- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
I-PREFR: Inverse PCR-Based Restriction Enzyme FRee Unidirectional Strategy for Rapid Markerless Chromosomal Gene Deletion and Reconstitution in Bacteria Using Suicide Vectors
Published: Vol 15, Iss 10, May 20, 2025 DOI: 10.21769/BioProtoc.5314 Views: 2343
Reviewed by: Emilia KrypotouJoyce ChiuDamián Lobato-Márquez

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Development of a Gene Replacement Method for the Filamentous Bacterium Leptothrix cholodnii SP-6
Tatsuki Kunoh [...] Nobuhiko Nomura
Apr 20, 2023 2384 Views
Efficient Genetic Transformation and Suicide Plasmid-mediated Genome Editing System for Non-model Microorganism Erwinia persicina
Tingfeng Cheng [...] Lei Zhao
Mar 20, 2024 2424 Views
A Practical CRISPR-Based Method for Rapid Genome Editing in Caulobacter crescentus
Xuezhou Yuan [...] Jingxian Sun
Nov 5, 2025 1807 Views
Abstract
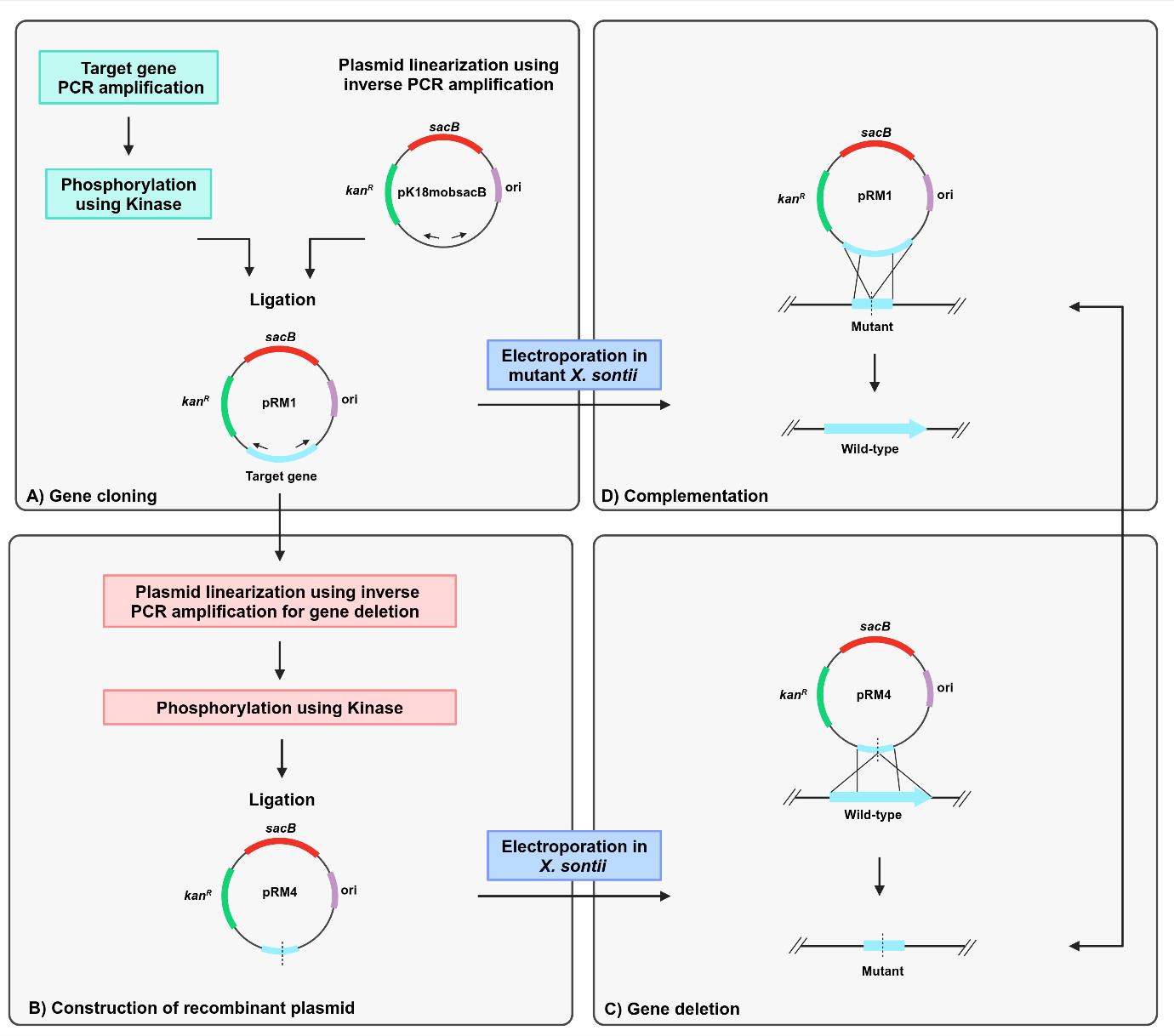
The standard protocols for allelic exchange using homologous recombination deploy suicide vectors with negative selection markers. However, the use of multiple restriction enzymes to generate sticky ends in the vector and the insert for cloning is time-consuming, resource-intensive, and challenging. The advent of next-generation proofreading enzymes is enabling researchers to routinely carry out long-range PCR. Hence, amplifying 5–6 kb of complete low-complex DNA cloning vectors and 2–3 kb of complex genomic regions is much easier. Here, we report a simple, accurate, rapid, and unidirectional approach for chromosomal in-frame gene deletion and complementation by reconstitution of the full-length gene without using any restriction enzymes. The method requires long-range PCR using Phusion polymerase to linearize the vector and amplify the target gene to create a recombinant vector (pRM1) and further inverse PCR amplification of pRM1 to create a recombinant vector (pRM4) with a deleted version of the gene. The cloning steps involve the use of kinase and ligase for phosphorylation and ligation steps, respectively. The recombinant plasmid, pRM4, is finally transformed into electrocompetent cells of Xanthomonas sontii, a gram-negative phytobacterium, for final genomic integration/excision to obtain an in-frame gene deletion mutant (PPL1RM15). Gene reconstitution for complementation is carried out by electroporating the deletion mutant with the recombinant plasmid (pRM1) carrying the wild-type allele. Clean gene mutation, allele restoration, and plasmid excision are confirmed using whole-genome sequencing.
Key features
• The protocol is cost-effective and simple, eliminating the need for restriction enzymes and multiple sets of lengthy primers with restriction sites.
• The protocol requires only kinase, ligase, and polymerase, along with three sets of standard-sized desalted primers.
• The protocol is unidirectional; no need to create two different recombinant plasmids for gene deletion and complementation by reconstitution of the full-length gene.
• The difference in size between empty and recombinant vectors facilitates the easy screening of transformed Escherichia coli colonies through colony PCR.
• The strategy can be applied to any bacteria using a suitable suicide vector with appropriate positive and negative selection markers.
