
a. In one FACS tube (referred to as control), add 1 drop of SpectraComp® unmixing Controls beads.
b. In another FACS tube (referred to as viability), add 1 drop of ViaComp® beads.
Note: Shake each bead vial before use.
c. For viability with the ViaComp® beads, add 2 μL of Live/Dead Fixable Aqua dye to the viability FACS tube.
d. Add 5 μL of IGDCC3 antibody to the control FACS tube.
e. Vortex both FACS tubes.
f. Incubate both tubes at room temperature for 30 min in the dark.
g. Add 2 mL of D-PBS to each 5 mL FACS tube.
h. Centrifuge both tubes at 300× g for 5 min with low braking and discard the supernatant.
i. Add 2 mL of D-PBS to each 5 mL FACS tube.
j. Centrifuge both tubes at 300× g for 5 min with low braking and completely discard the supernatant.
k. Add 500 μL of FACS buffer to each FACS tube and place at 4 °C until ready to be acquired by FACS.
7. Readying the FACSAria Fusion
Note: Specific training is required to perform this step and to operate FACS-specific equipment. In most cases, the sorting process will be performed by qualified scientific staff. The staff is responsible for the maintenance of the cytometer. However, the user will need to give out some extra information specific to this sort (specify the cell population type) and help the cell sorter operator in planning the sorting session (schedule a time).
a. The nozzle size used is a minimum of 100 μm to 135 μm. This allows for the lowest sorting pressure (maximum 20 psi) to ensure better cell survivability.
b. The cold block of the sorter should be set to 4 °C.
c. Materials needed for this step:
- Samples in FACS tubes (prepared in E1–5)
- Compensation controls (prepared in E6)
- Sorting tubes (FACS tubes with 1 mL of FACS buffer) in accordance with the number of samples.
- Extra FACS tubes and extra FACS buffer
F. Induction of sorted phrenic-like MNPCs into motor neurons (MNs): Days 18–24
1. Preparation of medium, coated flasks, and coverslips (Day 17)
a. On Day 17, prepare PLO/laminin-coated flasks (as described in B1a–e) and motor neuron differentiation medium (Step 4, see Recipes).
2. Plating of sorted MNPCs (Day 18)
a. On Day 18, following FACS, seed sorted cells at a concentration of 1 × 106 cells/mL on T25 flasks coated with PLO and laminin.
b. For each flask, add 5 mL of motor neuron differentiation medium (Step 4) and 5 μL of ROCK inhibitor.
c. Swirl the flask to spread cells and medium evenly across the surface.
d. Incubate flasks at 37 °C and 5% CO2 for 6 days.
e. On Day 19, observe cultures under the microscope: most cells should have attached to the bottom of the flask.
f. Aspirate and change motor neuron differentiation medium (Step 4) to eliminate debris and dying cells that have not attached.
g. Change medium (Step 4) every other day until ready for the next step (on Day 24).
G. Maturation of sorted phrenic-like MNs: Days 24–30+
1. Preparation of medium and dPGA/Matrigel-coated coverslips (Day 23)
a. On Day 23, prepare motor neuron maturation medium (Step 5, see Recipes).
b. On the same day, prepare 24-well plates with PLO/laminin-coated coverslips (as described in C1c–g) to use for immunocytochemical characterization of the FACS-sorted immature MNs after 25 days of culture.
c. On the same day, prepare 24-well plates with dPGA/Matrigel-coated coverslips to use for immunocytochemical characterization of the mature MNs after 32 days of culture.
d. Add 300 μL/coverslip of dPGA (at a concentration of 50 µg/mL in PBS) and swirl the plate to spread the dPGA solution evenly across the surface.
e. Incubate for at least 1 h at 37 °C and 5% CO2.
f. During this 1-h incubation, thaw Matrigel on ice (1 h before use) and prepare one conical tube of cold DMEM/F12 (keep on ice).
g. Aspirate the excess dPGA solution and rinse coverslips with PBS.
h. Add thawed Matrigel to the cold DMEM/F12 medium (at 1/100) and mix well.
i. Immediately add 300 μL of the cold diluted Matrigel per coverslip.
j. Swirl plate to spread the Matrigel solution evenly across the surface and incubate for at least 2 h.
k. Before use (the next day), gently tilt the plate to allow the excess Matrigel solution to collect on one side of each well and aspirate without scratching the coated surface.
