- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
The Establishment of 3D Polarity-Reversed Organoids From Human Endometrial Tissue as a Model for Infection-Induced Endometritis
Published: Vol 15, Iss 12, Jun 20, 2025 DOI: 10.21769/BioProtoc.5349 Views: 1746
Reviewed by: Alessandro DidonnaSérgio RibeiroAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling
Michele Bertacchi [...] Michèle Studer
Jun 20, 2025 2796 Views

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3751 Views
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1249 Views
Abstract
Endometritis is a prevalent gynecological condition, often resulting from bacterial infections, which poses significant risks to women’s reproductive health, including recurrent pregnancy loss, spontaneous abortion, and intrauterine adhesions. While conventional in vitro models have provided valuable insights into the pathogenesis of bacterial-induced endometritis, they often fail to replicate the complex cellular architecture and microenvironment of the endometrium due to species-specific differences and variations in the menstrual cycle. In this study, we present a novel organoid-based culture system that establishes a bacterial-induced endometritis model using endometrial organoids derived from primary epithelial cells. This protocol involves culturing endometrial organoids in a Matrigel-based three-dimensional matrix, followed by infection with Escherichia coli at a defined multiplicity of infection (MOI). The model effectively recapitulates key pathological features of bacterial-induced endometritis, including disruption of the epithelial barrier, release of inflammatory cytokines, and cellular damage. By preserving epithelial polarity, this approach offers enhanced physiological relevance, improves host–pathogen interaction studies, and provides a robust platform for evaluating potential therapeutic interventions.
Key features
• Establishes apical-out endometrial organoids to model pathogen-induced endometritis via natural infection routes.
• Utilizes primary human endometrial epithelial cells to preserve cellular diversity and mimic the native endometrial microenvironment.
• Provides a versatile platform for investigating host–pathogen interactions and evaluating potential therapeutic interventions in bacterial-induced endometritis.
• Developed apical-out endometrial organoids to better mimic tissue structure and enhance pathogen infection for host–pathogen interaction studies.
Keywords: Endometrial organoidBackground
Endometritis is a bacterial infection that leads to the disruption of the endometrial epithelial barrier, causing a range of adverse effects. With the growth of assisted reproductive technologies, gaining a better understanding of endometritis has become increasingly important, especially in developing models that assess how pathogenic bacteria impact the endometrial barrier and its receptivity [1–2]. In vitro models have become essential for exploring the mechanisms underlying endometritis and the interactions between the host and pathogens [3]. Cell-based systems provide a controlled environment that closely replicates human endometrial cells and physiological conditions [4]. These models allow for the examination of immune responses, pathogen evasion strategies, and the role of virulence factors in disease progression [5]. Additionally, in vitro platforms are crucial for evaluating the effectiveness and toxicity of therapeutic compounds in the early stages of drug development, reducing the dependency on expensive animal models. However, despite the growing interest in advanced culture systems for studying endometrial infections, there is still a lack of comprehensive reviews on these methodologies.
Research on endometrial models has evolved from the use of in vitro cell lines to animal models. In vitro 2D culture systems limit essential cell–cell interactions required to replicate the in vivo microenvironment [6–8]. Additionally, the use of non-human primates in research poses challenges, including high costs, specialized resources, and ethical concerns. Significant anatomical and physiological differences between human and mouse reproductive systems necessitate careful consideration of interspecies variations in research [9]. Recent advancements in the development of self-organizing three-dimensional organoids have addressed these limitations [10–11]. Following enzymatic digestion, endometrial tissue forms hollow, spherical organoids in specialized media, preserving genetic integrity during cell division and differentiation. These organoids can also recover secretory functions post-cryopreservation [12–13]. Furthermore, the epithelial cells of endometrial organoids (EOs) show positive responses to estrogen and progesterone, opening new avenues for research into endometrial-related disorders [5,14]. Traditional 2D endometrial cell cultures have limited lifespans and rapidly lose their phenotypic and hormonal responsiveness. In contrast, organoids preserve these characteristics and demonstrate remarkable expansion potential, overcoming the senescence typically observed in primary cell cultures [15–16]. Endometrial organoids hold promise for uncovering key intracellular signaling pathways and providing insights into the overall morphological features of tissues, thus offering a deeper understanding of human endometrial physiology and pathology at the cellular level [17–18]. When endometrial organoids are cultured in Matrigel, their apical surface faces inward, mimicking the native structure of the endometrium. In this polarized state, the epithelial barrier function is likely intact, with tight cell junctions and minimal antigen exposure, resulting in lower susceptibility to pathogen infection. However, when Matrigel is removed and the organoids are transferred to low-adhesion plates, the polarity is reversed, and the epithelial surface is exposed outward. This change increases the likelihood of pathogen interaction, as the exposed cell surface may facilitate pathogen attachment. The outward-facing polarity may also lead to looser cell junctions or expose specific receptors, further promoting pathogen adhesion and infection.
In endometritis, pathogens first encounter the surface of the endometrial epithelium before initiating infection. The organoids we have constructed resemble the deeper glandular structures of the endometrium, where the epithelial surface is located at the center of the glands. In humans, under the influence of hormones, these glands gradually move upward and extend, eventually forming the endometrial epithelium, with the epithelial surface becoming exposed. Therefore, we reverse the polarity of the organoids to replicate this process, ensuring that the epithelial surface is on the exterior, which is critical for subsequent experimental procedures, such as pathogen infection studies. This polarity reversal allows for a more accurate simulation of how pathogens interact with the surface epithelium during infection, improving the relevance and reliability of our model. Our novel endometrial organoid infection model addresses these limitations by optimizing bacterial exposure parameters, enhancing infection consistency, and maintaining organoid viability. This improved model closely mimics the in vivo endometrial microenvironment, providing a robust platform for investigating host–pathogen interactions, inflammatory responses, and potential therapeutic interventions. Additionally, this protocol may be adapted for studying other bacterial pathogens or exploring the impact of various environmental factors on endometrial health.