Keywords: Markerless gene deletionGraphical overview
Background
Restriction enzyme–mediated cloning is complicated and requires multiple time-consuming steps. The first part is the introduction of suitable restriction sites in the target gene PCR product and the vector for cloning. Second, it is required to create distinct recombinant vectors for deletion and complementation through gene reconstitution [1]. The plasmid pK18mobsacB is a cloning vector that facilitates markerless gene deletion in gram-negative bacteria through homologous recombination [2]. The vector has a kanR marker, which confers kanamycin resistance, and a sacB gene, which confers sucrose sensitivity through the levan synthesis in the presence of sucrose [3]. A deleted version of the target gene is generated using primers with restriction sites and then cloned into pK18mobsacB, followed by allelic exchange through homologous recombination. Gene complementation is carried through reconstitution by amplification and restriction enzyme–mediated cloning of the full-length gene in pk18mobsacB and further homologous recombination in the deletion mutant.
Here, we describe a protocol for gene deletion and complementation through reconstitution in Xanthomonas sontii, a gram-negative bacterium and rice endophyte, using a pK18mobsacB-based inverse-PCR strategy without using any restriction enzyme. The standard M13 region of pK18mobsacB was used to design inverse primers. The amplified target gene was phosphorylated with kinase and ligated with the linear plasmid. Further, to generate the in-frame deletion mutant, primers facing tail-to-tail situated 300–400 bp inside from the ends of the target gene were used to amplify the recombinant plasmid, excluding the gene region to be deleted. This was followed by kinase treatment and ligation. The recombinant plasmid with the deleted gene was electroporated into the Xanthomonas cells, leading to the deletion of the target gene in the chromosome through homologous recombination. In a similar way, the recombinant plasmid with the target gene was electroporated into the gene deletion mutant of Xanthomonas sontii, leading to the restoration of the full-length target gene in the chromosome through homologous recombination.
This unidirectional method is fast, accurate, and marker-free and can be easily used to delete any gene in the chromosome and carry out chromosomal complementation through allele reconstitution independent of the reliability of restriction sites or enzymes. Further considering the average length of a gene in bacteria (i.e., 1–2 kb), the protocol is applicable for a typical gene size. Moreover, the protocol is developed in a non-model organism with high GC content. Hence, it will readily apply to organisms with much lower GC contents. This protocol is applicable in both gram-positive and gram-negative bacteria with suitable suicide vectors with negative selection markers. Counterselection markers like sacB mediating sucrose toxicity are well-suited for gram-negative bacteria and a few gram-positive bacteria like Corynebacterium and Mycobacterium [2,4]. However, for other gram-positive bacteria, the mutated version of the phenylalanine tRNA transferase (pheS*) protein [5], mazF toxin [6], or any other suitable negative selection markers can be deployed.
Materials and reagents
Biological materials
1. Xanthomonas sontii PPL1T [7]
2. Escherichia coli Top10 cells (Invitrogen, catalog number: C404010)
3. pK18mobsacB plasmid [2]
Reagents
1. Quick-DNATM Fungal/Bacterial Miniprep kit (Zymo Research, catalog number: D6005)
2. GeneJET Plasmid Miniprep kit (Thermo Scientific, catalog number: K0502)
3. 5× FIREPol® master mix (Solis BioDyne, catalog number: 04-12-00115)
4. Nuclease-free water (MP Biomedicals, catalog number: 112450204)
5. Agarose (Sigma-Aldrich, catalog number: A9539)
6. Ethidium bromide solution (Sigma-Aldrich, catalog number: E1510)
7. 6× DNA loading buffer (Real-Gene Labs, catalog number: 530001)
8. Quick-load® 100 bp DNA ladder (New England Biolabs Inc., catalog number: N0467S)
9. Quick-load® 1 kb DNA ladder (New England Biolabs Inc., catalog number: N0468S)
10. GeneJET Gel Extraction kit (Thermo Scientific, catalog number: K0691)
11. GeneJET PCR Purification kit (Thermo Scientific, catalog number: K0701)
12. Phusion DNA polymerase (Thermo Scientific, catalog number: F-530S)
13. 5× Phusion HF buffer (Thermo Scientific, catalog number: F-518)
14. dNTP mix (Fermentas, catalog number: R0192)
15. T4 Polynucleotide kinase (New England Biolabs Inc., catalog number: M0201S)
16. 10× T4 DNA ligase buffer with 10 mM ATP (New England Biolabs Inc., catalog number: B0202A)
17. T4 DNA ligase (New England Biolabs Inc., catalog number: M0202S)
18. Kanamycin sulfate (Sigma-Aldrich, catalog number: 60615)
19. 0.22 μm PVDF filter (Whatman, catalog number: 9913-2502)
20. 5 mL syringe (Dispo Van)
21. 0.025 μM desalted primers (Sigma-Aldrich)
22. DifcoTM nutrient agar (BD, catalog number: 213000)
23. DifcoTM nutrient broth (BD, catalog number: 234000)
24. DifcoTM LB broth, Miller (BD, catalog number: 244620)
25. DifcoTM LB agar, Miller (BD, catalog number: 244520)
26. Sucrose (Sigma-Aldrich, catalog number: S0389)
27. Calcium chloride (CaCl2) dihydrate (Sigma-Aldrich, catalog number: C5080-500G)
28. Glycerol (Qualigens, catalog number: Q15457)
29. Tris base (Sigma-Aldrich, catalog number: 648310-500GM)
30. Glacial acetic acid (Qualigens, catalog number: Q11007)
31. EDTA disodium dihydrate (Sisco Research Lab. Pvt. Ltd., catalog number: 43272)
Solutions
1. Nutrient agar with 5% sucrose (see Recipes)
2. 50× TAE buffer (see Recipes)
3. Kanamycin sulfate stock 50 mg/mL (see Recipes)
4. 300 mM sucrose (see Recipes)
5. 100 mM CaCl2 + 15% glycerol (see Recipes)
6. 100 mM CaCl2 (see Recipes)
Recipes
1. Nutrient agar with 5% sucrose
| Reagent | Quantity or volume |
|---|---|
| Nutrient agar | 23 g |
| Sucrose | 50 g |
| Distilled water | 1,000 mL |
Note: Sterilize media by autoclaving at 121 °C for 15 min.
2. 50× TAE buffer
| Reagent | Quantity or volume |
|---|---|
| Tris base | 242 g |
| Glacial acetic acid | 57.1 mL |
| 0.5 mM EDTA | 18.612 g |
| Distilled water | Up to 1,000 mL |
Note: 50× TAE is diluted with distilled water (1:50) to make 1× TAE for the preparation of agarose gel.
3. Kanamycin sulfate stock 50 mg/mL
| Reagent | Quantity or volume |
|---|---|
| Kanamycin sulfate | 250 mg |
| Distilled water | 5 mL |
Note: Filter sterilize the solution with a 0.22 μm syringe filter.
4. 300 mM Sucrose
| Reagent | Quantity or volume |
|---|---|
| Sucrose | 5.134 g |
| Distilled water | 50 mL |
Note: Filter sterilize the solution with a 0.22 μm syringe filter.
