- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Expression and Purification of the Human Voltage-Gated Proton Channel (hHv1)
Published: Vol 15, Iss 12, Jun 20, 2025 DOI: 10.21769/BioProtoc.5344 Views: 1930
Reviewed by: Alessandro DidonnaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Novel Protein Purification Approach Using Elastin-Like Polypeptides (ELP) With His-Tag Assistance
Young Kee Chae and Han Bin Shin
Jun 20, 2025 3011 Views
Thermus thermophilus CRISPR Cas6 Heterologous Expression and Purification
Junwei Wei [...] Yingjun Li
Jul 20, 2025 2120 Views
Prokaryotic Expression and Purification of the hSox2-HMG Domain
Lijie Yang [...] Jingjun Hong
Aug 20, 2025 2307 Views
Abstract
The voltage-gated proton channel (Hv1) is a membrane protein that dissipates acute cell proton accumulations. To understand the molecular mechanisms explaining Hv1 function, methods for purifying the protein are needed. Previously, methods were developed for expressing and purifying human Hv1 (hHv1) in yeast and later in bacteria. However, these methodologies produced low protein yields and had high production costs, considerably limiting their usefulness. The protocol described in this work was developed to overcome those limitations. hHv1 is overexpressed in bacteria, solubilized with the detergent Anzergent 3–12, and purified by immobilized metal affinity chromatography (IMAC) and size-exclusion chromatography (SEC). Our protocol produced higher protein yields at lower costs than previously published methodologies.
Key features
• hHv1, containing a poly-His tag followed by an enterokinase cutting site in its N-terminus, is overexpressed in E. coli by autoinduction.
• The detergent Anzergent 3–12 is used to solubilize and purify hHv1 using nickel-immobilized metal affinity chromatography (IMAC).
• The entire procedure can be performed in 6 days.
Keywords: Voltage-gated proton channel (Hv1)Graphical overview
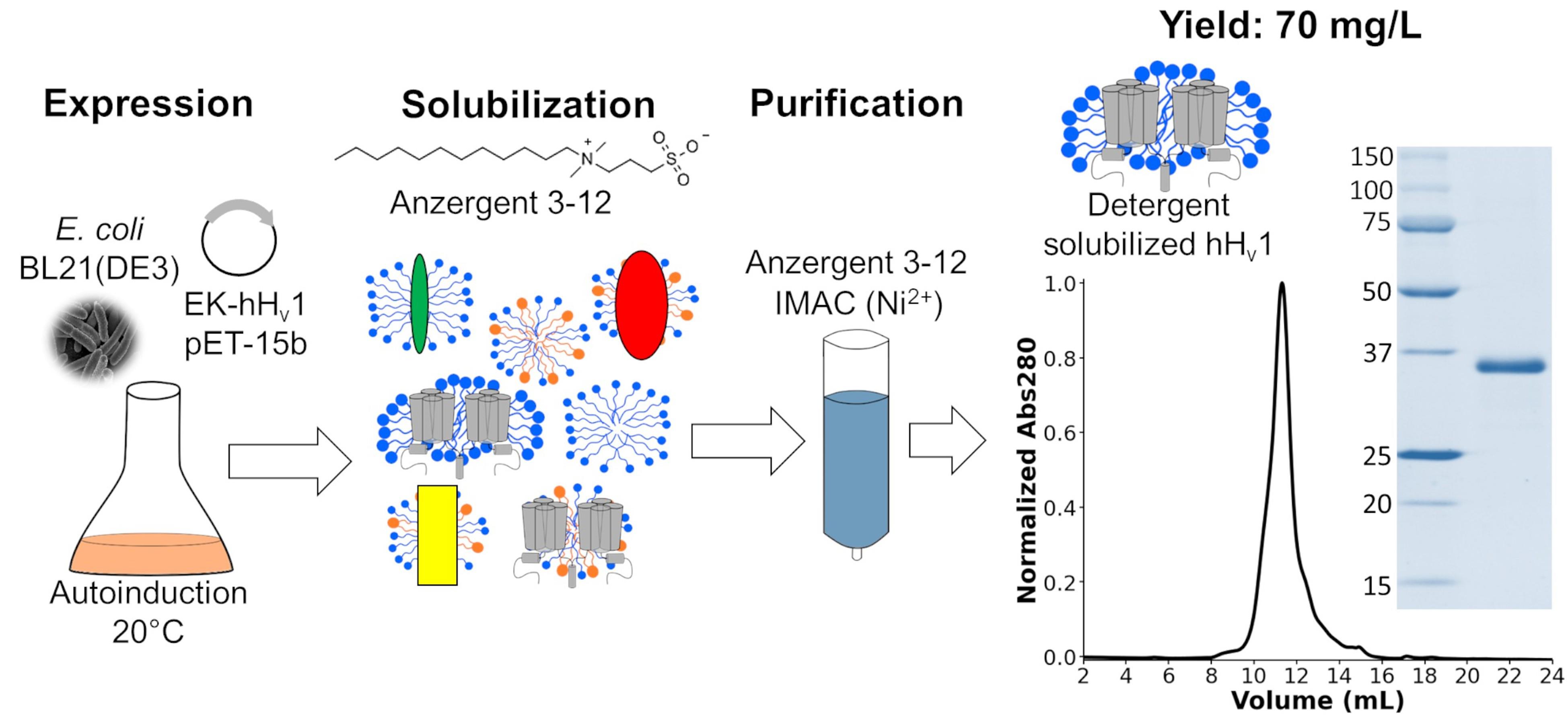
Background
The voltage-gated proton channel (Hv1) is a membrane protein that contains a highly selective permeation pathway for protons [1,2]. The opening of this proton permeation pathway is regulated by voltage [3,4], pH gradients [4,5], mechanical forces [6], and ligand binding [6–9]. The structural basis of these Hv1 biophysical properties is still poorly understood, including its permeation pathway location, sensitivity to pH gradients, mechanosensitivity, cooperativity of opening, and ligand binding sites. This missing knowledge is needed to use Hv1 as a therapeutic target for diseases such as immune disorders [10], diabetes [11], inflammatory pain [12], and cancer [13]. Most biophysical and structural studies require high quantities of stable and functional recombinant Hv1, which has been challenging to achieve. Initially, insect cells were used to express a chimeric recombinant mouse Hv1 to obtain its structure by X-ray crystallography [14]; later, the human Hv1 (hHv1) was expressed and purified using yeasts [15]. Finally, a new methodology was developed to produce hHv1 in E. coli for an electron paramagnetic resonance (EPR) study [16]. Other authors have used this last protocol to study hHv1 using nuclear magnetic resonance (NMR) [17] and single-molecule Förster energy transfer (sm-FRET) [18]. Nevertheless, this protocol had two main limitations: i) the protein yield was low (0.7 mg per liter of culture), and ii) the expensive detergents Fos-choline 12 and 14 were used to purify the protein. Recognizing these limitations, we optimized each step of the protocol to produce a new method that increased the protein yield up to 70 mg per liter of culture and decreased costs using the more economical detergent Anzergent 3–12. The final purified hHv1 protein is stable and functional [19]. Here, we describe such a protocol in detail to accelerate hHv1 research.