Materials and reagents
Biological materials
1. 0.5 × 1.0 cm human endometrial tissue (generated in patients undergoing hysteroscopic surgery)
2. Escherichia coli (ATCC-25922)
Reagents
1. Collagenase II (Sigma, catalog number: C2-28-100MG)
2. Collagenase IV (Sigma, catalog number: C4-28-100MG)
3. Y-27632 (MCE, catalog number: H9. Y-10071/CS-0131)
4. Matrigel (Corning, catalog number: 356255)
5. DMEM/F12 (HyClone, catalog number: SH30272.01)
6. B27 (Gibco, catalog number: 17504-044)
7. N2 supplement (Gibco, catalog number: 17502-048)
8. A83-01 (MCE, catalog number: HY-10432)
9. EGF (MCE, catalog number: HY-P7109)
10. Rspondin-1 (MCE, catalog number: HY-P7114)
11. Noggin (MCE, catalog number: HY-P7051A)
12. Dimethyl sulfoxide (DMSO) (Sigma, catalog number: D2650)
13. TrypLE Express (Gibco, catalog number: 12604-013)
14. Cell recovery solution (Corning, catalog number: 354270)
15. Antibiotic-antifungal (MCE, catalog number: HY-K1058)
16. LB broth (Solarbio, catalog number: L8291)
17. Fetal bovine serum (FBS) (Gibco, catalog number: A5669710)
18. Cryopreservation solution (Gibco, catalog number: 12648010)
19. PBS (Gibco, catalog number: 10010001)
20. Advanced DMEM/F-12 (Gibco, catalog number: 12634028)
21. Estradiol (MCE, catalog number: HY-B0141)
22. Progesterone (MCE, catalog number: HY-N0437)
Solutions
1. Endometrial organoid culture medium (see Recipes)
2. Collagenase type IV (2× and 1× solutions) (see Recipes)
3. Collagenase type II (2× and 1× solutions) (see Recipes)
Recipes
1. Endometrial organoid culture medium
First, prepare the growth factor stock solutions. Dissolve 50 μg of EGF powder in 500 μL of ddH2O to obtain a 100 μg/mL EGF solution. Aliquot 100 μL per tube and store at -20 °C for up to 3 months. Further dilute 50 μL of the 100 μg/mL EGF solution with 450 μL of ddH2O to achieve a final concentration of 10 μg/mL, and store under the same conditions. Similarly, dissolve 50 μg of Noggin powder in 500 μL of ddH2O to obtain a 100 μg/mL Noggin solution and store at -20 °C for up to 3 months. Dissolve 50 μg of R-spondin-1 powder in 500 μL of ddH2O to prepare a 100 μg/mL R-spondin-1 solution and store at -20 °C for up to 3 months. For small-molecule inhibitors, dissolve 5 mg of A83-01 powder in 2.5 mL of DMSO to obtain a 2 mg/mL (4.7 mM) A83-01 stock solution and store at -20 °C for up to 3 months. Similarly, dissolve 5 mg of Y-27632 powder in 1 mL of DMSO to prepare a 5 mg/mL (20 mM) Y-27632 stock solution and store at -20 °C for up to 3 months. Next, prepare the endometrial organoid culture medium by adding 400 μL of B27, 200 μL of N2, 100 μL of EGF (10 μg/mL), 20 μL of Noggin (100 μg/mL), 20 μL of R-spondin-1 (100 μg/mL), 2.1 μL of A83-01 (4.7 mM), and 10 μL of Y-27632 (20 mM) into 19.2 mL of DMEM/F12. Mix thoroughly with a pipette and store at 4 °C until use (maximum 3 days).
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM/F12 | - | 19.2 mL |
| B27 (50×) | 1× | 400 μL |
| N2 (100×) | 1× | 200 μL |
| EGF (10 μg/mL) | 50 ng/mL | 100 μL |
| Noggin (100 μg/mL) | 100 ng/mL | 20 μL |
| Rspondin-1 (100 μg/mL) | 100 ng/mL | 20 μL |
| A83-01 (4.7 mM) | 0.5 μM | 2.1 μL |
| Y-27632 (20 mM) | 10 μM | 10 μL |
2. Collagenase type IV
Dilute 0.02 g of collagenase type IV powder in 10 mL of PBS and sterilize the solution by passing it through a 0.22 μm filter. 2× aliquots can be stored at -20 °C for ≤ 6 months. Bring collagenase type IV (1×) to room temperature before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Collagenase type IV | 2 mg/mL | 0.02 g |
| PBS | 1× | 10 mL |
| Total (optional) | 2 mg/mL | 10 mL |
3. Collagenase type II
Dilute 0.02 g of collagenase type II powder in 10 mL of PBS and filter sterilize. 2× aliquots can be stored at -20 °C for ≤ 6 months. Bring collagenase type II (1×) to room temperature before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Collagenase type II | 2 mg/mL | 0.02 g |
| PBS | 1× | 10 mL |
| Total (optional) | 2 mg/mL | 10 mL |
Laboratory supplies
1. 15 mL conical tubes (Thermo, catalog number: 339659)
2. 50 mL conical tubes (Thermo, catalog number: 0644320)
3. 10 μL pipette tips (Thermo, catalog number: 9400303)
4. 300 μL pipette tips (Thermo, catalog number: 9401253)
5. 1,000 μL pipette tips (Thermo, catalog number: 9401115)