5. 100 mM CaCl2 + 15% glycerol
| Reagent | Quantity or volume |
|---|---|
| CaCl2·2H2O | 0.735 g |
| Glycerol | 7.5 mL |
| Distilled water | Up to 50 mL |
6. 100 mM CaCl2
| Reagent | Quantity or volume |
|---|---|
| CaCl2·2H2O | 1.47 g |
| Distilled water | 100 mL |
Equipment
1. Micropipettes (Eppendorf)
2. Nanodrop (DeNovix, catalog number: DS-11 FX)
3. UV Transilluminator (Wealtac, model: HD-25, 365 nm)
4. Gel electrophoresis assembly (Bio-Rad, model: 200/2.0)
5. Mastercycler gradient (Eppendorf, catalog number: 950000023)
6. Centrifuge (Eppendorf, model: 5810 R)
7. Microcentrifuge (Sigma, model: 1-15P)
8. Thermomixer comfort (Eppendorf, catalog number: 5355 000.011)
9. Electroporator (Bio-Rad, catalog number: 1652660)
10. Incubators at 28 °C and 37 °C (Innova®, model: Innova® 42R Inc/Ref shaker)
Software and datasets
1. AmplifX v2.0.7 [8]
2. Primer3 v0.4.0 (https://primer3.ut.ee/)
3. MEGA-X v10.2.6 [9]
4. NEBiocalculator (https://nebiocalculator.neb.com/)
Procedure
A. Primer design
1. Design primers for the PCR amplification of the target gene (P1 and P2). Design tail-to-tail facing primers positioned 300–400 bp inside from the 3’ and 5’ ends of the target gene (P3 and P4). Inverse PCR amplification using these primers will result in the deletion of a significant portion of the gene (Figure 1, Table 1).
Note: Primers can be designed in a similar way, including upstream and downstream regions of the gene for complete gene deletion.
2. Design primers facing tail-to-tail using the M13 forward and M13 reverse regions for inverse PCR amplification and linearization of pK18mobsacB (P7 and P8).
3. Design primers for PCR amplification of kanR (P9 and P10) and sacB (P11 and P12) markers of pK18mobsacB to screen integrants. Additionally, design primers for PCR amplification of the target gene, including extra 100–200 bp both upstream and downstream to screen deletion mutants (P5 and P6).
4. The nucleotide sequences of the primers used in this protocol were mentioned in our earlier publication [10].
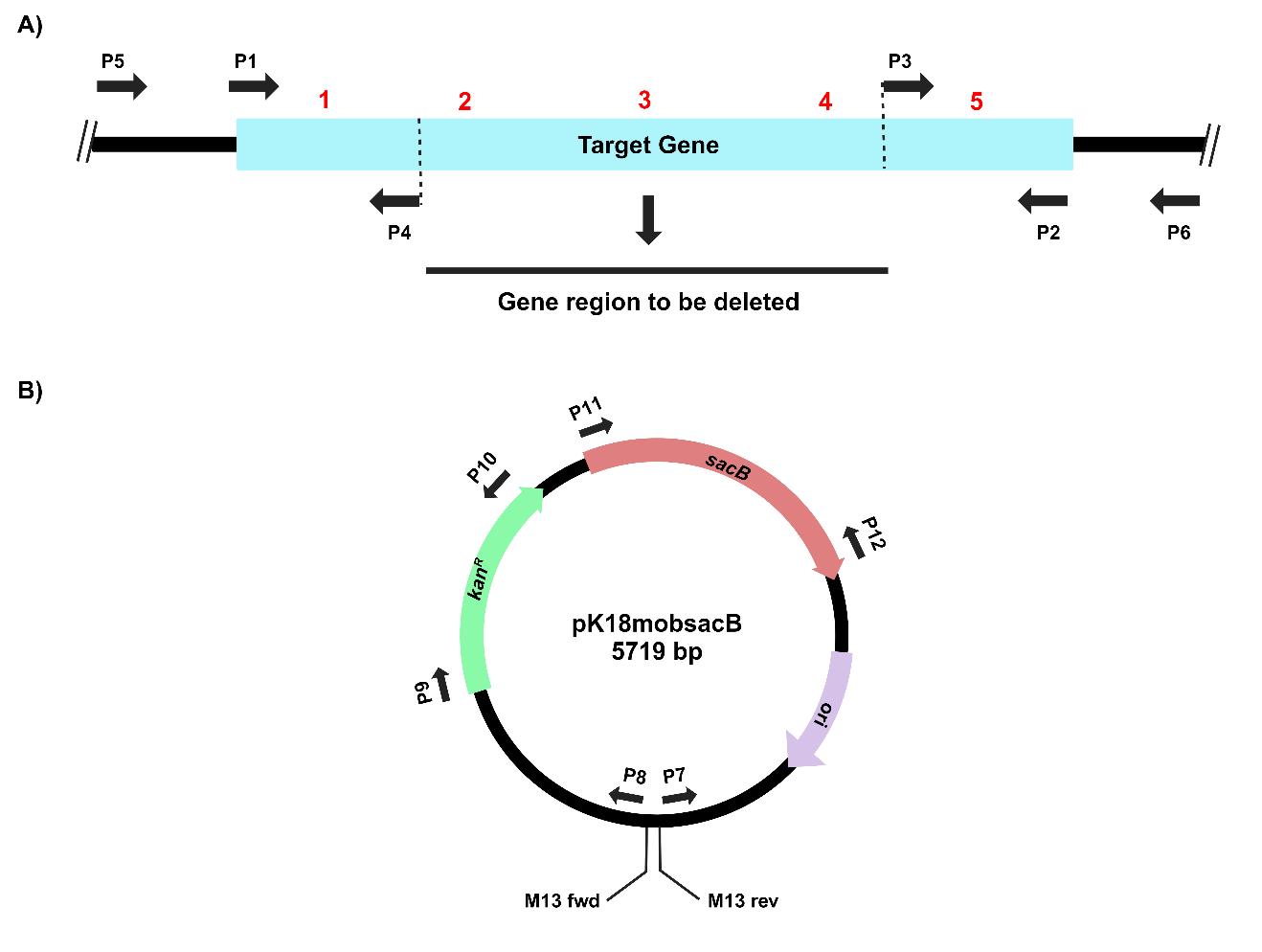
Figure 1. Primer design for PCR amplifications of (A) the target gene, virD4, and (B) the suicide vector, pK18mobsacB, for gene cloning, deletion, and complementation by reconstitution. The thick black lines depict DNA backbones for the chromosome and the plasmid.
Table 1. Primers used in this study
| S. No. | Primer pair | Primer name | GC content (%) | Melting temperature (Tm°) | Size (bp) | Purpose |
| 1 | P1 | VirD4_3_F | 42.1 | 58.9 | 19 | Target gene (virD4) amplification |
| 2 | P2 | VirD4_5_R | 50 | 57 | 18 | |
| 3 | P3 | VirD4_inv_F_2 | 50 | 57.9 | 18 | Linearization of pRM1 |
| 4 | P4 | VirD4_inv_R | 55.5 | 61.7 | 18 | |
| 5 | P5 | VirD4_idnt_F | 66.6 | 67.2 | 18 | Detection of gene deletion mutation in X. sontii PPL1 |
| 6 | P6 | VirD4_idnt_R | 55 | 66.6 | 20 | |
| 7 | P7 | Set1_M13_FP | 52.9 | 58.7 | 17 | Linearization of pK18mobsacB |
| 8 | P8 | Set1_M13_RP | 47.3 | 58.1 | 19 | |
| 9 | P9 | KanR_F | 55 | 64.8 | 20 | Kanamycin resistance gene amplification |
| 10 | P10 | KanR_R | 50 | 68.8 | 20 | |
| 11 | P11 | sacB_F | 50 | 66.3 | 20 | sacB gene amplification |
| 12 | P12 | sacB_R | 45 | 64.3 | 20 |
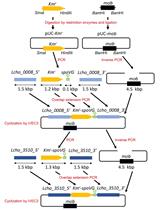
B. Target gene cloning in pK18mobsacB
The process for inverse PCR–based gene cloning using the suicide vector pK18mobsacB is depicted in Figure 2.