2. Passaging of sorted MNs (Day 24)
a. On Day 24, aspirate Step 4 medium and rinse with D-PBS (10 mL/dish).
b. Aspirate D-PBS, add 5 mL of gentle cell dissociation reagent per culture dish, and incubate 5 min at 37 °C and 5% CO2.
c. Detach cells by pipetting up/down with a 1,000 μL pipette.
d. Collect cells and dissociation solution into a 15 mL conical tube and add 10 mL of DMEM/F12 to stop the reaction.
e. Centrifuge cells at 1,300× g for 3 min at room temperature. Discard supernatant and resuspend cells in 1 mL of DMEM/F12.
f. Count and seed cells at a concentration of 30,000 cells/mL on coverslips coated with dPGA/Matrigel. Keep some cells to be plated on PLO/laminin-coated coverslips for immunocytochemical characterization of immature MNs (see G3).
g. For each coverslip, add 300 μL of motor neuron maturation medium (Step 5) and 0.3 μL of ROCK inhibitor.
h. Swirl the plate to spread cells and medium evenly across the surface.
i. Incubate plate at 37 °C and 5% CO2 for 6+ days.
j. The next day (Day 25), observe cultures under the microscope: most cells should have attached to the surface of the coverslip.
k. Aspirate and change motor neuron maturation medium (Step 5) to eliminate debris and dying cells that have not attached.
l. Change medium (Step 5) every other day until ready to fix cells on Day 30+ (as described in C4) for subsequent immunochemical characterization of the resulting phrenic-like MNs (see G5) or until ready to use for another test.
3. MNs plating for immunocytochemical characterization (Day 24)
a. Seed cells at 30,000 cells/mL on PLO/laminin-coated coverslips in a 24-well plate.
b. Add 300 μL of motor neuron differentiation medium (Step 4) and 0.3 μL of ROCK inhibitor per well.
c. Swirl plate to spread cells and medium evenly across the coverslip surface.
d. Incubate plate(s) at 37 °C and 5% CO2 for 1 day, before fixing cells (as described in C4) on Day 25 for subsequent immunocytochemistry (see G4).
4. Immunocytochemical characterization of post-FACS immature phrenic-like MNs