6. Nunclon multi dish 24 wells (Thermo, catalog number: 142475)
7. Nunc EasYDish 100 mm (Thermo, catalog number: 150466)
8. Ultra-low attachment plates (Corning, catalog number: 3473)
9. Cryogenic vials (Corning, catalog number: CLS430488)
10. Agar plates (Corning, catalog number: 1.46231)
11. Filters (Corning, catalog number: CLS431220)
12. Cryovials (BRAND, catalog number: Z334081)
13. Pipettes (Thermo, catalog number: 4651080N)
14. Pipette tips (Thermo, catalog number: 9401113)
Equipment
1. Refrigerator (2–8 °C)
2. Freezer (-20 °C, -80 °C)
3. Water bath (37 °C) (KUANSON, model: J-HH-2A)
4. NordicSafe® Class II biological safety cabinet (ESCO, model: NC2-L)
5. Laboratory centrifuge with rotors for 15 and 50 mL conical tubes (Thermo, catalog number: 75009740)
6. HeracellTM VIOS 160i CO2 incubator (Thermo, catalog number: 51033547)
7. Forceps (Thermo, catalog number: DS0399-0002)
8. Cell counter (MARIENFELD, catalog number: AP-0650010)
9. Microscope (OLYMPUS, catalog number: IX53)
10. Bacterial incubator (Thermo, catalog number: 360)
11. Spectrophotometer (Thermo, catalog number: 840-297000)
12. Cell shaker (Thermo, catalog number: 889501D)
Procedure
A. Endometrial tissue isolation and digestion
1. Prepare 10 culture dishes (100 mm) in advance to clean the endometrial tissues.
2. Prepare PBS supplemented with 4 μL of 10 μM Y-27632 and 100 μL of antibiotic-antifungal (100×; final concentrations: penicillin 100 U/mL, streptomycin 0.1mg/mL, and amphotericin B 0.25 μg/mL) per 10 mL of PBS. Use 10 mL of this supplemented PBS to wash the endometrial tissue in each dish. All procedures should be performed in a biosafety cabinet.
Note: Tissue samples should preferably be processed for isolation on the same day. If immediate processing is not possible, they can be stored at 4 °C overnight to preserve viability.
3. Gently agitate the tissue during washing to remove clots and debris. Perform 10 sequential washes (Figure 1A).
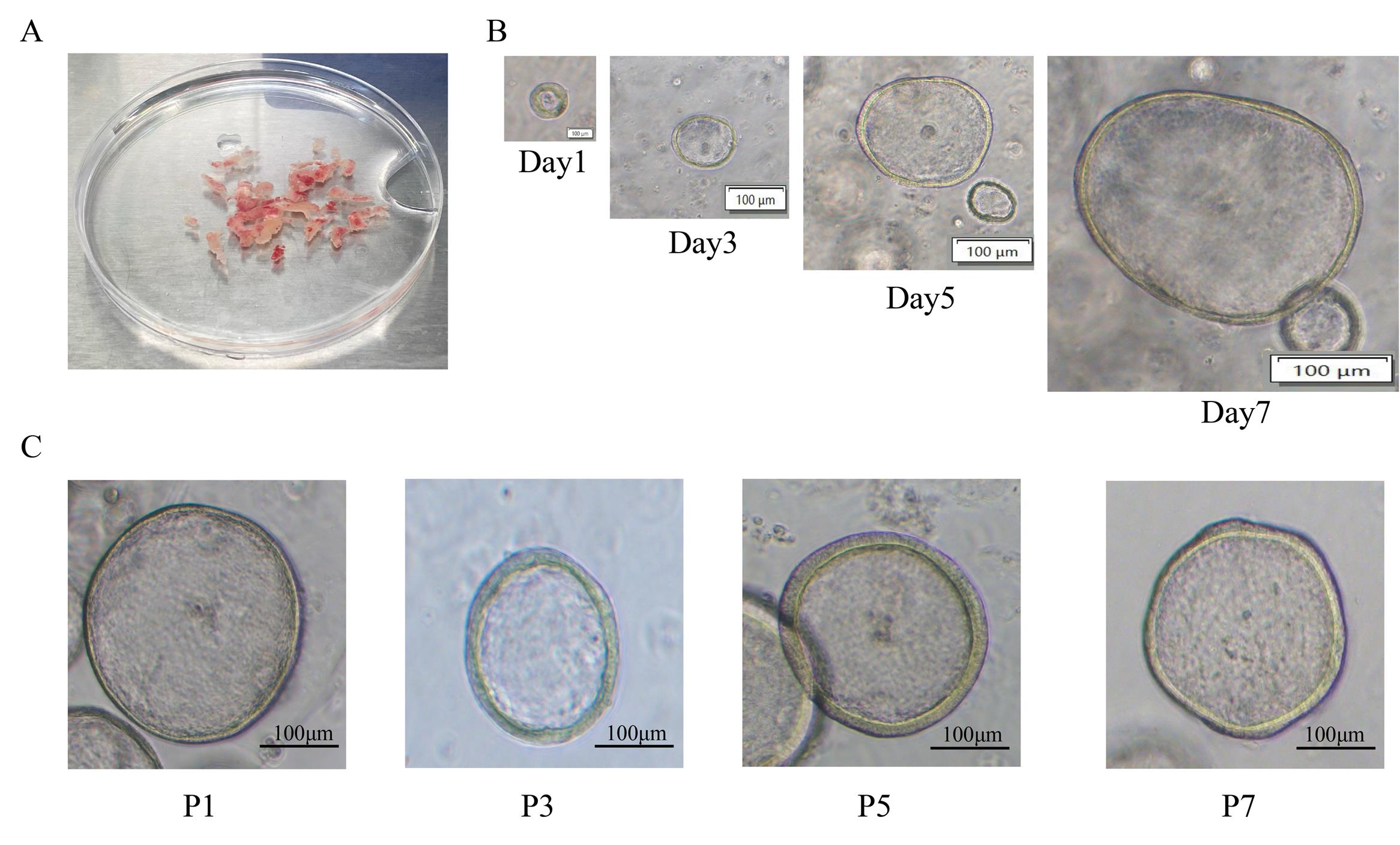
Figure 1. Morphology of endometrial organoids during tissue isolation, digestion, and culture. (A) Representative images of endometrial tissue morphology after PBS washing. (B) Brightfield images of endometrial organoids at Days 1, 3, 5, and 7 of culture. Scale bar: 100 μm. (C) Brightfield images of endometrial organoids at passages P1, P3, P5, and P7 following passaging. Scale bar: 100 μm.