Materials and reagents
Biological materials
1. BL21-Gold(DE3) competent cells (Agilent, catalog number: 200131)
2. pHis-EK-hHv1.pET15-b (source and sequence in Dataset S1)
3. Mouse α-penta-His antibody (Qiagen, catalog number: 34660)
4. StarBright Blue 700 goat α-mouse IgG (Bio-Rad, catalog number: 12004158)
Reagents
1. Tryptone (Fisher, catalog number: BP1421)
2. Yeast extract (Fisher, catalog number: BP1422)
3. NaCl (Sigma-Aldrich, catalog number: S9888)
4. PEG (Sigma-Aldrich, catalog number: P3640)
5. MgSO4·7H2O (Sigma-Aldrich, catalog number: M-9397)
6. MgCl2·6H2O (Fisher, catalog number: BP214)
7. Glycerol (Fisher, catalog number: G33-4)
8. Sterile-filtered DMSO (GoldBio, catalog number: D-361)
9. Ampicillin (sodium) (GoldBio, catalog number: A-301)
10. KCl (Fisher, catalog number: P217)
11. Glucose (Sigma-Aldrich, catalog number: G6152)
12. 1,000× trace metal mixture (Teknova, catalog number: T1001)
13. Na2HPO4·7H2O (Sigma-Aldrich, catalog number: S9390)
14. KH2PO4 (Sigma-Aldrich, catalog number: P5655)
15. NH4Cl (Sigma-Aldrich, catalog number: 213330)
16. Na2SO4 (Sigma-Aldrich, catalog number: 239313)
17. Glycerol (Fisher, catalog number: G33-4)
18. α-lactose monohydrate (Sigma-Aldrich, catalog number: L2643)
19. Tris buffer (Invitrogen, catalog number: 15504020)
20. Sodium dodecyl sulfate (SDS) (Bio-Rad, catalog number: 1610302)
21. Bromophenol blue sodium salt (Sigma-Aldrich, catalog number: B7021)
22. 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
23. 10% v/v antifoam 204 (Teknova, catalog number: A6427)
24. Phenylmethanesulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626)
25. Isopropanol (Fisher, catalog number: A416)
26. Benzamidine hydrochloride hydrate (Sigma-Aldrich, catalog number: B6506)
27. Lysozyme egg white (GoldBio, catalog number: L-040)
28. DNase I, bovine pancreas (GoldBio, catalog number: D-300)
29. 10× Tris/glycine/SDS running buffer (Bio-Rad, catalog number: 1610772)
30. 12% Mini-PROTEAN® stain-freeTM protein gels (Bio-Rad, catalog number: 4568045)
31. Precision plus protein unstained standards (Bio-Rad, catalog number: 1610363)
32. Precision plus protein dual color standards (Bio-Rad, catalog number: 1610374)
33. Ethanol (Fisher, catalog number: BP28184)
34. 5× Transfer buffer (Bio-Rad, catalog number: 10026938)
35. KCl (Fisher, catalog number: P217)
36. Tween 20 (Bio-Rad, catalog number: 1706531)
37. Low-fat dry milk (Milkman)
38. TCEP HCl (GoldBio, catalog number: TCEP)
39. Anzergent 3–12 (Anatrace, catalog number: AZ312)
40. Imidazole (Thermo Scientific Chemicals, catalog number: 396745000)
41. HCl (Fisher, catalog number: AC423795000)
Solutions
1. Luria-Bertani broth (Miller) (LB) (see Recipes)
2. 1 M MgSO4 (see Recipes)
3. 1 M MgCl2 (see Recipes)
4. 50% glycerol (see Recipes)
5. Transforming and Storage Solution (TSS) (see Recipes)
6. 100 mg/mL ampicillin (see Recipes)
7. 3 M KCl (see Recipes)
8. 4 M NaCl (see Recipes)
9. 40% (w/v) glucose (see Recipes)
10. Super optimal broth with catabolite repression (SOC) (see Recipes)
11. Terrific broth (see Recipes)
12. 50× M solution (see Recipes)
13. 50 × 512 solution (see Recipes)
14. Complete autoinduction medium (AIM) (see Recipes)
15. 1 M Tris-HCl (see Recipes)
16. 4× SDS-PAGE sample buffer (see Recipes)
17. 10× buffer-H1 (see Recipes)
18. 26 mg/mL PMSF (see Recipes)
19. 200 mM benzamidine (see Recipes)
20. Lysozyme solution (see Recipes)
21. 2.5 mg/mL DNase I (see Recipes)
22. 10× Phosphate-Buffered Saline (PBS) (see Recipes)
23. PBS-Tween (PBS-T) (see Recipes)
24. Blocking solution (see Recipes)
25. 4 M NaCl (see Recipes)
26. 0.5 M TCEP (see Recipes)
27. 20% w/v Anzergent 3–12 (see Recipes)
28. Buffer-H2 (see Recipes)
29. 5 M imidazole (see Recipes)
30. Washing buffer (see Recipes)
31. Elution buffer (see Recipes)
Recipes
Note: Solutions listed below can be stored at room temperature unless indicated otherwise.
1. Luria-Bertani broth (Miller) (LB)
Prepare 500 mL of LB medium by adding the components listed below in an autoclavable bottle and dissolving them in 500 mL of ultra-pure (type 1) water. Sterilize by autoclaving at 121 °C for 30 min.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Tryptone | 10 g/L | 5 g |
| NaCl | 10 g/L | 5 g |
| Yeast extract | 5 g/L | 2.5 g |
2. 1 M MgSO4
Dissolve 24.6 g of MgSO4·7H2O in 100 mL of ultra-pure (type 1) water. Sterilize by autoclaving at 121 °C for 30 min.
3. 1 M MgCl2
Dissolve 20.3 g of MgCl2·6H2O in 100 mL of ultra-pure (type 1) water. Sterilize by autoclaving at 121 °C for 30 min.
4. 50% glycerol
Dissolve 50 mL of glycerol in 100 mL of ultra-pure (type 1) water. Sterilize by autoclaving at 121 °C for 30 min.
5. Transforming and storage solution (TSS)
Prepare 50 mL of TSS medium by dissolving the components listed below in 47.5 mL of LB medium. Adjust the pH of the solution to 6.5. Sterilize by filtration. Finally, add 2.5 mL of filter-sterilized DMSO in sterile conditions (final concentration 5% v/v). Store at 4 °C.
| Reagent | Final concentration | Quantity |
|---|---|---|
| PEG | 10% w/v | 5 g |
| 1 M MgSO4 | 20 mM | 1 mL |
| 1 M MgCl2 | 20 mM | 1 mL |
6. 100 mg/mL ampicillin
Dissolve 5 g of ampicillin in 50 mL of ultra-pure (type 1) water. Sterilize by filtration. Store at 4 °C.
7. 3 M KCl
Dissolve 22.36 g of KCl in 100 mL of ultra-pure (type 1) water.
8. 4 M NaCl
Dissolve 23.38 g of NaCl in 100 mL of ultra-pure (type 1) water.