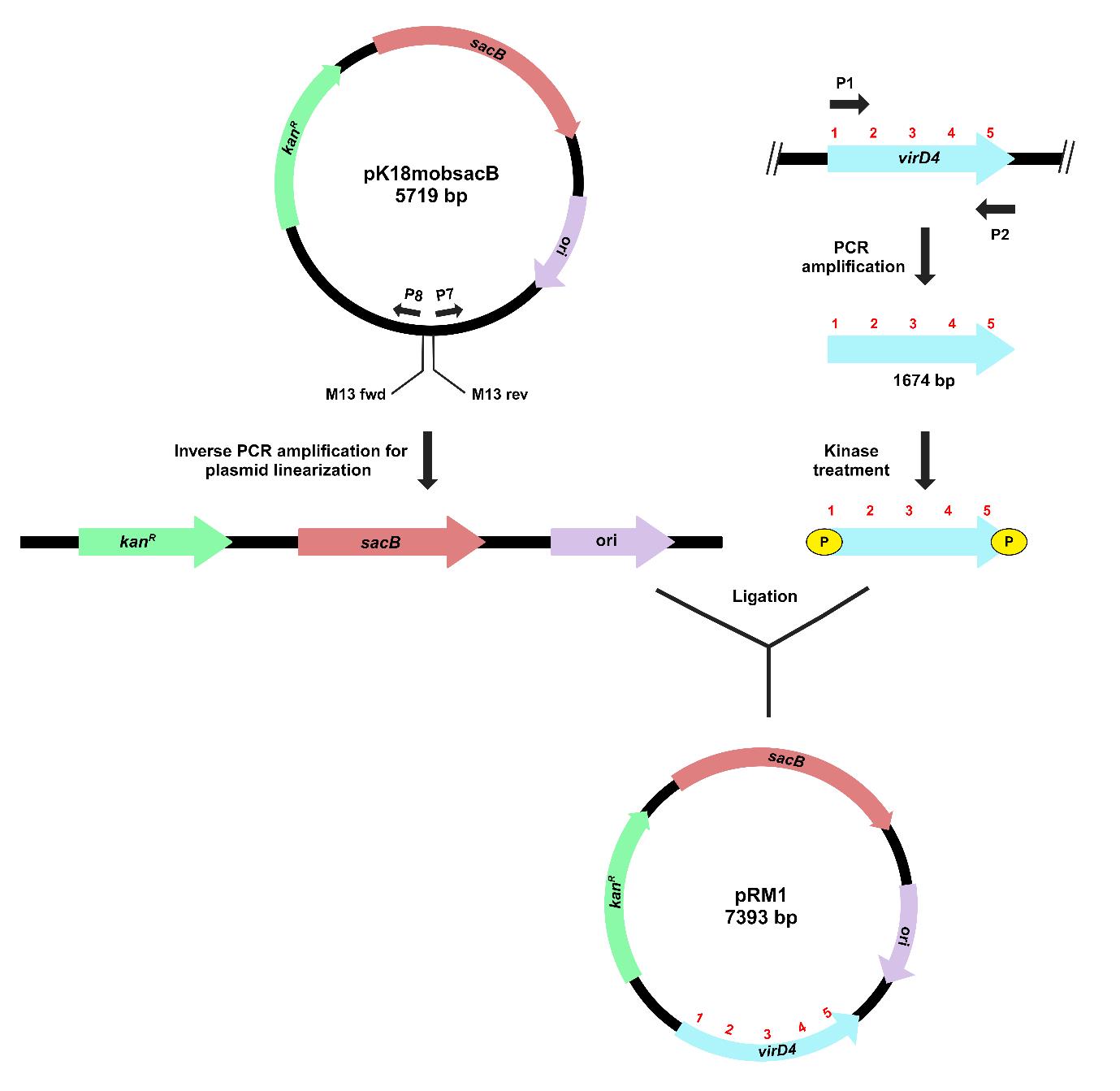
Figure 2. Schematic representation of target gene cloning in pK18mobsacB, using inverse PCR-based strategy. Outward-facing primers (P7 and P8) amplify and linearize the plasmid (pK18mobsacB). Kinase treatment of the target gene (virD4) creates phosphorylated ends for ligation with the linearized plasmid. The recombinant plasmid, pRM1, contains a complete copy of the virD4 gene.
1. Streak PPL1 on nutrient agar (NA) and incubate at 28 °C for 24–48 h. Inoculate a single colony in 10 mL of nutrient broth (NB) and incubate overnight at 28 °C at 180 rpm. Isolate genomic DNA using Quick-DNATM Fungal/Bacterial Miniprep kit and quantify using a Nanodrop.
2. PCR-amplify the target gene (virD4) with the P1 and P2 primers using Phusion DNA polymerase. Set up a 20 μL volume PCR reaction as shown in Table 2 with the following conditions: (i) initial denaturation at 98 °C for 30 s, followed by (ii) 30 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 30 s, and extension at 72 °C for 45 s, and (iii) final extension at 72 °C for 10 min.
Table 2. Reaction mixture for PCR amplification of the target gene.
| Component | Volume/concentration |
|---|---|
| 5× Phusion HF buffer (contains MgCl2) | 4 μL |
| 10 mM dNTPs | 0.4 μL |
| 10 μM forward primer (P1) | 1 μL |
| 10 μM reverse primer (P2) | 1 μL |
| Phusion DNA polymerase | 0.2 μL |
| Template DNA | 2 μL (108 ng/µL) |
| Nuclease-free water | 11.4 μL |
| Total | 20 μL |
3. Confirm a 1,674 bp PCR-amplified gene on a 1% agarose gel using the 1 kb ladder under the UV transilluminator. Purify the PCR product using the GeneJET PCR Purification kit and quantify it using a Nanodrop.
4. Streak Escherichia coli culture with pK18mobsacB on LB agar with kanamycin sulfate (50 μg/mL) and incubate at 37 °C for 18 h. Inoculate a single colony in 10 mL of LB broth with kanamycin sulfate (50 μg/mL) and incubate overnight at 37 °C at 200 rpm. Isolate the plasmid using the GeneJET Plasmid Miniprep kit and quantify it using a Nanodrop.