4. Transfer the washed tissue into DMEM/F12 culture medium for further processing.
5. Using pre-sterilized forceps, cut the tissue into small fragments (~0.1 × 0.1 cm).
6. Transfer the dissected tissue pieces into a 50 mL sterile centrifuge tube.
7. Centrifuge the tissue at 100× g for 5 min at 4 °C.
8. Using a pipette, carefully discard the supernatant without disturbing the tissue pellet.
Notes:
1. Before digestion, thoroughly wash the tissue to remove blood clots. Incomplete cleaning may affect subsequent experiments.
2. Ensure that the endometrial tissue remains on ice throughout the entire procedure to preserve cellular integrity and viability.
9. Add 3 mL of enzyme digestion solution to the tissue pellet. Prepare the digestion solution by mixing Collagenase II and Collagenase IV at a final concentration of 1 mg/mL in a 1:1 ratio.
10. Incubate the mixture in a 37 °C water bath for 30 min.
11. Gently agitate the mixture intermittently to ensure uniform exposure of the tissue to the enzyme solution.
12. After 30 min, terminate the digestion by adding 6mL of DMEM/F12 culture medium prewarmed to 37 °C, which is twice the volume of the digestion mixture.
13. Centrifuge the mixture at 100× g for 5 min at 4 °C.
14. Carefully discard the supernatant without disturbing the pellet. The tissue is now ready for further processing.
B. Organoid seeding and establishment
1. After the endometrial tissue is separated and digested, the cells are counted using a hemocytometer. Harvest endometrial cells from culture and resuspend them in organoid culture medium pre-cooled to 4 °C at a concentration of 10,000 cells per 50 μL of Matrigel. Mix the suspension with Matrigel to achieve a final Matrigel concentration of 70%.
2. Using a pipette, dispense 50 μL of the cell suspension into each well of a 24-well plate, with three droplets per well. The droplet is 50 μL in total per well.
3. Incubate the plate in a 37 °C incubator with 5% CO2, placing it in an inverted position for 10 min, then returning it to the upright position for 20 min to allow organoid establishment.
4. Add 500 μL of prewarmed (37 °C) endometrial organoid culture medium to each well.
5. Change the endometrial organoid culture medium every three days to maintain optimal organoid growth (Figure 1B).
Notes:
1. Ensure Matrigel droplets are evenly spaced to prevent merging during incubation.
2. Handle Matrigel quickly to maintain its consistency and prevent premature polymerization.
3. Minimize disturbances to the plate during the initial incubation period to ensure proper organoid formation.
C. Medium change for organoids and organoid passaging
1. Prewarm the organoid culture medium and PBS at 37 °C before use.
2. Tilt the 24-well plate gently.
3. Carefully aspirate the old medium using a 1 mL pipette tip, ensuring the tip does not touch the Matrigel layer.
4. Add 500 μL of prewarmed PBS to each well to wash the organoids, ensuring that the plate remains horizontal.
5. Aspirate and discard the PBS.
6. Add 500 μL of prewarmed fresh organoid culture medium to each well.
7. Return the culture plate to the 37 °C incubator for continued incubation.
8. On Day 7 of endometrial organoid culture, wash the organoids once with prewarmed PBS.
9. Add 800 μL of cold cell recovery solution to each well.
10. Gently scrape the bottom of the 24-well plate using a bent 10 μL pipette tip to detach the Matrigel layer.
Note: For organoids remaining at the bottom, wash the well again with cold cell recovery solution to recover them.
11. Rinse the pipette tip with FBS to reduce cell loss and transfer the collected suspension into a 15 mL centrifuge tube.
12. Incubate the tube on ice at 4 °C with gentle shaking at 80 rpm for 1–1.5 h.
13. Centrifuge the suspension at 100× g for 5 min at 4 °C.
14. Discard the supernatant and resuspend the pellet in 4 mL of pre-cooled PBS.
15. Centrifuge again at 100× g for 5 min and carefully discard the supernatant while keeping the sample at 4 °C.
16. Resuspend the pellet in 200 μL of cold DMEM/F12.
17. Pipette the suspension up and down approximately 30–80 times to dissociate the organoids. Monitor the size of the cell clusters under a microscope and stop pipetting once 10–20 clusters are visible.
Note: Inspect for residual Matrigel. If residual gel remains, repeat the washing step with 4 mL of DMEM/F12, centrifuge, and discard the supernatant.
18. Resuspend the final pellet in 50 μL of a mixture of organoid culture medium and Matrigel, then transfer the 50 μL suspension into each single well of a 24-well plate.
19. Incubate the plate for 30 min in a 37 °C incubator with 5% CO2 to allow organoid establishment. If most organoids remain intact, proceed to the next passage (Figure 1C).
Note: To monitor organoid integrity during pipetting:
i. If extensive fragmentation occurs, add 1 mL of TrypLE to the pellet.
ii. Incubate the suspension at 37 °C for 3 min.
iii. Neutralize TrypLE by adding 500 μL of AdF12.
iv. Centrifuge at 100× g for 5 min, discard the supernatant, resuspend the pellet in 600 μL of AdF12, centrifuge again at 100× g for 5 min, and discard the supernatant.
D. Cryopreservation and thawing of endometrial organoids
1. Prewarm PBS to 37 °C.
2. On Day 8 of endometrial organoid culture, wash the organoids once with prewarmed PBS to remove residual medium.
3. Add 800 μL of cold cell recovery solution to each well of a 24-well plate.
4. Gently scrape the bottom of the well using a curved 10 μL pipette tip to detach the Matrigel.
5. Collect detached organoids into a 15 mL centrifuge tube. If organoids remain at the bottom, wash the well again with cold cell recovery solution and transfer the liquid to the same tube.