9. 40% (w/v) glucose
Dissolve 20 g of glucose in 50 mL of ultra-pure (type 1) water. Sterilize by filtration.
10. Super optimal broth with catabolite repression (SOC)
Prepare 100 mL of SOC medium by dissolving the components listed below in ultra-pure (type 1) water. Adjust the pH of the solution to 7.0. Sterilize by filtration. Aliquot in 50 mL and store at 4 °C.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Tryptone | 20 g/L | 2 g |
| Yeast extract | 5 g/L | 0.5 g |
| 4 M NaCl | 10 mM | 250 μL |
| 3 M KCl | 2.5 mM | 83.33 μL |
| 1 M MgCl2 | 10 mM | 1 mL |
| 1 M MgSO4 | 10 mM | 1 mL |
| 40% (w/v) glucose | 20 mM | 900.9 μL |
11. Terrific broth
Prepare 960 mL of terrific broth medium by dissolving the components listed below in ultra-pure (type 1) water. Sterilize by autoclaving at 121 °C for 30 min.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Tryptone | 12 g/L | 12 g |
| Yeast extract | 24 g/L | 24 g |
12. 50× M solution
Prepare 1 L of solution by dissolving the components listed below in ultra-pure (type 1) water. Sterilize by autoclaving at 121 °C for 30 min. Filter the solution in sterile conditions to avoid crystal formation.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Na2HPO4·7H2O | 1.25 M | 335.1 g |
| KH2PO4 | 1.25 M | 170 g |
| NH4Cl | 2.5 M | 134 g |
| Na2SO4 | 0.25 M | 35.5 g |
13. 50× 512 solution
Prepare 1 L of solution by dissolving the components listed below in ultra-pure (type 1) water. Sterilize by filtration.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Glycerol | 25% w/v | 250 g |
| Glucose | 5% w/v | 50 g |
| α-lactose monohydrate | 10% w/v | 105.2 g |
14. Complete autoinduction medium (AIM)
Prepare 1 L of AIM by adding the components listed below to 960 mL of sterile terrific broth medium under sterile conditions.
| Reagent | Final concentration | Quantity |
|---|---|---|
| 1 M MgSO4 | 2 mM | 2 mL |
| 1,000× trace metal mixture | 0.2× | 200 μL |
| 50× 512 solution | 1× | 20 mL |
| 50× M solution | 1× | 20 mL |
| 100 mg/mL ampicillin | 0.4 mg/mL | 4 mL |
15. 1 M Tris-HCl
Dissolve 12.1 g of Tris buffer in 70 mL ultra-pure (type 1) water. Adjust to the desired pH with HCl. Complete to 100 mL with ultra-pure (type 1) water. Sterilize by autoclaving at 121 °C for 30 min.
16. 4× SDS-PAGE sample buffer
Prepare 50 mL of solution by adding the components listed below and complete the final volume with ultra-pure (type 1) water. Aliquot 1 mL in 1.5 mL microcentrifuge tubes and store at -20 °C. This recipe is for preparing a reducing buffer. Replace the 2-mercaptoethanol with ultra-pure (type 1) water for a non-reducing buffer.
| Reagent | Final concentration | Quantity |
|---|---|---|
| 1 M Tris pH 6.8 | 250 mM | 12.5 mL |
| SDS | 8% w/v | 4 g |
| Glycerol | 40% v/v | 20 mL |
| Bromophenol blue sodium salt | 0.4% w/v | 200 mg |
| 2-Mercaptoethanol | 20% v/v | 10 mL |
17. 10× buffer-H1
Prepare 1 L of solution by dissolving the components listed below in ultra-pure (type 1) water. Adjust the pH of the solution to 8.0. To prepare 1× solution, dilute 10 times with ultra-pure (type 1) water and adjust the pH to 8.0 if necessary.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Tris | 500 mM | 60.6 g |
| NaCl | 1.5 M | 87.7 g |
18. 26 mg/mL PMSF
Dissolve 1.3 g of PMSF in 50 mL of isopropanol. Store at room temperature, protected from light.
19. 200 mM benzamidine
Dissolve 1.64 g of benzamidine hydrochloride hydrate in 50 mL of cold ultra-pure (type 1) water. Aliquot and store at -20 °C.
20. Lysozyme solution
Prepare 50 mL of solution by supplementing cold 1× buffer-H1 with the components listed below. Prepare just before use and keep at 4 °C.
| Reagent | Final concentration | Quantity |
|---|---|---|
| 200 mM benzamidine | 1 mM | 250 μL |
| 26 mg/mL PMSF | 0.17 mg/mL | 327 μL |
| 2-Mercaptoethanol | 2 mM | 7 μL |
| Lysozyme | 0.5 mg/mL | 25 mg |
21. 2.5 mg/mL DNase I
Prepare 20 mL of solution by dissolving the components listed below in cold, ultra-pure (type 1) water. Aliquot 1 mL in 1.5 mL microcentrifuge tubes and store at -20 °C.
| Reagent | Final concentration | Quantity |
|---|---|---|
| 1 M Tris pH 7.5 | 20 mM | 0.8 mL |
| 1 M MgCl2 | 1 mM | 40 μL |
| DNase I | 2.5 mg/mL | 100 mg |
| Glycerol | 50% v/v | 20 mL |
22. 10× phosphate-buffered saline (PBS)
Prepare 1 L of solution by dissolving the components listed below in ultra-pure (type 1) water. Adjust the pH of the solution to 7.5. To prepare 1× solution, dilute 10 times with ultra-pure (type 1) water.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Na2HPO4·7H2O | 100 mM | 26.8 g |
| NaCl | 1.37 M | 80 g |
| KCl | 27 mM | 2 g |
| KH2PO4 | 18 mM | 2.4 g |
23. PBS-Tween (PBS-T)
Add 0.5 mL of Tween 20 to 1 L of 1× PBS (0.05% Tween 20).
24. Blocking solution
Dissolve 2.5 g of low-fat dry milk in 50 mL of PBS-T.
25. 4 M NaCl
Dissolve 233.76 g of NaCl in 1 L of ultra-pure (type 1) water.
26. 0.5 M TCEP
Prepare 40 mL of solution by dissolving 5.733 g of TCEP-HCl in 25 mL of chilled ultra-pure (type 1) water. Bring the solution’s pH to 7.0 with a 10 M NaOH solution (around 7.5 mL). Complete the final volume to 40 mL, aliquot, and store at -20 °C.
27. 20% w/v Anzergent 3–12
Prepare 50 mL of solution by dissolving 10 g of Anzergent 3–12 in 50 mL of ultra-pure (type 1) water. Store at 4 °C.
28. Buffer-H2
Prepare 100 mL of solution by adding the components listed below in ultra-pure (type 1) water. Adjust the pH of the solution to 8.0. Since the 1× buffer-H1 contains 150 mM NaCl, adding 350 mM NaCl results in a final concentration of 500 mM NaCl.
| Reagent | Final concentration | Quantity |
|---|---|---|
| 10× buffer-H1 | 1× | 10 mL |
| 4 M NaCl | 350 mM (500 mM total) | 8.75 mL |
| 20% w/v Anzergent 3–12 | 0.4% w/v | 2 mL |
29. 5 M imidazole
Prepare 200 mL of solution by dissolving 68.08 g of imidazole in 100 mL of ultra-pure (type 1) water. Adjust pH to 8.0. Complete volume to 200 mL with ultra-pure (type 1) water.