5. Linearize pK18mobsacB using inverse M13 region primers (P7 and P8). Set up a 20 μL PCR amplification reaction using Phusion DNA polymerase as in Table 3. The PCR amplification conditions are as follows: (i) 98 °C for 30 s, followed by 30 cycles of (ii) 98 °C for 10 s, (iii) 60 °C for 30 s, and (iv) 72 °C for 3 min, and (iii) final extension at 72 °C for 10 min.
Table 3. Reaction mixture for PCR amplification and linearization of the vector.
| Component | Volume/concentration |
|---|---|
| 5× Phusion HF buffer (contains MgCl2) | 4 μL |
| 10 mM dNTPs | 0.4 μL |
| 10 μM forward primer (P7) | 1 μL |
| 10 μM reverse primer (P8) | 1 μL |
| Phusion DNA polymerase | 0.2 μL |
| Template DNA | 1 μL (82 ng/µL) |
| Nuclease-free water | 12.4 μL |
| Total | 20 μL |
6. Confirm plasmid linearization on a 0.8% agarose gel with the 1 kb ladder by visualizing a 5,719 bp band under the UV transilluminator. Excise the gel band and purify it using the GeneJET Gel Extraction kit. Quantify the plasmid using a Nanodrop.
7. Set up a 50 μL kinase reaction of the PCR-purified target gene as given in Table 4. Incubate the reaction mixture at 37 °C for 30 min, followed by heat-inactivation at 65 °C for 20 min.
Table 4. Components used for the kinase treatment of the target gene.
| Component | Volume/concentration |
|---|---|
| DNA (target gene) | 19 μL (3.453 pmol of 5’ termini) |
| T4 DNA ligase buffer with ATP (10×) | 5 μL |
| T4 PNK | 1 μL |
| Nuclease-free water | 25 μL |
| Total | 50 μL |
8. Set up a 20 μL ligation reaction with the kinase-treated target gene and linearized pK18mobsacB in a 3:1 ratio as given in Table 5. Incubate the reaction mixture at 16 °C overnight, followed by heat inactivation at 65 °C for 10 min.
Table 5. Components used for the ligation reaction.
| Component | Volume/concentration |
|---|---|
| Vector (pK18mobsacB) | 5 μL (0.055 pmol) |
| Insert | 2.5 μL (0.174 pmol) |
| T4 DNA ligase buffer with ATP (10×) | 2 μL |
| T4 DNA ligase | 1 μL |
| Nuclease-free water | 9.5 μL |
| Total | 20 μL |
9. Streak E. coli Top10 cells on LB agar plates and incubate them at 37 °C for 18 h. Inoculate a single colony in 100 μL of LB broth and prepare chemically competent cells using the previously described protocol [11].
10. For transformation, transfer 10 μL of the ligated product to 100 μL of chemically competent Top 10 cells and incubate on ice for 15 min, followed by transfer at 42 °C for 60 s and again on ice for 2 min. Add 1 mL of LB broth media to the cells and incubate at 37 °C at 200 rpm for 1 h. Pellet down the cells at 6,000× g for 5 min, discard the supernatant, and resuspend the pellet in 100 μL of LB broth. Plate the suspension on LB agar (50 μg/mL kanamycin sulfate) and incubate at 37 °C. Transformed colonies will appear after 18–24 h.
11. To screen the transformed colonies, perform PCR amplification of the target gene (virD4) using 5× FIREPol® master mix with P1 and P2 primers as per the following PCR conditions: (i) initial denaturation at 95 °C for 5 min, followed by (ii) 30 cycles of denaturation at 95 °C for 40 s, annealing at 60 °C for 45 s, and extension at 72 °C for 1 min 30 s, and (iii) final extension at 72 °C for 10 min. The details of the PCR reaction mixture are provided in Table 6.
Table 6. Reaction mixture for PCR amplification of the target gene in transformed colonies.
| Component | Volume/concentration |
|---|---|
| 5× FIREPol® master mix | 4 μL |
| 5 μM forward primer (P1) | 1 μL |
| 5 μM reverse primer (P2) | 1 μL |
| Template DNA | - |
| Nuclease-free water | - |
| Total | 20 μL |
Note: The volume of nuclease-free water and template DNA is adjusted for each sample. The 5× FIREPol® master mix is a pre-mix solution of DNA polymerase, MgCl2, dNTPs, and buffer.
12. Confirm the PCR product by observing a 1,674 bp DNA band on a 1% agarose gel under the UV transilluminator.
C. Deletion of target gene region in recombinant pK18mobsacB
The method for gene deletion using inverse PCR in the recombinant pK18mobsacB is outlined in Figure 3.
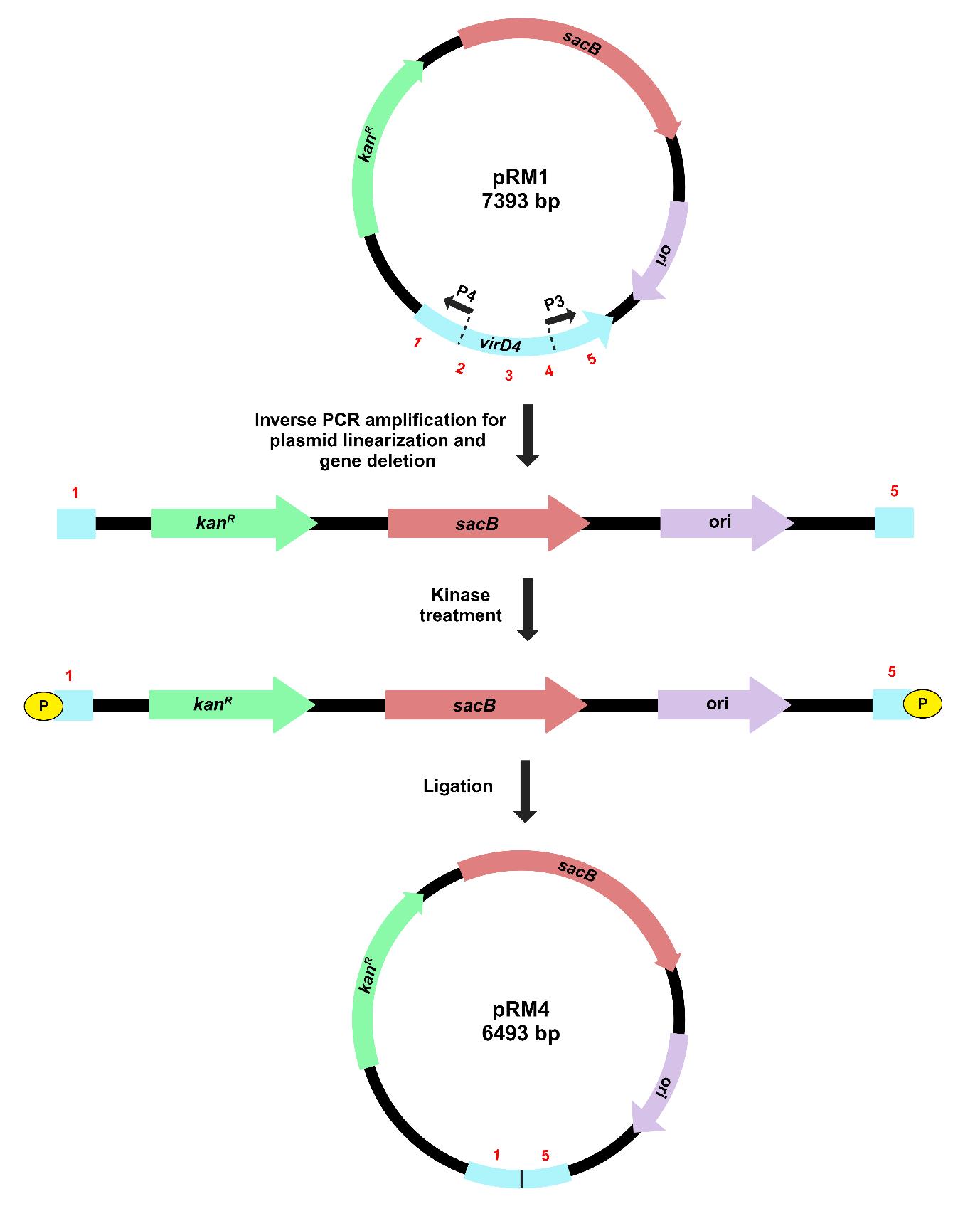
Figure 3. Illustration of target gene deletion in recombinant pK18mobsacB, using the inverse PCR–based strategy. Inverse primers (P3 and P4) amplify and linearize pRM1, resulting in the deletion of a significant portion of the virD4 gene. Kinase treatment of linearized pRM1 creates phosphorylated ends for plasmid circularization through self-ligation. The recombinant plasmid, pRM4, carries a gene deletion of the virD4 gene.