6. Rinse the pipette tip with FBS and transfer the recovered suspension into a centrifuge tube.
7. Place the tube in a 4 °C ice box and rotate at 80 rpm for 1.5 h to fully dissolve the Matrigel.
8. Centrifuge at 100× g for 5 min at 4 °C.
9. Discard the supernatant and wash the pellet with 4 mL of pre-cooled PBS.
10. Centrifuge again under the same conditions and discard the supernatant.
11. Resuspend the pellet in 200 μL of cold DMEM/F12 and pipette 50 times to break up the organoids.
12. Examine the organoids under a microscope to ensure most remain intact, with cluster sizes of 20–30 cells. Organoids should be slightly larger than those used for passaging.
13. Add 1 mL of cryopreservation solution to the resuspended organoid clusters.
14. Transfer the suspension into cryogenic vials and immediately store them in a -80 °C freezer for long-term preservation.
E. Thawing of endometrial organoids
1. Retrieve a cryogenic vial from the -80 °C freezer.
2. Immediately place the vial in a 37 °C water bath.
3. Gently shake the vial until the contents are completely thawed.
Caution: Avoid prolonged exposure to high temperatures to minimize cell damage.
4. Transfer the thawed organoid suspension into a pre-cooled 15 mL centrifuge tube.
5. Slowly add 5 mL of prewarmed organoid culture medium while gently mixing.
6. Centrifuge at 100× g for 5 min at room temperature and discard the supernatant.
7. Retain the pellet containing organoids.
8. Add 2 mL of prewarmed organoid culture medium containing 10 μM Y-27632.
9. Gently pipette up and down to resuspend the organoids.
10. Observe under a microscope to ensure the organoids remain intact.
11. Place the Matrigel on ice to pre-chill and liquefy.
12. Mix the organoid suspension with Matrigel to achieve a final Matrigel concentration of ~70%.
13. Pipette a total of 50 μL of the mixture into each prewarmed well of a 24-well plate, dividing it into three equal Matrigel droplets per well. Ensure that the total volume per well is 50μL.
14. Invert the plate and incubate at 37 °C for 10 min, then return it to the upright position for an additional 20 min to allow full polymerization.
15. Slowly add 500 μL of prewarmed organoid culture medium to each well.
16. Incubate the plate in a 37 °C, 5% CO2 incubator.
17. Replace with fresh organoid culture medium every 3 days to support growth.
F. Hormone treatment
1. After the 3-day establishment period, treat the organoids by adding 500 μL of organoid culture medium containing 10 nM estradiol (E2) to each well; use three wells per group.
2. As a control, add 500 μL of organoid culture medium without estradiol to the designated wells and continue culturing for the same number of days as the estrogen and progesterone treatment.
3. Incubate the plate in a 37 °C incubator with 5% CO2 for 3 days.
4. After the initial 3-day treatment, carefully aspirate the medium from each well without disturbing the Matrigel droplets.
5. Add 500 μL of organoid culture medium containing 10 nM estradiol (E2) and 1 μM progesterone (P4).
Note: Treat the organoids with E2 alone for 3 days, followed by E2 + P4 for 4 days.
6. Incubate the organoids in a 37 °C incubator with 5% CO2 for an additional 4 days.
7. Treat each condition in triplicate wells for reproducibility.
8. Prepare an additional fourth well for each condition for histological analysis.
G. Generation and propagation of apical-out endometrial organoids (AO-EOs)
1. Aspirate the growth medium from the cultured endometrial organoids.
2. Add 1 mL of cell recovery solution pre-cooled to 4 °C to the culture to facilitate organoid dissociation.
3. Using wide-bore 1 mL pipette tips, transfer the organoid–cell mixture into Eppendorf tubes.
4. Gently rotate the tubes using a cell shaker at 60 rpm at 4 °C for 1 h to allow complete dissociation.
5. Centrifuge the dissociated organoids at 100× g for 5 min at room temperature to pellet the cells.
6. Carefully aspirate the supernatant and wash the pellet with 7 mL of cold DMEM/F12 to remove any residual recovery solution; perform the entire procedure on ice.
7. Resuspend the washed organoid pellet in 5 mL of organoid culture medium to enhance cell survival and prevent anoikis in suspension cultures.
8. Seed the resuspended organoids into ultra-low attachment plates to promote the formation of apical-out endometrial organoids.
9. Gently agitate the culture twice daily using a pipette to prevent organoid clumping.
10. Replace the growth medium every other day to maintain optimal conditions for organoid proliferation and apical-out orientation (Figure 2).