30. Washing buffer
Prepare 100 mL of solution by adding the components listed below in ultra-pure (type 1) water. Adjust the pH of the solution to 8.0. Since the 1× buffer-H1 contains 150 mM NaCl, adding 350 mM NaCl results in a final concentration of 500 mM NaCl. Supplement with 2 mM TCEP (400 μL of 0.5 M TCEP) and 0.17 mg/mL PMSF (654 μL of 26 mg/mL PMSF) just before use.
| Reagent | Final concentration | Quantity |
|---|---|---|
| 10× buffer-H1 | 1× | 10 mL |
| 4 M NaCl | 350 mM (500 mM total) | 8.75 mL |
| 5 M imidazole | 50 mM | 1 mL |
| 20% w/v Anzergent 3–12 | 0.4% w/v | 2 mL |
31. Elution buffer
Prepare 100 mL of solution by adding the components listed below in ultra-pure (type 1) water. Adjust the pH of the solution to 8.0. Since the 1× buffer-H1 contains 150 mM NaCl, adding 350 mM NaCl results in a final concentration of 500 mM NaCl. Supplement with 2 mM TCEP (400 μL of 0.5 M TCEP) just before use.
| Reagent | Final concentration | Quantity |
|---|---|---|
| 10× buffer-H1 | 1× | 10 mL |
| 4 M NaCl | 350 mM (500 mM total) | 8.75 mL |
| 5 M imidazole | 500 mM | 10 mL |
| 20% w/v Anzergent 3–12 | 0.4% w/v | 2 mL |
Laboratory supplies
1. Pyrex® round media storage bottles and reusable screw caps (Corning, catalog number: CLS1395)
2. Pyrex® VistaTM test tubes 25 × 150 mm (Corning, catalog number: 70800)
3. Foam plugs for test tubes and laboratory flasks (Chemglass Life Sciences, catalog number: CGE-1490)
4. Pyrex® baffled shaker flasks (Corning, catalog number: 4444)
5. Sterile 15 mL and 50 mL conical polypropylene centrifuge tubes (Thermo Scientific, catalog number: 339650)
6. Sterile 1.5 mL microcentrifuge tubes (Corning, catalog number: MCT-150-C)
7. Falcon® sterile polypropylene round-bottom tubes (Corning, catalog number: 352059)
8. PYREX® 2800 mL Fernbach-style culture flask with baffles (Corning, catalog number: 4423-2XL)
9. Glass beads, acid-washed (Sigma-Aldrich, catalog number: G8772)
10. 0.6 mL microcentrifuge tubes (Corning, catalog number: MCT-060-C)
11. 1 L polycarbonate bottle assembly (Beckman Coulter, catalog number: C31600)
12. PVDF membrane (Bio-Rad, catalog number: 1620264)
13. His-Pur Ni-NTA resin (Thermo Scientific, catalog number: 88222)
14. Econo-Pac® chromatography column (Bio-Rad, catalog number: 7321010)
15. Vivaspin® Turbo 15 PES centrifugal concentrator (Sartorius, catalog number: VS15T32)
16. ENrich SEC 650 column (Bio-Rad, catalog number: 7801650)
17. Disposable polystyrene cuvettes (Bio-Rad, catalog number: 2239955)
Equipment
1. Synergy UV R water purification system (Millipore, model: SYNSVR000)
2. Incubator shaker (Infors HT, model: Multitron, SM100116-HC)
3. Refrigerated centrifuge (Beckman Coulter, model: Avanti J-26 XPI)
4. JS-5.3 AllSpin swinging-bucket rotor and buckets (Beckman Coulter, catalog number: 368690)
5. Water bath (Fisher Scientific, model: 202S)
6. Spectrophotometer to measure OD600 (Implen, model: DiluPhotometer OD600)
7. Benchtop microcentrifuge (Fisher Scientific, model: accuSpin Micro 17R)
8. J-LITE JLA-8.1000 fixed-angle aluminum rotor (Beckman Coulter, catalog number: 363688)
9. Digital sonifier (Branson, model: SFX 250)
10. Ultracentrifuge (Beckman, model: Optima XL-80K)
11. Type 45 Ti fixed-angle titanium rotor (Beckman Coulter, catalog number: 339160)
12. Electrophoresis chamber (Bio-Rad, catalog number: 1658004)
13. Power supply (Bio-Rad, catalog number: 1645050)
14. Gel Doc EZ system (Bio-Rad, catalog number: 1708270)
15. Stain-free sample tray (Bio-Rad, catalog number: 1708274)
16. Trans-Blot® TurboTM transfer system (Bio-Rad, catalog number: 1704150)
17. ChemiDoc MP imaging system (Bio-Rad, catalog number: 12003154)
18. NGC Quest 10 Plus chromatography system (Bio-Rad, catalog number: 7880003)
19. Spectrophotometer (Thermo Scientific, model: NanoDrop Lite)
20. Steam sterilizer autoclave (Market Forge, model: STM-E)
Procedure
A. Preparation of chemically competent cells
Note: We have observed that fresh competent cells produce higher yields of recombinant protein in E. coli. Therefore, we routinely prepare our own competent cells using the procedure by Chung and Miller [20].
1. Starting from the frozen BL21-Gold(DE3) cells stock, transfer some bacteria using a pipette tip to grow a preculture in 5 mL of LB medium without antibiotics overnight at 37 °C and 250 rpm in a sterile Pyrex VistaTM test tube. To ensure the medium is sterile, prepare a control tube with 5 mL of LB medium without cells and incubate it under the same conditions as the culture.
Critical: All materials and solutions should be sterile. Procedures should be performed under sterile conditions under a Bunsen burner flame. Sterility when working with bacteria is critical to obtain final high protein yields.
2. The next day, dilute the saturated culture 1:100 in LB with no antibiotics. For instance, add 250 μL of saturated culture in 25 mL of LB in a 125 mL baffled shaker flask or 500 μL of saturated culture in 50 mL of LB in a 250 mL baffled shaker flask.
Note: The optical density at 600 nm (OD600) of the saturated culture is between 4.5 and 5.0.
3. Grow cells at 37 °C and 250 rpm until OD600 = 0.5 (around 2 h) by adding 1 mL of the culture in a cuvette and measuring its optical density at 600 nm. In the meantime, chill tubes to receive the culture on ice. Turn on and chill the centrifuge to 4 °C so it is cold to harvest cells later.
Critical: Do not overgrow cells.
4. Transfer the culture flask to ice and incubate for 30 min.
Critical: All subsequent steps are carried out at 4 °C. Keeping the cells cold is critical to obtain high transformation efficiencies.
5. Transfer cells to a sterile chilled conical tube and centrifuge at 1,200× g for 10 min at 4 °C.
6. Gently resuspend the cells in 1/10 of the initial harvested volume with chilled TSS (for instance, for 15 mL of harvested culture, resuspend in 1.5 mL of TSS).