1. Isolate the recombinant plasmid (pRM1) from transformed E. coli Top10 cells using the GeneJET Plasmid Miniprep kit and quantify it using a Nanodrop.
2. PCR-amplify the recombinant plasmid using Phusion DNA polymerase and inverse primers (P3 and P4) to linearize it and create the target gene deletion. Set up a 20 μL PCR amplification reaction as mentioned in Table 7. The PCR conditions are as follows: (i) initial denaturation at 98 °C for 30 s, followed by (ii) 30 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 30 s, and extension at 72 °C for 3 min, and (iii) final extension at 72 °C for 10 min.
Table 7. Reaction mixture for PCR amplification and linearization of the recombinant plasmid
| Component | Volume/concentration |
|---|---|
| 5× Phusion HF buffer (contains MgCl2) | 4 μL |
| 10 mM dNTPs | 0.4 μL |
| 10 μM forward primer (P3) | 1 μL |
| 10 μM reverse primer (P4) | 1 μL |
| Phusion DNA polymerase | 0.2 μL |
| Template DNA | 0.5 μL (125 ng/µL) |
| Nuclease-free water | 12.9 μL |
| Total | 20 μL |
3. Confirm the plasmid linearization on a 0.8% agarose gel with the 1 kb ladder and by visualizing a 6,493 bp band in the UV transilluminator. Cut the gel band and perform gel extraction using the GeneJET Gel Extraction kit. Quantify it using a Nanodrop.
4. Set up a 50 μL kinase reaction of the linearized recombinant plasmid as given in Table 8. Incubate the reaction mixture at 37 °C for 30 min, then heat-inactivate at 65 °C for 20 min.
Table 8. Components used for the kinase treatment of the recombinant plasmid
| Component | Volume/concentration |
|---|---|
| DNA (linearized pRM1) | 18 μL (0.290 pmol of 5’ termini) |
| T4 DNA ligase buffer with ATP (10×) | 5 μL |
| T4 PNK | 1 μL |
| Nuclease-free water | 26 μL |
| Total | 50 μL |
5. For self-ligation of the linearized recombinant plasmid, add 2.5 μL of T4 DNA ligase to the 50 μL kinase reaction after heat inactivation. Incubate at 16 °C overnight, followed by heat inactivation at 65 °C for 10 min.
6. Transform the chemically competent Top 10 cells by adding 10 μL of ligation mixture to 100 μL of cells following the same procedure as mentioned in step B10.
7. Screen the colonies for the presence of the gene deletion carrying the recombinant plasmid by PCR amplification of the target gene with the same conditions as specified in step B11.
8. Confirm gene deletion by running the PCR product on a 1.2% agarose gel with the 100 bp ladder and visualizing the 774 bp DNA band under the UV transilluminator.
D. Chromosomal deletion of the target gene in X. sontii
The process for gene deletion in X. sontii PPL1 through homologous recombination is depicted in Figure 4. The plasmid is incorporated into the bacterial genome during the first homologous recombination. The colonies from the first homologous recombination will grow on kanamycin due to the expression of kanR, and sucrose will have a negative impact on them as sacB expression leads to the synthesis of levan, which is fatal to gram-negative bacteria [2]. After the second homologous recombination, the plasmid will be excised from the chromosome, leading to either wild-type or mutant colonies. The colonies will now be sensitive to kanamycin and grow normally on sucrose.
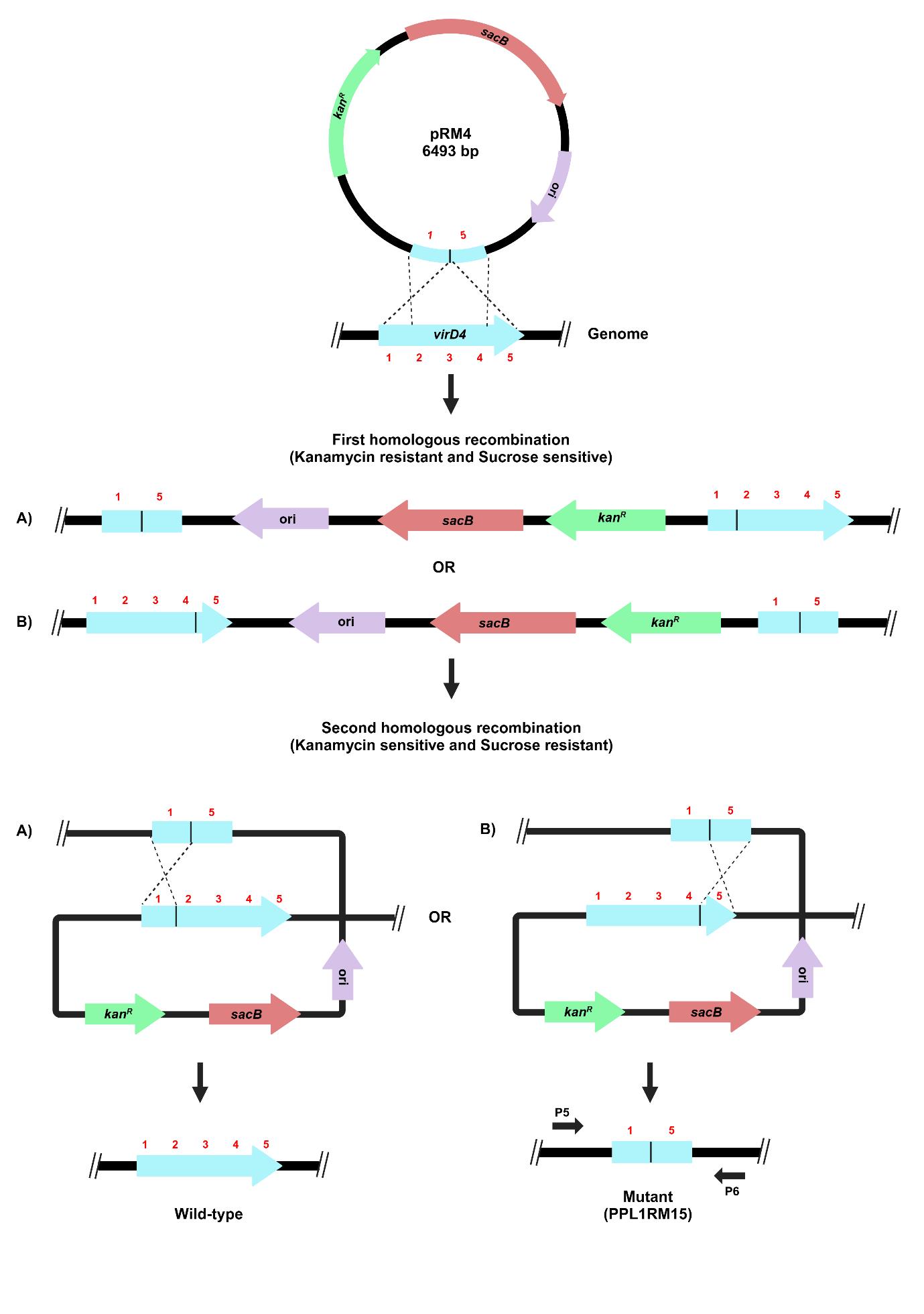
Figure 4. Schematic representation of target gene deletion in X. sontii through homologous recombination. Target gene deletion carrying plasmid, pRM4, was electroporated in X. sontii PPL1. Strains from the first homologous recombination event were screened on kanamycin, while those from the second homologous recombination event were screened on sucrose.