For quality control characterization of immature post-FACS MNs by immunochemistry, follow the steps described in C5, with the following specific antibodies: mouse anti-ISL1 and mouse anti-HB9 in combination, together with rabbit anti-LHX3 and guinea pig anti-SCIP.
Note: See an example of the post-FACS phrenic-like MNs in Figure 2.
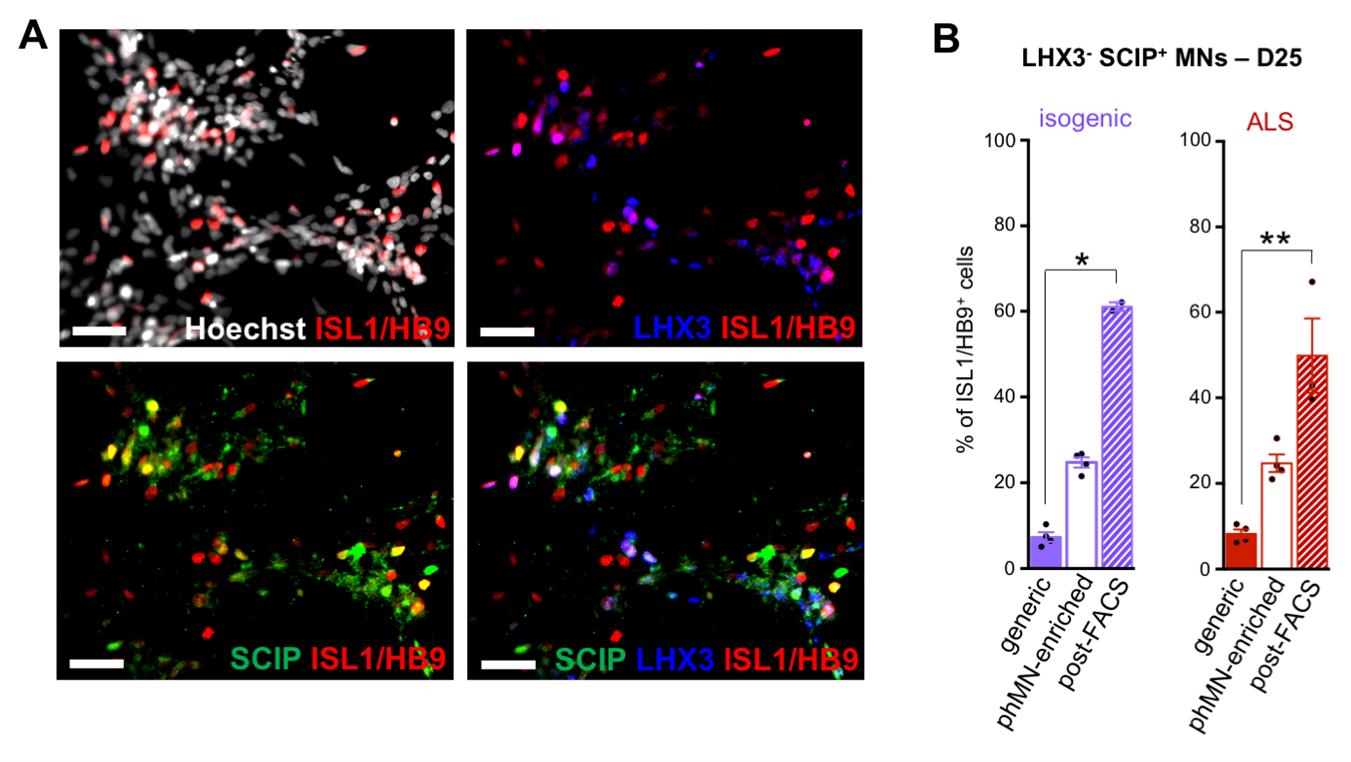
Figure 2. Characterization of FACS-sorted immature motor neurons (MNs) after 25 days in culture: enrichment in SCIP-positive phrenic MNS (phMNs). (A) Representative images of IGDCC3-positive cells, 3 days after FACS sorting of MN cultures derived from ALS CS29 hiPSCs (D25). Cultures were co-stained with the known pan-MN marker HB9/ISL1 (red), the anti-LHX3 (blue), and the anti-SCIP antibody (green). Scale bars = 50 μm. (B) Quantification of phMNs, identified as ISL1/HB9+ SCIP+ LHX3-, as percentages of the number of ISL1/HB9+ cells in generic MN cultures (solid bars), non-FACS phMN cultures (hollow bars), or post-FACS phMN cultures (hatched bars) derived from CS29 isogenic (purple) or ALS (red) after 25 days of differentiation. Comparison with generic MN cultures (derived as described previously [20,22]) allows for analysis of changes in the proportion of phMNs. Kruskal–Wallis nonparametric test and Dunn’s post-hoc multiple comparisons test; *p < 0.05; **p < 0.005; n = 3 cultures (with >500 cells in random fields for each culture). Adapted from Thiry et al. Communications Biology [22], DOI: 10.1038/s42003-024-05925-z (Figure 4).
5. Immunocytochemical characterization of post-FACS mature phrenic-like MNs
For quality control characterization of mature post-FACS phrenic-like MNs by immunochemistry, follow the steps described in C5 with the following specific antibodies: mouse anti-ChAT together with guinea pig anti-SCIP, and either rabbit anti-HOXA5, rabbit anti-FOXP1, or rabbit anti-LHX3.
Note: See an example of the mature post-FACS phrenic-like MNs in Figure 3.