7. Aliquot 120 μL of cells in sterile chilled 1.5 mL centrifuge tubes, flash-freeze, and store at -80 °C. These aliquots can be used for three months if kept at -80 °C.
Note: As reported by Chung and Miller [20], the typical transformation efficiency of these cells is ~107 CFU/μg DNA.
B. Transformation
1. Add 1 μL of the pHis-EK-hHv1.pET15-b plasmid (100–200 ng of DNA) to a chilled sterile polypropylene round-bottom tube on ice.
2. Thaw one aliquot of BL21-Gold(DE3) competent cells on ice.
3. Add 100 μL of competent cells to the bottom of the tube where the DNA was placed. Gently mix three times.
4. Incubate the cells with DNA for 5 min on ice.
5. Apply thermal shock by placing the tube in a water bath at 42 °C for 45 s.
6. Incubate cells on ice for 2 min at 4 °C.
7. Add 1 mL of SOC at room temperature to the tube.
8. Grow cells for 1 h at 37 °C and 250 rpm.
9. Inoculate cells in a 125 mL baffled flask containing 25 mL of LB media supplemented with 0.2% (w/v) glucose [125 μL of 40% (w/v) glucose] and 0.4 mg/mL ampicillin (100 μL of 100 mg/mL ampicillin). Grow overnight at 37 °C and 250 rpm.
C. Protein expression
Protein expression is accomplished by using the protocol for autoinduction by Studier with minor modifications [21]. Before starting the culture for expression, 960 mL of sterile terrific broth must be prepared in a 2.8 L baffled flask the previous day.
1. Prepare complete AIM (see Recipes) on the day of the experiment.
2. Dilute the saturated preculture 1:100 in the complete AIM (10 mL in 1 L).
3. Grow the culture at 37 °C and 250 rpm until the OD600 = 1.5 (around 2–3 h).
3. Transfer the culture to a shaker previously set to 20 °C.
4. Grow overnight at 20 °C and 250 rpm.
D. Cell harvesting and lysis
1. Determine the OD600 and collect samples for western blot.
a. Measure the OD600 of a 1:20 dilution of the culture (50 μL in 950 μL of LB media). Recover the OD600 of the culture by multiplying the measured OD600 by 20.
b. Take an aliquot (V) of the culture in a 1.5 mL microcentrifuge tube using the following equation: V (μL) = 800/OD600.
c. Centrifuge the sample in a benchtop microcentrifuge at 3,500× g for 5 min at 4 °C.
d. Discard the supernatant and lyse bacteria by adding 250 μL of 1× SDS-PAGE sample buffer.
Caution: Make sure to dissolve the cell pellet by pipetting it with the sample buffer. Be careful not to accidentally lose the pellet in the pipette tip during this procedure.
e. Add 300 μL of glass beads to the extract (measured with a 0.6 mL microcentrifuge tube). Vortex the sample for 2 s, 20 times.
f. Store samples at -20 °C.
2. Add 500 μL of 10% v/v antifoam 204 to the culture and mix.
3. Transfer the culture to a 1 L polycarbonate bottle to harvest cells at 4,000× g for 30 min at 4 °C.
4. Discard the supernatant and collect the cell pellet in a previously weighed 50 mL conical polypropylene centrifuge tube. Take note of the cell pellet mass (should be around 20 g).
5. Resuspend the pellet in a final volume of 40 mL with cold lysozyme solution and incubate the cell suspension for 30 min at 4 °C with gentle rotation. Store at -80 °C.
6. Thaw the cell extracts in a water bath at room temperature.
7. Supplement the thawed extract with 0.17 mg/mL PMSF, 1 mM benzamidine, 5 mM MgCl2, 2 mM 2-mercaptoethanol, and 12.5 μg/mL DNase I.
8. Incubate the extract for 1 h at 4 °C with gentle rotation.
9. Dilute the extract with cold 1× buffer-H1 supplemented with 0.17 mg/mL PMSF, 1 mM benzamidine, and 2 mM 2-mercaptoethanol to a final concentration of 0.2 g of cell pellet per milliliter.
10. Sonicate the extract in Time Mode for a total ON time of 6 min and 60% amplitude in a water-ice bath, with 7-s ON and 15-s OFF cycles.
Critical: Keep the temperature low (close to 4 °C). Use a glass container and the ice-water bath to optimize heat transfer. Also, set the sonifier tip deep enough and close to the base to avoid the formation of bubbles.
11. Centrifuge the sonicated extract at 100,000× g (30,000 rpm in a Type 45 Ti rotor) for 1 h at 4 °C.
12. Resuspend the membrane pellet with the help of a brush in cold 1× buffer-H1 supplemented with 0.17 mg/mL PMSF and 1 mM benzamidine. Measure the final volume of the membrane extract and take note of the concentration (mass of cell pellet per milliliter). The concentration should be between 0.5 and 0.4 g/mL.
13. Aliquot the membrane extract and store it at -80 °C.
E. Gel electrophoresis and western blot
Note: Before proceeding with protein purification, we strongly recommend checking that the protein was expressed by performing a western blot against the His-tag.
1. Perform an SDS-PAGE electrophoresis of the samples prepared before harvesting cells (step D1).
a. Open and install a 12% Mini-PROTEAN® Stain-Free™ protein gel according to the manufacturer’s instructions.
b. Use 6 μL of a 1:1 mixture of unstained standards and dual color standards as the protein ladder.
c. Load 15–20 μL of sample.
d. Run electrophoresis at 120 V for 70 min.
e. Disassemble the gel, activate, and image the stain-free gel (Figure 1A).
f. Place the gel in 1× transfer buffer and incubate with gentle agitation for at least 5 min.
2. Activate a PVDF membrane for the western blot:
a. Cover the membrane with ethanol (10–15 mL).
b. Discard the ethanol and add enough 1× transfer buffer to cover the membrane completely.
c. Incubate with gentle agitation at room temperature for at least 5 min.
Caution: Manipulate the PVDF membrane using clean gloves and forceps.
3. Transfer the proteins from the gel to the membrane following the instructions of the Trans-® TurboTM transfer system. Use a constant 2.5 A for 4 min.
4. Incubate the membrane in 50 mL of fresh blocking solution for 1 h at room temperature with gentle agitation.
5. Remove the milk and add the primary antibody (50 μL of mouse α-penta-His antibody in 15 mL of PBS-T). Incubate overnight at 4 °C with gentle agitation.
6. Next day, remove the primary antibody solution and wash the membrane with PBS-T.
a. Wash the membrane by adding, manually agitating, and discarding PBS-T three times.
b. Add PBS-T and incubate at room temperature for 5 min with gentle agitation.
c. Repeat the two previous steps three times.
7. Discard the last wash and add the secondary antibody (2 μL of StarBright Blue 700 goat α-mouse IgG in 15 mL of PBS-T). Incubate for 1 h at room temperature with gentle agitation.
8. Remove the secondary antibody solution and wash the membrane with PBS-T.
a. Wash the membrane by adding, manually agitating, and discarding PBS-T three times.
b. Add PBS-T and incubate at room temperature for 5 min with gentle agitation.
c. Repeat the two previous steps three times.