1. Extract the pK18mobsacB plasmid (pRM4) carrying the deleted version of the virD4 gene using the GeneJET Plasmid Miniprep kit and quantify it using a Nanodrop.
2. Streak PPL1 on NA and incubate at 28 °C for 24–48 h. Inoculate a single colony in 6 mL of NB media and incubate at 28 °C at 180 rpm until O.D.600nm reaches 0.7–0.8. Prepare electrocompetent cells of PPL1 using 300 mM sucrose, following the previously described protocol [12].
3. For electroporation, mix 1 μL (50–100 ng) of plasmid in 100 μL of electrocompetent cells and transfer to 2 mm electroporation cuvettes (Bio-Rad) on ice and subject to electroporation at 2.5 kV, 25 μF, and 200 Ω using the Gene Pulser Xcell electroporation system (Bio-Rad). Afterward, add 1 mL of NB to the cells in a 5 mL microcentrifuge tube and incubate at 28 °C at 180 rpm for 2 h. Pellet down cells at 6,000× g for 5 min, resuspend in 100 μL of NB, and plate on NA (50 μg/mL kanamycin sulfate). Incubate the plate at 28 °C for 24–48 h.
4. Screen colonies for the integration of recombinant pK18mobsacB (pRM4) into the bacterial genome (first homologous recombination) by PCR amplification of kanR (primers, P9 and P10) and sacB genes (primers, P11 and P12) using the following PCR conditions with 5× FIREPol® Master Mix: (i) initial denaturation at 95 °C for 5 min, followed by (ii) 30 cycles of denaturation at 95 °C for 30 s, annealing at 66 °C for 30 s, and extension at 72 °C for 45 s, and (iii) final extension at 72 °C for 10 min. Components for the PCR reaction mixture are given in Table 9.
Table 9. Reaction mixture for PCR amplification of marker genes to confirm the first homologous recombination.
| Component | Volume/concentration |
|---|---|
| 5× FIREPol® master mix | 4 μL |
| 5 μM forward primer (P9/P11) | 1 μL |
| 5 μM reverse primer (P10/P12) | 1 μL |
| Template DNA | - |
| Nuclease-free water | - |
| Total | 20 μL |
5. Confirm PCR products by running on a 1.2% agarose gel with the 100 bp ladder and visualizing ~300–400 bp DNA bands for kanR and sacB genes under the UV transilluminator.
6. Passage the confirmed strain from the first homologous recombination event through NB for five generations; then, plate on NA + 5% sucrose. Incubate the plate at 28 °C for 24–48 h.
7. Screen colonies for gene deletion (second homologous recombination) using PCR amplification of the region ~200 bp upstream to ~180 bp downstream of the virD4 gene sequence (primers, P5 and P6) as given in Table 10. The PCR amplification conditions using 5× FIREPol® Master Mix are as follows: (i) initial denaturation at 95 °C for 5 min, followed by (ii) 30 cycles of denaturation at 95 °C for 30 s, annealing at 66 °C for 45 s, and extension at 72 °C for 1 min 30 s, and (iii) final extension at 72 °C for 10 min.
Table 10. Reaction mixture for PCR amplification of the target gene along with the upstream and downstream regions to confirm gene deletion in X. sontii
| Component | Volume/concentration |
|---|---|
| 5× FIREPol® master mix | 4 μL |
| 5 μM forward primer (P5) | 1 μL |
| 5 μM reverse primer (P6) | 1 μL |
| Template DNA | - |
| Nuclease-free water | - |
| Total | 20 μL |
Note: The volume of nuclease-free water and template DNA is adjusted for each sample.
8. Confirm PCR product by running on a 1% agarose gel with the 100 bp ladder and visualizing a 1,161 bp DNA band for the gene deletion mutant under the UV transilluminator.
E. Chromosomal complementation of the deletion mutant in X. sontii
For gene complementation by reconstitution, the recombinant plasmid (pRM1) containing the complete copy of the target gene from the initial cloning step was electroporated into the mutated strain (PPL1RM15). The process for gene complementation in the gene deletion mutant of X. sontii through homologous recombination is depicted in Figure 5.