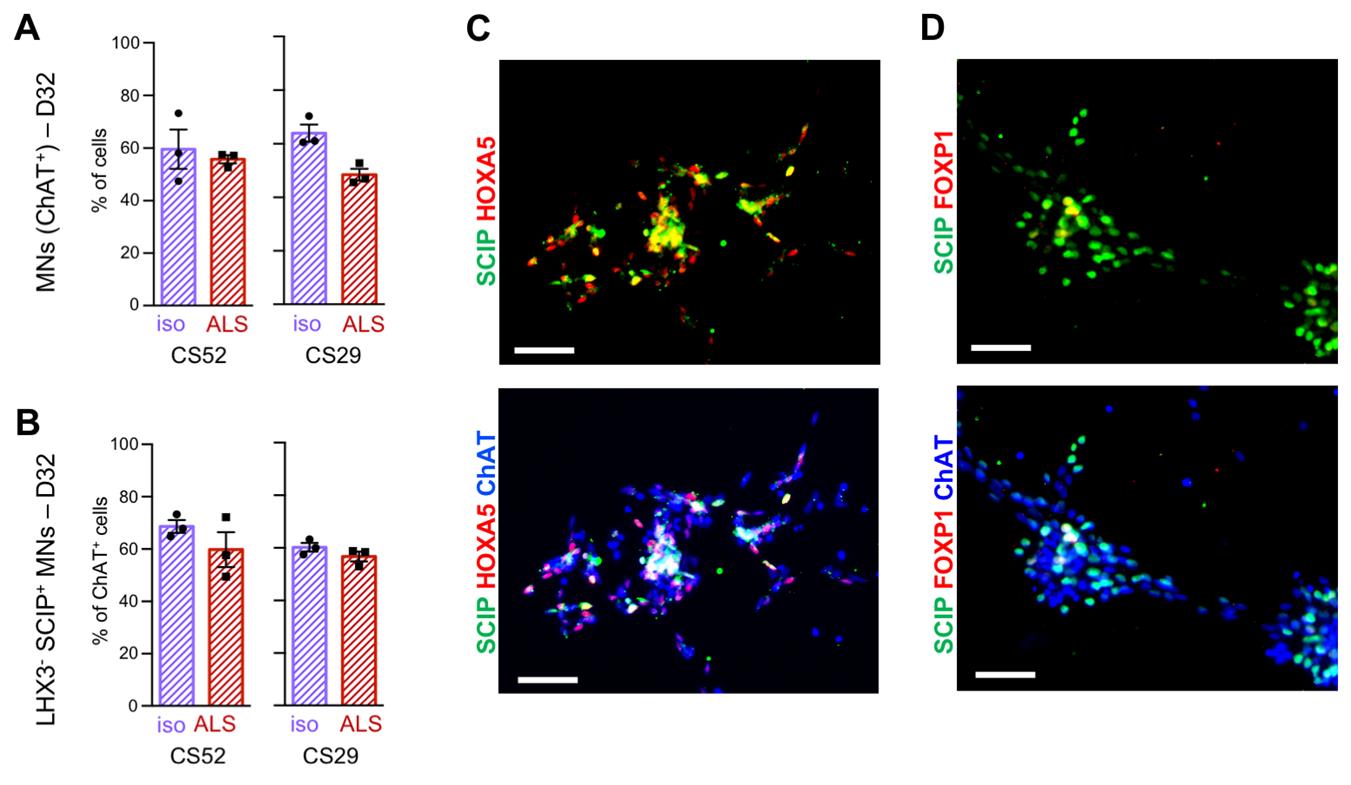
Figure 3. Characterization of mature post-FACS phrenic-like motor neurons (phMNs) after 32 days in culture. (A) Quantification of MNs identified as ChAT + cells, as percentages of the total number of cells, 2 weeks after FACS sorting of ALS CS52 and CS29 iPSCs-derived MN cultures (D32). (B) Quantification of phMNs identified as ChAT+/LHX3−/SCIP+, as percentages of the number of ChAT + cells, 2 weeks after FACS sorting of ALS CS52 and CS29 induced pluripotent stem cell (iPSC)-derived MN cultures (D32). (C–D). Representative images of MNs co-stained with the anti-ChAT (blue), the anti-SCIP antibody (green), and either the anti-HOXA5 antibody (red, C) or the anti-FOXP1 antibody (red, D), 2 weeks after FACS sorting of ALS CS52 iPSC-derived MN cultures (D32). SCIP-positive phMNs express HOXA5 but not FOXP1. Scale bars = 50 µm. Adapted from Thiry et al. Communications Biology [22], DOI: 10.1038/s42003-024-05925-z (Figure 4 and Supplementary Figure 6).