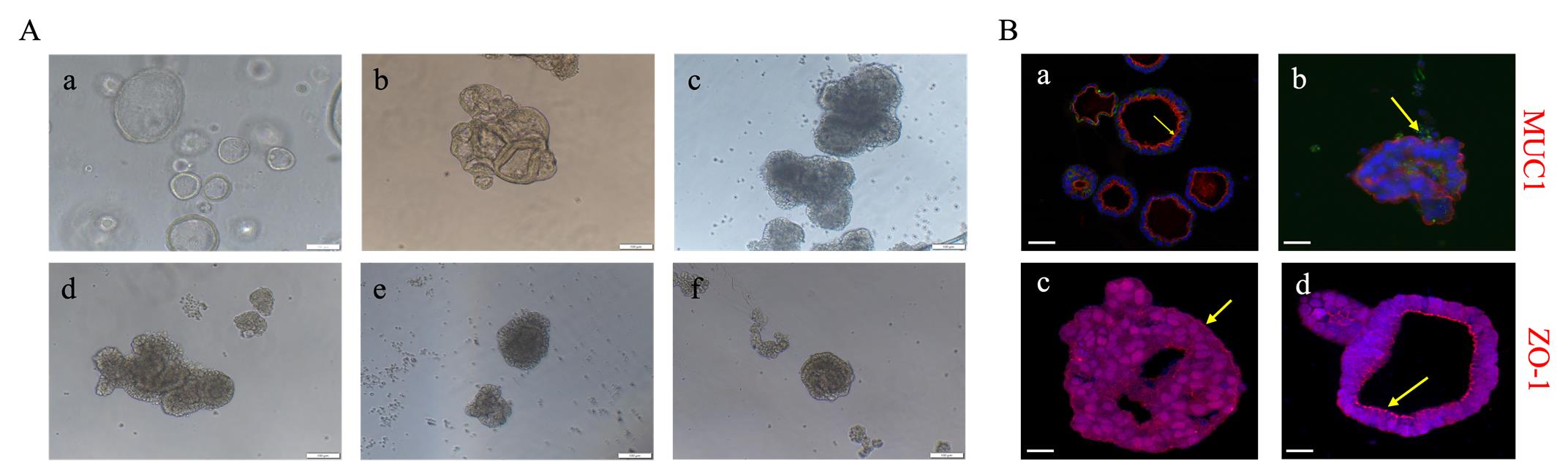
Figure 2. Formation process of apical-out endometrial organoids. (A) Sequential process of apical-out transformation in endometrial organoids: (a) before apical-out, (b) 1.5 h after apical-out induction, (c) 1 day, (d) 2 days, (e) 4 days, and (f) 6 days post-induction. Scale bar: 100 μm. (B) Immunofluorescence staining of MUC1 and ZO-1 in endometrial organoids before and after apical-out transformation. (a) MUC1 staining of endometrial organoids; (b) MUC1 staining of polarity-reversed endometrial organoids; (c) ZO-1 staining of endometrial organoids; (d) ZO-1 staining of polarity-reversed endometrial organoids. The arrows indicate red fluorescence signals of MUC1 and ZO-1. Scale bar: 20 μm.
H. Co-culture of organoids with bacteria
1. Obtain Escherichia coli (ATCC-25922) from the Microecology Laboratory of Beijing Obstetrics and Gynecology Hospital.
2. Streak the bacterial strain onto LB agar plates and incubate at 37 °C to allow colony formation.
3. Pick isolated colonies from the agar plates and inoculate them into 5 mL of LB broth.
4. Incubate the bacterial culture at 37 °C with shaking at 120 rpm to promote bacterial growth.
5. Measure the bacterial concentration using a spectrophotometer by determining the optical density at 600 nm (OD600).
6. Ensure bacteria are in their exponential growth phase to maximize viability and infectivity.
7. Adjust the bacterial suspension to achieve a multiplicity of infection (MOI) of 1. Each organoid contains approximately 1 × 103 cells, and the required bacterial load for infection is 1.0 × 103 CFU per organoid. Prepare the bacterial suspension by diluting the 0.5 McFarland standard bacterial solution (1.0 × 108 CFU/mL) in the corresponding culture medium volume to achieve a final bacterial concentration of 1.0 × 103 CFU per organoid.
8. Resuspend the prepared AO-EOs in organoid culture medium.
9. Combine the EOs with bacterial cells in a tissue culture incubator.
10. Rotate the co-culture at 40rpm for 3 h at 37 °C to promote bacterial interaction.
11. Following incubation, remove excess bacterial cells by washing the organoids five times with 10 mL of PBS via centrifugation at 80× g to minimize non-adherent bacteria.
12. Incubate the co-cultured organoids in 2 mL of fresh organoid culture medium for a recovery period of 24 h.
13. At predetermined time points, collect 500 μL of the culture medium supernatant (Figure 3).
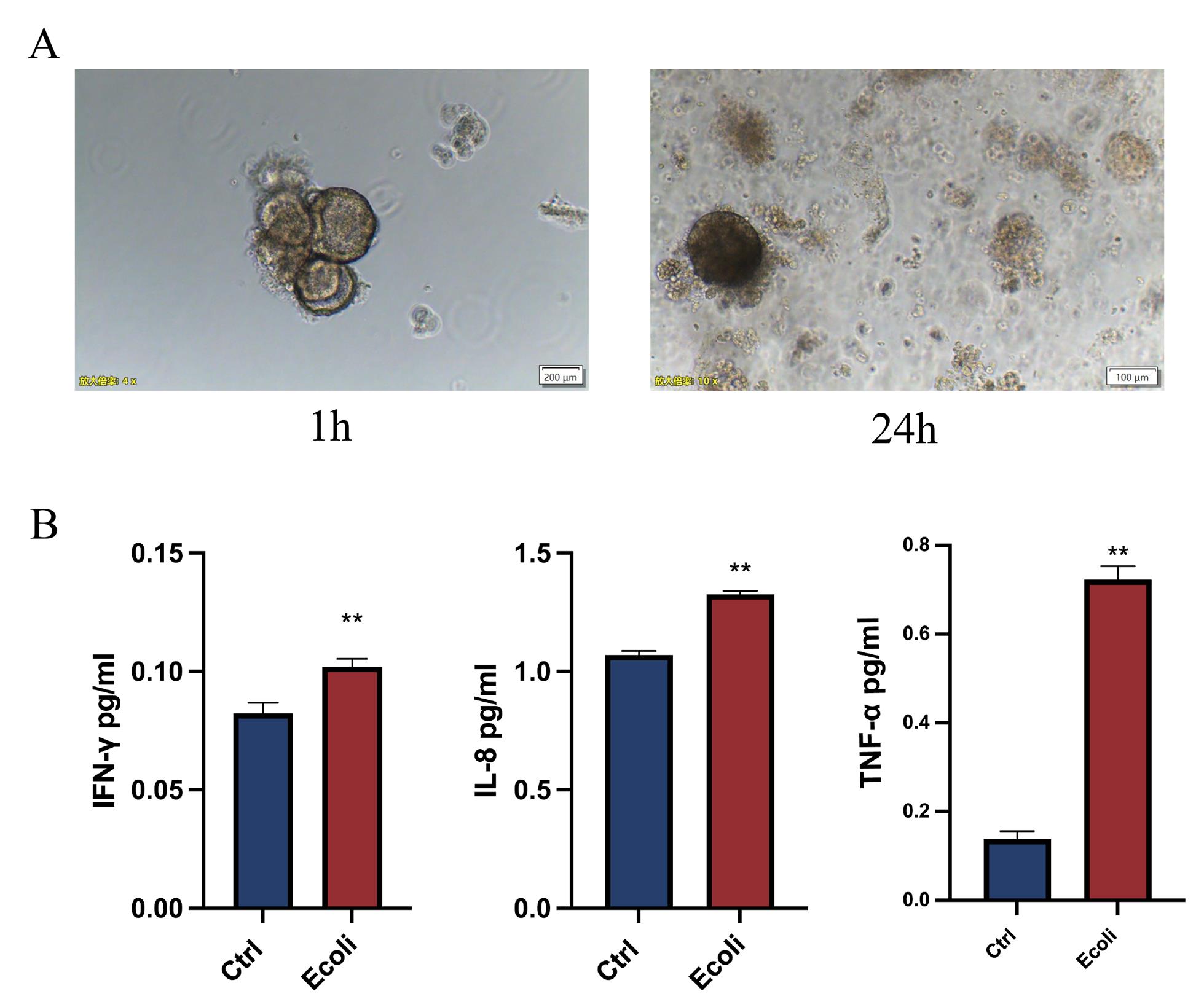
Figure 3. Escherichia coli infection in endometrial organoids. (A) Brightfield images of endometrial organoids at 1 h and 24 h post-infection with E. coli. Scale bar: 200 μm. (B) Expression levels of inflammatory factors compared between endometrial organoids infected with E. coli and uninfected controls. The results are shown as the mean ± SD and are representative of three independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article(s):
• Zhang et al. [19] Investigating bacteria-induced inflammatory responses using novel endometrial epithelial gland organoid models. Front Reprod Health (Figures 1–6).
• Zhang et al. [20] Comparative analysis of organoid, air-liquid interface, and direct infection models for studying pathogen–host interactions in endometrial tissue. Sci Rep (Figures 1–3).
General notes and troubleshooting
General notes