9. Image the membrane with a ChemiDoc MP imaging system following the equipment instructions for capturing the fluorescent signal of the secondary antibody (Figure 1B).
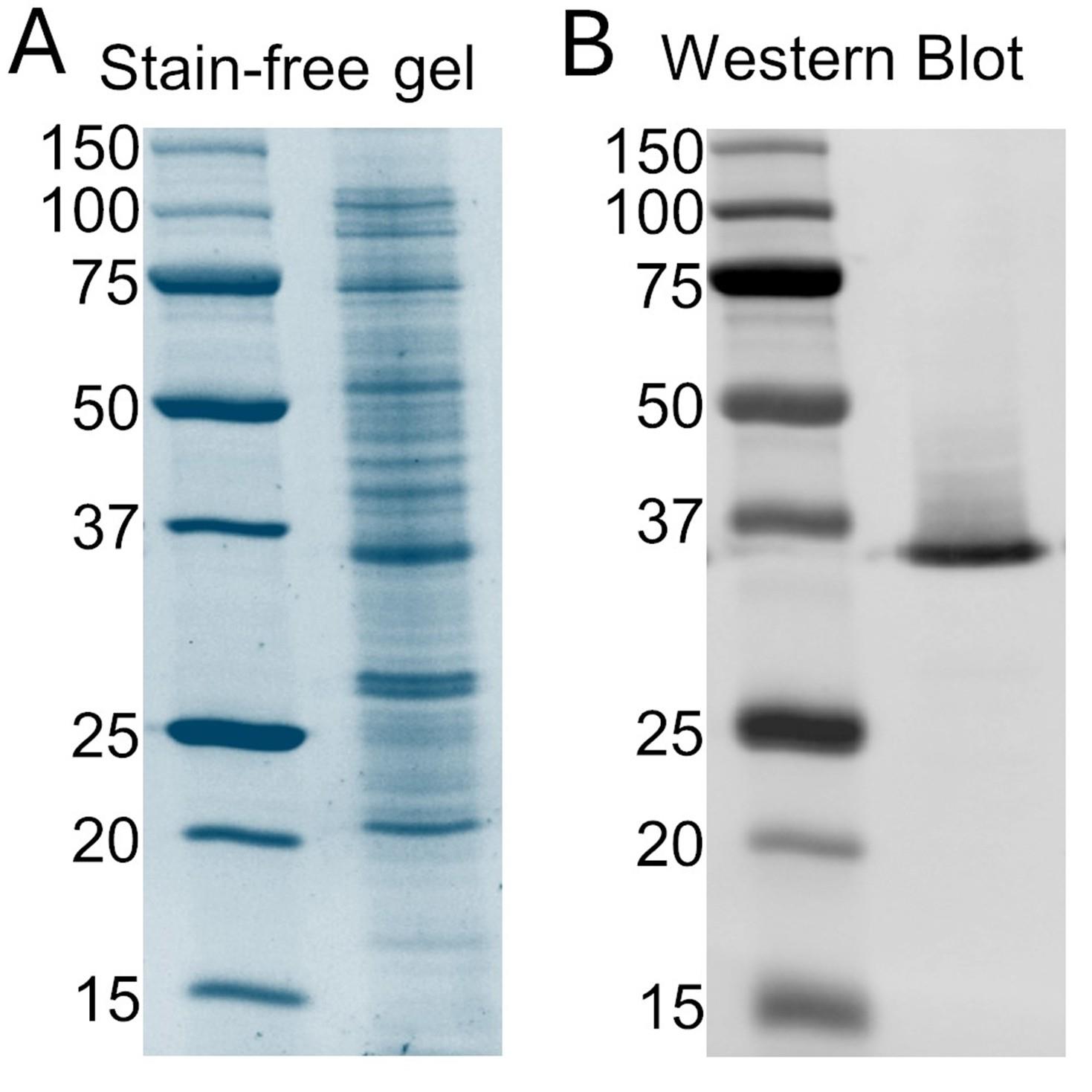
Figure 1. Expression of EK-hHv1. (A) Stain-free gel of cellular extract. The left lane is the protein ladder, and the right lane corresponds to the cellular extract sample. (B) Western blot against the poly-His tag of the cellular extract. The left lane is the protein ladder, and the right lane corresponds to the cellular extract sample. hHv1: human voltage-gated proton channel.
F. Solubilization
1. Thaw the membrane extract in a water bath at room temperature. To solubilize the protein, first determine the final volume needed to have the extract at a final concentration of 0.16 g/mL. Using this final volume, add 500 mM NaCl, 12.5 μg/mL DNase, 5 mM MgCl2, 2 mM TCEP, 0.17 mg/mL PMSF, 1 mM benzamidine, and 1.5% w/v Anz3–12. Complete the volume with 1× buffer-H1. As an example, the volumes added from the stock solutions for solubilizing 10 mL of a membrane extract at an initial concentration of 0.32 g/mL are shown in Table 1.
Table 1. Solubilization example. For the solubilization of 25 mL of membrane extract at 0.32 g/mL, the final volume should be 50 mL. Below are the volumes added from the stock solutions to solubilize this extract.
| Stock solution | Final concentration | Volume |
|---|---|---|
| 0.32 g/mL membrane extract | 0.16 g/mL | 25 mL |
| 200 mM benzamidine | 1 mM | 250 μL |
| 26 mg/mL PMSF | 0.17 mg/mL | 327 μL |
| 1 M MgCl2 | 5 mM | 250 μL |
| 2.5 mg/mL DNase I | 12.5 μg/mL | 250 μL |
| 4 M NaCl | 500 mM | 6.25 mL |
| 0.5 M TCEP | 2 mM | 200 μL |
| 20% w/v Anzergent 3–12 | 1.5% w/v | 3.75 mL |
| 1× buffer-H1 | 13.7 mL |
2. Incubate the solution for 1 h at room temperature with gentle rotation.
3. Centrifuge the solution at 100,000× g (30,000 rpm in a Type 45 Ti rotor) for 1 h at 4 °C.
G. Purification
1. Pack and equilibrate a Ni-NTA gravity-flow column:
a. Pour 10 mL of Ni-NTA resin per liter of culture (20 mL of a 50% slurry) into a gravity-flow column and drain the storage buffer.
b. Wash the column with at least 10 column volumes (CVs) of ultra-pure (Type 1) water.
c. Equilibrate the column with 6 CVs of buffer-H2.
2. Binding in batch:
a. Filter the supernatant of the centrifuged solubilized membrane extract with a 0.45 μm membrane filter and supplement with fresh 1 mM benzamidine.
b. Mix the supernatant with the equilibrated resin and incubate for 1 h at room temperature with gentle rotation.
c. Collect the resin in a gravity-flow column.
3. Wash the resin with 10 CVs of washing buffer.
4. Elution of the purified EK-hHv1:
a. Add one CV of elution buffer. Collect the eluate. Usually, this eluate contains only small amounts of EK-hHv1 protein.
b. Stop the flow of the column and add one CV of elution buffer. Incubate the column for 10 min at room temperature.
c. Collect the eluate.
d. Repeat the two previous steps 8 times to recover a total of 10 CV of eluate containing purified EK-hHv1.