Data analysis
Detailed description of data processing and analyses including statistical tests, criteria for data inclusion/exclusion, number of biological and technical replicates for each experiment and description of single-cell RNA sequencing analysis of the resulting cell cultures is provided in the Methods section of the following paper: Thiry L. et al. [DOI: 10.1038/s42003-024-05925-z], Generation of human iPSC-derived phrenic-like motor neurons to model respiratory motor neuron degeneration in ALS, Communications Biology [22].
Validation of protocol
This protocol has been used and validated in the following research article:
Thiry. et al. [22], Generation of human iPSC-derived phrenic-like motor neurons to model respiratory motor neuron degeneration in ALS. Commun Biol.
As a minimum requirement, quality control through immunocytochemistry characterization and validation of the cells should be performed after each key derivation step, as described in this protocol.
General notes and troubleshooting
General notes
1. Each culture medium can be prepared in advance and kept at 4 °C, in the dark, for a maximum of 6 days.
2. All cell culture manipulations must be performed under a sterile hood by a person with appropriate training.
3. Always change the medium the day after passaging or thawing cells, to remove debris and dying cells.
4. Retinoic acid is extremely sensitive to UV light, air, and oxidizing agents, especially in solution. Always use new aliquots to make a working solution.
5. Gentle cell dissociation reagent, DMEM/F12, and culture medium should be warmed up before use: place at room temperature under the sterile hood for 1–2 h before use. Alternatively, these solutions can be placed in the 37 °C water bath 30 min before use.
6. Matrigel, PLO/laminin, and dPGA/Matrigel-coated plates/coverslips can be used up to 1 day after coating, as long as they are placed in the dark at 37 °C, 5% CO2 until ready for use. Note that the plates/coverslips must not dry out before use.
7. Cell counting was performed using an automated cell counter (LUNA-IITM Automated Cell Counter, see Equipment), allowing for viability assessment, rapid execution, and reproducibility.
8. After NPC, MNPC, and MN derivation steps, cellular molecular identity should be characterized by immunocytochemistry, as described, to validate the procedure.
Troubleshooting
Problem 1: hiPSC-derived MNs coalesce into large cell aggregates, and eventually detach from their substrate after prolonged culture, thus making long-term studies technically challenging.
Possible cause: This situation is most likely due to the degradation of the protein-based substrate (Matrigel or laminin) commonly used for coating [23].
Solution: To overcome this issue, use a non-peptide polymer substrate that was shown to improve primary neuron culture conditions (dPGA) [23], in combination with Matrigel, as described in C2a.
Problem 2: hiPSC-derived MNs detach from the coverslip during the immunocytochemistry procedure.
Possible cause: Even when using dPGA/Matrigel-coated coverslips, mature MN cultures are very fragile and can easily detach when submitted to successive rinsing steps.
Solution: Each rinsing step must be performed very gently: do not aspirate to remove excess PBS, blocking solution, or antibody solution. Instead, absorb excess solution by gently tapping the side of the coverslip on a KimwipeTM and add the next solution with a pipette, as carefully as possible.
Problem 3: Sorted MNPCs do not attach/proliferate after FACS.
Possible cause: There were not enough sorted IGDCC3-positive MNPCs.
Solution: Increase the amount of MNPCs submitted to FACS.
Acknowledgments
L.T. performed all experiments, data analysis and figure preparation and wrote the first draft of the manuscript. J.S. performed FACS experiments and wrote FACS-associated parts of this manuscript. S.S., T.D., and L.T. conceived the study plan. S.S. designed the experiments and supervised data analysis and manuscript writing. These studies were funded in part by grants to S.S. from the Canadian Institutes for Health Research and Fonds de la recherche en Sante-Quebec under the frame of E-Rare-3, the ERA-Net for Research on Rare Diseases. T.M.D. was supported by the Canada First Research Excellence Fund, awarded through the HBHL initiative at McGill University, the CQDM’s Health Collaborations Accelerator Fund program, and a project grant from CIHR (PJT – 169095). S.S. is a Distinguished James McGill Professor of McGill University. This protocol was described and validated in the following original research paper: Thiry L. et al. (DOI: 10.1038/s42003-024-05925-z), Generation of human iPSC-derived phrenic-like motor neurons to model respiratory motor neuron degeneration in ALS, Communications Biology [22]. This paper is licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Competing interests
The authors declare no conflicts of interest.