1. Handling and storage of Matrigel: Matrigel should be handled with care as it can degrade when exposed to temperatures above 4 °C. Always store it on ice and work quickly when preparing organoid culture setups to prevent premature solidification. If using Matrigel for embedding during infection, ensure that the temperature is kept controlled (e.g., using ice) to prevent it from prematurely solidifying during organoid seeding.
2. Medium and supplements: The culture medium used for both organoid maintenance and infection should be carefully prepared to ensure consistency. Variations in supplement concentration (e.g., EGF, FGF-10, and R-Spondin-1) can significantly affect organoid growth and immune responses. Always prepare fresh batches of medium and consider adjusting growth factor concentrations if the organoids show signs of stress or insufficient growth.
3. Weekend work can be avoided by increasing medium volumes during medium changes on Fridays (4 mL/well for 6-well plates and 1 mL/well for 24-well plates).
Troubleshooting
Problem 1: Organoids do not form or fail to grow properly.
Possible cause: Inadequate Matrigel concentration or improper culture medium composition.
Solution: Ensure that Matrigel is diluted to the correct concentration (typically 60%–70%) and that the culture medium contains all necessary growth factors and supplements. Check the consistency of the medium, including EGF, FGF-10, and R-Spondin-1, and ensure they are freshly prepared.
Problem 2: Inconsistent bacterial infection or poor adhesion to organoid epithelial cells.
Possible cause: Incorrect bacterial concentration or improper MOI (multiplicity of infection).
Solution: Confirm that bacteria are in their exponential growth phase and that the bacterial concentration is accurately measured (e.g., OD600). Ensure the MOI is appropriate (usually 1:1) and that organoids are properly suspended during infection, possibly using a rotation shaker to enhance bacterial contact.
Problem 3: High levels of organoid apoptosis or cell death post-infection.
Possible cause: Excessive bacterial load or insufficient immune response activation.
Solution: Reduce the bacterial concentration and MOI to prevent overwhelming the organoids. Consider adjusting the duration of infection to allow for controlled immune response activation. Additionally, verify that the appropriate immune modulators (e.g., TLR ligands) are included in the culture medium to balance immune activation without causing excessive cell death.
Acknowledgments
X.Z. conducted the research and drafted the manuscript. L.Z. assisted with manuscript revision and the construction of figures and tables. Z.H.L. and L.Y.F. contributed to the conceptual framework, supervised the study, and revised the manuscript. All authors have read and approved the final manuscript. This work was supported by the R&D Program of the Beijing Municipal Education Commission (KM202410025006). This protocol is adapted from Xin Zhang et al. [19,20].
Competing interests
The authors declare that they have no competing interests.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki. All analyses involving patient and human samples adhered to the guidelines and procedures of the Beijing Obstetrics and Gynecology Hospital, Capital Medical University. The Institutional Review Board (IRB number: 2023-KY-011-02) approved the appropriate licenses and protocols, and informed consent was obtained from all participating patients.