5. Concentrate the desired amount of protein with a 50 kDa cutoff centrifugal filter by centrifuging at 1,200× g and 4 °C to at least 1 mL. The duration of this step will depend on the amount of protein to be concentrated.
6. Inject the sample into an ENrich SEC 650 column, which has been previously equilibrated with 1.5 CVs of buffer-H2 at room temperature. The EK-hHv1 protein will elute at around 11.0 mL (Figure 2).
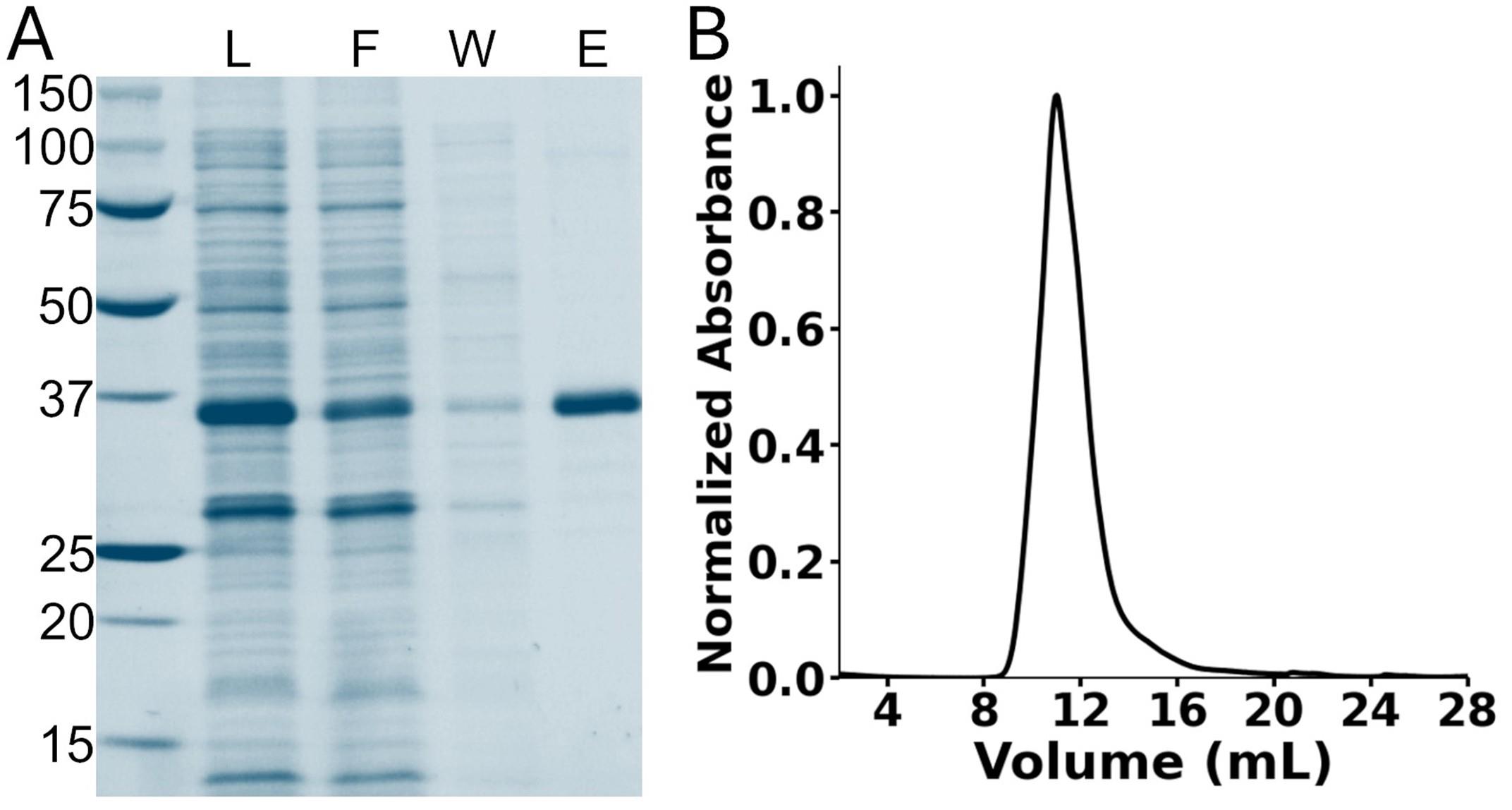
Figure 2. Purification of EK-hHv1. (A) Stain-free gel of the nickel immobilized metal affinity chromatography purification steps. The first lane corresponds to the protein ladder. L: Load; F: flowthrough; W: washing; E: elution. (B) Chromatogram of the size-exclusion chromatography of the purified EK-hHv1. hHv1: human voltage-gated proton channel.
7. Determine the EK-hHv1 protein concentration (C) by measuring the absorbance at 280 nm (Abs280) and using the equation C (mg/mL) = 1.20 (Abs280).
8. Concentrate the EK-hHv1 with a 50 kDa cutoff centrifugal filter to 5 mg/mL, aliquot, flash-freeze, and store at -80 °C.
Validation of protocol
This protocol has been used and validated in the following research article:
Carmona et al. [19]. A novel method for expressing and purifying large quantities of functional and stable human voltage‐gated proton channel (hHv1). Protein Science (Figures 1–5).
General notes and troubleshooting
General notes
1. Purification can be performed with 150 mM NaCl instead of 500 mM NaCl, but the protein yield will decrease from 70 mg/L to 30 mg/L [19].
Troubleshooting
Problem 1: Low protein expression.
Possible causes: Low efficiency of transformation of competent cells. Contamination of sterile media.
Solutions: Prepare new competent cells. Replace the media.
Problem 2: Eluted EK-hHv1 is contaminated.
Possible cause: The washing buffer is draining too fast from the column.
Solution: Add the washing buffer in two CV steps, incubating for 5 min between steps.
Supplementary information
The following supporting information can be downloaded here:
1. Dataset S1. Information and sequence of the pHis-EK-hHv1.pET15-b
Acknowledgments
Conceptualization, E.M.C. and L.G.C.; Investigation, E.M.C. and D.M.C. Writing—Original Draft, E.M.C.; Writing—Review & Editing, E.M.C. and L.G.C.; Funding acquisition, E.M.C. and L.G.C.; Supervision, L.G.C. This work was supported by the NIH 1R35GM149523-02 and the Welch Foundation BI-194 to L.G.C., and Comisión Nacional de Investigación Científica y Tecnológica (CONICYT)/Programa Formación de Capital Humano Avanzado/Doctorado Nacional/2017-21170395 to E.M.C. E.M.C. is a Pew Latin American Fellow.
This protocol was described and validated in Carmona et al. [19]. This protocol was developed from the experience gained in the works of Elberson et al. and Tilegenova et al. [22,23].
Competing interests
The authors declare no conflicts of interest.