References
- Loutfl Sami, A., Saeed, U. K., David, P. M., Kay, S. and Hiroshi, M. (1997). Effect of Noninvasive Positive-Pressure Ventilation on Survival in Amyotrophic Lateral Sclerosis. Ann Intern Med. 127: 450–453. https://doi.org/10.7326/0003-4819-127-6-199709150-00006.
- Al-Chalabi, A. and Hardiman, O. (2013). The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 9(11): 617–628. https://doi.org/10.1038/nrneurol.2013.203
- Nichols, N. L., Gowing, G., Satriotomo, I., Nashold, L. J., Dale, E. A., Suzuki, M., Avalos, P., Mulcrone, P. L., McHugh, J., Svendsen, C. N., et al. (2013). Intermittent Hypoxia and Stem Cell Implants Preserve Breathing Capacity in a Rodent Model of Amyotrophic Lateral Sclerosis. Am J Respir Crit Care Med. 187(5): 535–542. https://doi.org/10.1164/rccm.201206-1072oc
- Rousso, D. L., Gaber, Z. B., Wellik, D., Morrisey, E. E. and Novitch, B. G. (2008). Coordinated Actions of the Forkhead Protein Foxp1 and Hox Proteins in the Columnar Organization of Spinal Motor Neurons. Neuron. 59(2): 226–240. https://doi.org/10.1016/j.neuron.2008.06.025
- Philippidou, P., Walsh, C. M., Aubin, J., Jeannotte, L. and Dasen, J. S. (2012). Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat Neurosci. 15(12): 1636–1644. https://doi.org/10.1038/nn.3242
- Machado, C. B., Kanning, K. C., Kreis, P., Stevenson, D., Crossley, M., Nowak, M., Iacovino, M., Kyba, M., Chambers, D., Blanc, E., et al. (2014). Reconstruction of phrenic neuron identity in embryonic stem cell-derived motor neurons. Development. 141(4): 784–794. https://doi.org/10.1242/dev.097188
- Dasen, J. S., De Camilli, A., Wang, B., Tucker, P. W. and Jessell, T. M. (2008). Hox Repertoires for Motor Neuron Diversity and Connectivity Gated by a Single Accessory Factor, FoxP1. Cell. 134(2): 304–316. https://doi.org/10.1016/j.cell.2008.06.019
- Nichols, N. L., Satriotomo, I., Harrigan, D. J. and Mitchell, G. S. (2015). Acute intermittent hypoxia induced phrenic long-term facilitation despite increased SOD1 expression in a rat model of ALS. Exp Neurol. 273: 138–150. https://doi.org/10.1016/j.expneurol.2015.08.011
- Lladó, J., Haenggeli, C., Pardo, A., Wong, V., Benson, L., Coccia, C., Rothstein, J. D., Shefner, J. M. and Maragakis, N. J. (2006). Degeneration of respiratory motor neurons in the SOD1 G93A transgenic rat model of ALS. Neurobiol Dis. 21(1): 110–118. https://doi.org/10.1016/j.nbd.2005.06.019
- Smittkamp, S. E., Spalding, H. N., Brown, J. W., Gupte, A. A., Chen, J., Nishimune, H., Geiger, P. C. and Stanford, J. A. (2010). Measures of bulbar and spinal motor function, muscle innervation, and mitochondrial function in ALS rats. Behav Brain Res. 211(1): 48–57. https://doi.org/10.1016/j.bbr.2010.03.007
- Genabai, N. K., Kannan, A., Ahmad, S., Jiang, X., Bhatia, K. and Gangwani, L. (2017). Deregulation of ZPR1 causes respiratory failure in spinal muscular atrophy. Sci Rep. 7(1): 8295. https://doi.org/10.1038/s41598-017-07603-z
- Seven, Y. B., Nichols, N. L., Kelly, M. N., Hobson, O. R., Satriotomo, I. and Mitchell, G. S. (2018). Compensatory plasticity in diaphragm and intercostal muscle utilization in a rat model of ALS. Exp Neurol. 299: 148–156. https://doi.org/10.1016/j.expneurol.2017.10.015
- Hedlund, E., Karlsson, M., Osborn, T., Ludwig, W. and Isacson, O. (2010). Global gene expression profiling of somatic motor neuron populations with different vulnerability identify molecules and pathways of degeneration and protection. Brain. 133(8): 2313–2330. https://doi.org/10.1093/brain/awq167