References
- Singh, N. and Sethi, A. (2022). Endometritis - Diagnosis,Treatment and its impact on fertility - A Scoping Review. JBRA Assist Reprod.: e20220015. https://doi.org/10.5935/1518-0557.20220015
- Jiang, T. and Xu, X. (2024). Protective effect of Timosaponin AIII on Escherichia coli-induced endometritis in mice through inhibiting inflammatory response and regulating uterine microbiota structure. Int Immunopharmacol. 130: 111649. https://doi.org/10.1016/j.intimp.2024.111649
- Liu, J., Tang, X., Chen, L., Zhang, Y., Gao, J. and Wang, A. (2024). Microbiome dysbiosis in patients with chronic endometritis and Clostridium tyrobutyricum ameliorates chronic endometritis in mice. Sci Rep. 14(1): 12455. https://doi.org/10.1038/s41598-024-63382-4
- Tang, S., Parks, S. E., Liao, Z., Cope, D. I., Blutt, S. E. and Monsivais, D. (2023). Establishing 3D Endometrial Organoids from the Mouse Uterus. J Visualized Exp.: e3791/64448. https://doi.org/10.3791/64448
- Jiang, Y., Palomares, A. R., Munoz, P., Nalvarte, I., Acharya, G., Inzunza, J., Varshney, M. and Rodriguez-Wallberg, K. A. (2024). Proof-of-Concept for Long-Term Human Endometrial Epithelial Organoids in Modeling Menstrual Cycle Responses. Cells. 13(21): 1811.https://doi.org/10.3390/cells13211811
- Caliari, S. R. and Burdick, J. A. (2016). A practical guide to hydrogels for cell culture. Nat Methods. 13(5): 405–414. https://doi.org/10.1038/nmeth.3839
- Gęgotek, A., Atalay, S., Domingues, P. and Skrzydlewska, E. (2019). The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells. 8(9): 995. https://doi.org/10.3390/cells8090995
- Sebők, C., Tráj, P., Vörösházi, J., Mackei, M., Papp, M., Gálfi, P., Neogrády, Z. and Mátis, G. (2021). Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures. Cells. 10(8): 1910. https://doi.org/10.3390/cells10081910
- Lucas, E. S., Salker, M. S. and Brosens, J. J. (2013). Uterine plasticity and reproductive fitness. Reprod Biomed Online. 27(5): 506–514. https://doi.org/10.1016/j.rbmo.2013.06.012
- Leung, M. M., Swanton, C. and McGranahan, N. (2025). Integrating model systems and genomic insights to decipher mechanisms of cancer metastasis. Nat Rev Genet.: e1038/s41576–025–00825–2. https://doi.org/10.1038/s41576-025-00825-2
- Dobric, A. and Tape, C. J. (2025). High-dimensional signalling analysis of organoids. Curr Opin Cell Biol. 94: 102488. https://doi.org/10.1016/j.ceb.2025.102488
- Hwang, S. Y., Lee, D., Lee, G., Ahn, J., Lee, Y. G., Koo, H. S. and Kang, Y. J. (2024). Endometrial organoids: a reservoir of functional mitochondria for uterine repair. Theranostics. 14(3): 954–972. https://doi.org/10.7150/thno.90538
- Rizo, J. A., Ahmad, V., Pru, J. M., Winuthayanon, S., Challa, S., Kim, T. H., Jeong, J. W., Spencer, T. E. and Kelleher, A. M. (2025). Uterine organoids reveal insights into epithelial specification and plasticity in development and disease. Proc Natl Acad Sci USA. 122(5): e2422694122. https://doi.org/10.1073/pnas.2422694122
- Zhang, R., Yang, Y., Li, R., Ma, Y., Ma, S., Chen, X., Li, B., Li, B., Qi, X., Ha, C., et al. (2025). Construction organoid model of ovarian endometriosis and the function of estrogen and progesterone in the model. Sci Rep. 15(1): e1038/s41598–025–90329–0. https://doi.org/10.1038/s41598-025-90329-0
- Turco, M. Y., Gardner, L., Hughes, J., Cindrova-Davies, T., Gomez, M. J., Farrell, L., Hollinshead, M., Marsh, S. G. E., Brosens, J. J., Critchley, H. O., et al. (2017). Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 19(5): 568–577. https://doi.org/10.1038/ncb3516
- Mannelli, C., Ietta, F., Avanzati, A. M., Skarzynski, D. and Paulesu, L. (2015). Biological Tools to Study the Effects of Environmental Contaminants at the Feto–Maternal Interface. Dose-Response. 13(4): e1177/1559325815611902. https://doi.org/10.1177/1559325815611902
- Xu, Y., Hu, J., Lv, Q., Shi, C., Qiu, M., Xie, L., Liu, W., Yang, B., Shan, W., Cheng, Y., et al. (2023). Endometrium-derived mesenchymal stem cells suppress progression of endometrial cancer via the DKK1-Wnt/β-catenin signaling pathway. Stem Cell Res Ther. 14(1): 159. https://doi.org/10.1186/s13287-023-03387-4
- Hu, Z., Wu, Z., Liu, W., Ning, Y., Liu, J., Ding, W., Fan, J., Cai, S., Li, Q., Li, W., et al. (2024). Proteogenomic insights into early-onset endometrioid endometrial carcinoma: predictors for fertility-sparing therapy response. Nat Genet. 56(4): 637–651. https://doi.org/10.1038/s41588-024-01703-z
- Zhang, X., Zhang, L., Li, T., Zhang, Z., Shang, X., Bai, H., Liu, Y., Zong, X., Shang, C., Song, D., et al. (2024). nvestigating bacteria-induced inflammatory responses using novel endometrial epithelial gland organoid models. Front Reprod Health. 6: e1490520. https://doi.org/10.3389/frph.2024.1490520
- Zhang, X., Fan, L., Zhang, L. and Liu, Z. (2025). Comparative analysis of organoid, air-liquid interface, and direct infection models for studying pathogen–host interactions in endometrial tissue. Sci Rep. 15(1): 158531. https://doi.org/10.1038/s41598-025-93374-x
Article Information
Publication history
Received: Mar 18, 2025
Accepted: May 13, 2025
Available online: Jun 5, 2025
Published: Jun 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Zhang, X., Zhang, L., Fan, L. and Liu, Z. (2025). The Establishment of 3D Polarity-Reversed Organoids From Human Endometrial Tissue as a Model for Infection-Induced Endometritis. Bio-protocol 15(12): e5349. DOI: 10.21769/BioProtoc.5349.
Category
Cell Biology > Cell isolation and culture > 3D cell culture
Medicine > Inflammation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