References
- Ramsey, I. S., Moran, M. M., Chong, J. A. and Clapham, D. E. (2006). A voltage-gated proton-selective channel lacking the pore domain. Nature. 440(7088): 1213–1216. https://doi.org/10.1038/nature04700
- Sasaki, M., Takagi, M. and Okamura, Y. (2006). A Voltage Sensor-Domain Protein Is a Voltage-Gated Proton Channel. Science. 312(5773): 589–592. https://doi.org/10.1126/science.1122352
- Carmona, E. M., Larsson, H. P., Neely, A., Alvarez, O., Latorre, R. and Gonzalez, C. (2018). Gating charge displacement in a monomeric voltage-gated proton (Hv 1) channel. Proc Natl Acad Sci USA. 115(37): 9240–9245. https://doi.org/10.1073/pnas.1809705115
- Cherny, V. V., Markin, V. S. and DeCoursey, T. E. (1995). The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 105(6): 861–896. https://doi.org/10.1085/jgp.105.6.861
- Pathak, M. M., Tran, T., Hong, L., Joós, B., Morris, C. E. and Tombola, F. (2016). The Hv1 proton channel responds to mechanical stimuli. J Gen Physiol. 148(5): 405–418. https://doi.org/10.1085/jgp.201611672
- Kawanabe, A., Takeshita, K., Takata, M. and Fujiwara, Y. (2023). ATP modulates the activity of the voltage‐gated proton channel through direct binding interaction. J Physiol. 601(18): 4073–4089. https://doi.org/10.1113/jp284175
- Kawanabe, A. and Okamura, Y. (2014). The Activation Kinetics of the Voltage-Gated Proton Channel is Drastically Accelerated by Unsaturated Fatty Acids. Biophys J. 106(2): 744a. https://doi.org/10.1016/j.bpj.2013.11.4098
- Qiu, F., Chamberlin, A., Noskov, S. and Larsson, H. P. (2015). Molecular Mechanism of Zinc Inhibition on Voltage-Gated Proton Channel Hv1. Biophys J. 108(2): 367a. https://doi.org/10.1016/j.bpj.2014.11.2012
- Zhao, R., Dai, H., Arias, R. J., De Blas, G. A., Orta, G., Pavarotti, M. A., Shen, R., Perozo, E., Mayorga, L. S., Darszon, A., et al. (2021). Direct activation of the proton channel by albumin leads to human sperm capacitation and sustained release of inflammatory mediators by neutrophils. Nat Commun. 12(1): 3855. https://doi.org/10.1038/s41467-021-24145-1
- Capasso, M. (2014). Regulation of immune responses by proton channels. Immunology. 143(2): 131–137. https://doi.org/10.1111/imm.12326
- Pang, H., Wang, X., Zhao, S., Xi, W., Lv, J., Qin, J., Zhao, Q., Che, Y., Chen, L., Li, S. J., et al. (2020). Loss of the voltage-gated proton channel Hv1 decreases insulin secretion and leads to hyperglycemia and glucose intolerance in mice. J Biol Chem. 295(11): 3601–3613. https://doi.org/10.1074/jbc.ra119.010489
- Zhang, Q., Ren, Y., Mo, Y., Guo, P., Liao, P., Luo, Y., Mu, J., Chen, Z., Zhang, Y., Li, Y., et al. (2022). Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects. Cell Res. 32(5): 461–476. https://doi.org/10.1038/s41422-022-00616-y
- Alvear-Arias, J. J., Pena-Pichicoi, A., Carrillo, C., Fernandez, M., Gonzalez, T., Garate, J. A. and Gonzalez, C. (2023). Role of voltage-gated proton channel (Hv1) in cancer biology. Front Pharmacol. 14: e1175702. https://doi.org/10.3389/fphar.2023.1175702
- Takeshita, K., Sakata, S., Yamashita, E., Fujiwara, Y., Kawanabe, A., Kurokawa, T., Okochi, Y., Matsuda, M., Narita, H., Okamura, Y., et al. (2014). X-ray crystal structure of voltage-gated proton channel. Nat Struct Mol Biol. 21(4): 352–357. https://doi.org/10.1038/nsmb.2783
- Lee, S. Y., Letts, J. A. and MacKinnon, R. (2009). Functional Reconstitution of Purified Human Hv1 H+ Channels. J Mol Biol. 387(5): 1055–1060. https://doi.org/10.1016/j.jmb.2009.02.034
- Li, Q., Shen, R., Treger, J. S., Wanderling, S. S., Milewski, W., Siwowska, K., Bezanilla, F. and Perozo, E. (2015). Resting state of the human proton channel dimer in a lipid bilayer. Proc Natl Acad Sci USA. 112(44): e1515043112. https://doi.org/10.1073/pnas.1515043112
- Bayrhuber, M., Maslennikov, I., Kwiatkowski, W., Sobol, A., Wierschem, C., Eichmann, C., Frey, L. and Riek, R. (2019). Nuclear Magnetic Resonance Solution Structure and Functional Behavior of the Human Proton Channel. Biochemistry. 58(39): 4017–4027. https://doi.org/10.1021/acs.biochem.9b00471
- Han, S., Peng, S., Vance, J., Tran, K., Do, N., Bui, N., Gui, Z. and Wang, S. (2022). Structural dynamics determine voltage and pH gating in human voltage-gated proton channel. eLife. 11: e73093. https://doi.org/10.7554/elife.73093
- Carmona, E. M., Cortes, D. M. and Cuello, L. G. (2025). A novel method for expressing and purifying large quantities of functional and stable human voltage‐gated proton channel (hHv1). Protein Sci. 34(2): e70017. https://doi.org/10.1002/pro.70017
- Chung, C. T. and Miller, R. H. (1993). [43] Preparation and storage of competent Escherichia coli cells. Meth Enzymol.: 621–627. https://doi.org/10.1016/0076-6879(93)18045-e
- Studier, F. W. (2005). Protein production by auto-induction in high-density shaking cultures. Protein Expression Purif. 41(1): 207–234. https://doi.org/10.1016/j.pep.2005.01.016
- Elberson, B. W., Whisenant, T. E., Cortes, D. M. and Cuello, L. G. (2017). A cost-effective protocol for the over-expression and purification of fully-functional and more stable Erwinia chrysanthemi ligand-gated ion channel. Protein Expression Purif. 133: 177–186. https://doi.org/10.1016/j.pep.2017.03.006
- Tilegenova, C., Vemulapally, S., Cortes, D. M. and Cuello, L. G. (2016). An improved method for the cost-effective expression and purification of large quantities of KcsA. Protein Expression Purif. 127: 53–60. https://doi.org/10.1016/j.pep.2016.07.002
Article Information
Publication history
Received: Apr 8, 2025
Accepted: May 13, 2025
Available online: May 30, 2025
Published: Jun 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
How to cite
Carmona, E. M., Cortes, D. M. and Cuello, L. G. (2025). Expression and Purification of the Human Voltage-Gated Proton Channel (hHv1). Bio-protocol 15(12): e5344. DOI: 10.21769/BioProtoc.5344.
Category
Biochemistry > Protein > Isolation and purification
Biochemistry > Protein > Expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