- Fusco, A. F., Mccall, A. L., Dhindsa, J. S., Pucci, L. A. and Strickland, L. M. (2019). The Respiratory Phenotype of Rodent Models of Amyotrophic Lateral Sclerosis and Spinocerebellar Ataxia. Front Neuroinflamm Neurodegener. Dis. 3:100011. https://pubmed.ncbi.nlm.nih.gov/31893284/
- Chen, H., Qian, K., Du, Z., Cao, J., Petersen, A., Liu, H., Blackbourn, L. W., Huang, C. L., Errigo, A., Yin, Y., et al. (2014). Modeling ALS with iPSCs Reveals that Mutant SOD1 Misregulates Neurofilament Balance in Motor Neurons. Cell Stem Cell. 14(6): 796–809. https://doi.org/10.1016/j.stem.2014.02.004
- Amoroso, M. W., Croft, G. F., Williams, D. J., O'Keeffe, S., Carrasco, M. A., Davis, A. R., Roybon, L., Oakley, D. H., Maniatis, T., Henderson, C. E., et al. (2013). Accelerated High-Yield Generation of Limb-Innervating Motor Neurons from Human Stem Cells. J Neurosci. 33(2): 574–586. https://doi.org/10.1523/jneurosci.0906-12.2013
- Maury, Y., Côme, J., Piskorowski, R. A., Salah-Mohellibi, N., Chevaleyre, V., Peschanski, M., Martinat, C. and Nedelec, S. (2014). Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat Biotechnol. 33(1): 89–96. https://doi.org/10.1038/nbt.3049
- Li, X. J., Du, Z. W., Zarnowska, E. D., Pankratz, M., Hansen, L. O., Pearce, R. A. and Zhang, S. C. (2005). Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 23(2): 215–221. https://doi.org/10.1038/nbt1063
- Du, Z. W., Chen, H., Liu, H., Lu, J., Qian, K., Huang, C. L., Zhong, X., Fan, F. and Zhang, S. C. (2015). Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun. 6(1): 6626. https://doi.org/10.1038/ncomms7626
- Thiry, L., Hamel, R., Pluchino, S., Durcan, T. and Stifani, S. (2020). Characterization of Human iPSC-derived Spinal Motor Neurons by Single-cell RNA Sequencing. Neuroscience. 450: 57–70. https://doi.org/10.1016/j.neuroscience.2020.04.041
- Stifani, S., Blaumueller, C. M., Redhead, N. J., Hill, R. E. and Artavanis-Tsakonas, S. (1992). Human homologs of a Drosophila Enhancer of Split gene product define a novel family of nuclear proteins. Nat Genet. 2(2): 119–127. https://doi.org/10.1038/ng1092-119
- Thiry, L., Sirois, J., Durcan, T. M. and Stifani, S. (2024). Generation of human iPSC-derived phrenic-like motor neurons to model respiratory motor neuron degeneration in ALS. Commun Biol. 7(1): 238. https://doi.org/10.1038/s42003-024-05925-z
- Thiry, L., Clément, J. P., Haag, R., Kennedy, T. E. and Stifani, S. (2022). Optimization of Long-Term Human iPSC-Derived Spinal Motor Neuron Culture Using a Dendritic Polyglycerol Amine-Based Substrate. ASN NEURO. 14(1): e1177/17590914211073381. https://doi.org/10.1177/17590914211073381
Article Information
Publication history
Received: Apr 17, 2025
Accepted: May 26, 2025
Available online: Jun 10, 2025
Published: Jul 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Thiry, L., Sirois, J., Durcan, T. M. and Stifani, S. (2025). Derivation and Culture of Enriched Phrenic-Like Motor Neurons From Human iPSCs. Bio-protocol 15(13): e5363. DOI: 10.21769/BioProtoc.5363.
Category
Neuroscience > Sensory and motor systems > Cell isolation and culture
Stem Cell > Adult stem cell > Neural stem cell
Cell Biology > Cell isolation and culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