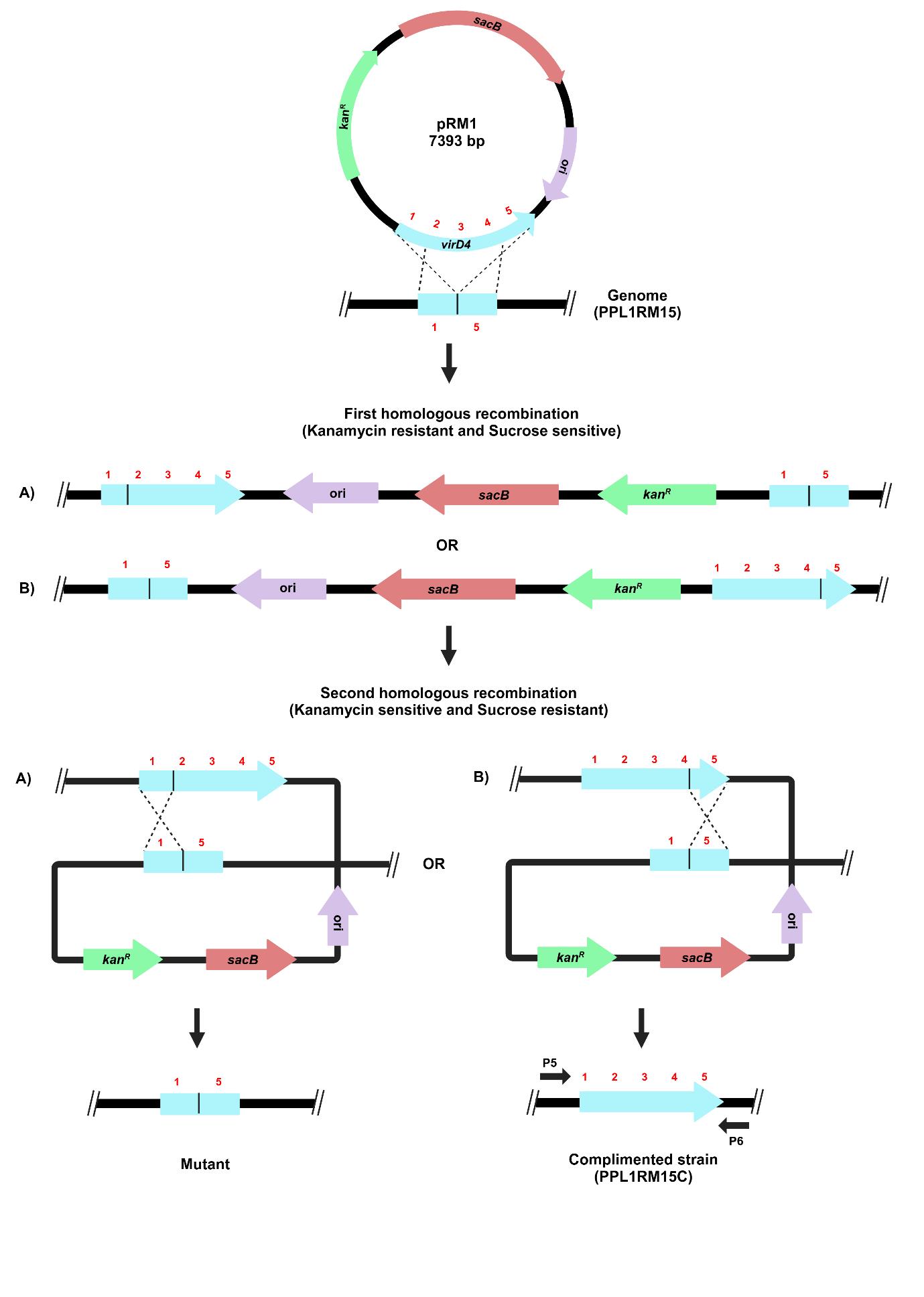
Figure 5. Schematic representation of the target gene complementation protocol through homologous recombination. The recombinant plasmid carrying a complete copy of the virD4 gene, pRM1, was electroporated in X. sontii PPL1RM15 (gene deletion mutant). Strains from the first homologous recombination event were screened on kanamycin, and those from the second homologous recombination event were screened on sucrose.
1. Electroporate the recombinant pk18mobsacB plasmid carrying the complete virD4 gene (pRM1) into electrocompetent cells of the gene deletion mutant (PPL1RM15) following steps D1–3.
2. Screen colonies for the integration of pRM1 into the bacterial genome of PPL1RM15 by PCR amplification of kanR and sacB genes, following steps D4–5.
3. Passage the confirmed strain through NB for five generations and plate on NA + 5% sucrose. Incubate the plates at 28 °C for 24–48 h.
4. Screen colonies after the second homologous recombination event by PCR amplification of the region ~200 bp upstream and ~180 bp downstream of the virD4 gene sequence, following step D7.
5. Confirm the PCR product by running on a 1% agarose gel with the 1 kb ladder and visualizing a 2,016 bp DNA band for gene complementation under the UV transilluminator.
Data analysis
Gene deletion mutant (PPL1RM15) and complemented strain of the mutant (PPL1RM15C) were further validated by whole genome sequencing, which also confirmed clean excision of pK18mobsacB. Whole genome sequencing was carried out using the NovaSeq6000 platform (Neuberg, India). Raw reads quality was assessed using FastQC v0.11.6 (https://qubeshub.org/resources/fastqc), and reads were trimmed using Trim-Galore v0.6.7 [13]. SPAdes v3.15.5 was used for de novo assembly [14]. Assembled genome quality was assessed using Quast v5.2.0 and Checkm v1.2.2 [15,16]. The approximate genome size of the mutant and complemented strain was 4.9 Mb, assembled in 77 and 73 contigs, respectively. The nucleotide sequences of the virD4 gene and pK18mobsacB plasmid were searched in the genomes of PPL1RM15 and PPL1RM15C using NCBI local BLASTn. The pK18mobsacB sequence was not detected in any of the genomes. A clean deletion of 900 bp of the virD4 gene was present in contig 18 (genomic coordinates: 55348-55813) in PPL1RM15, and a clean, complete copy of the virD4 gene was in contig 4 (genomic coordinates: 52202-53875) of PPL1RM15C (Figure 6). The whole genome sequences of PPL1RM15 and PPL1RM15C can be accessed on the Figshare link (https://figshare.com/s/19cfff61f91122f1b936).

Figure 6. Alignment of the virD4 gene in wild-type (PPL1), mutant (PPL1RM15), and complemented strains (PPL1RM15C).
General notes and troubleshooting
General notes
1. The use of E. coli S17-1 and bi-parental mating will further reduce the reliance on electroporation in resource-poor settings.
2. Custom primers with 5’ phosphate rather than a kinase reaction can also be used to increase the yield of 5’-phosphorylated PCR products.
3. In the case of a small-sized gene (~500 bp), flanking regions can be amplified to target the deletion of the complete gene.
4. In the case of bacteria in which colony PCR is difficult and not working, screening can be carried out by isolating genomic DNA.
5. Sanger sequencing of the target region can be used as an alternative to whole genome sequencing to confirm the final gene deletion mutation and complementation.
6. DpnI digests methylated parental DNA. During inverse PCR, there are chances of parental transformants, so it can be used to reduce such transformations and further enhance the efficiency of the protocol.
Troubleshooting
Problem 1: Low recovery of DNA after gel extraction and PCR purification.
Possible cause: Insufficient PCR product volume.
Solution: Multiple PCR reactions (three to four) can be set up and pooled to obtain high-yield DNA.
Problem 2: Reduced transformation efficiency following electroporation.
Possible cause: Insufficient recovery time due to variable generation time and post-electroporation stress response, including metabolic adjustments and recovery processes.
Solution: X. sontii has a short generation time, so the incubation time after electroporation is set to 2 h. This timing can be adjusted based on the generation time of different bacteria.
Problem 3: Primer dimer formation and nonspecific binding of the primers.
Possible cause: Complementary sequences in primers can result in self-annealing, and primers with similarity to multiple sequences can lead to nonspecific binding.
Solution: When designing primers, it is crucial to minimize the potential for dimer formation and nonspecific binding by using various primer design tools, such as AmplifX [8].
Problem 4: Empty vector colonies.
Possible cause: Vector self-ligation.
Solution: Phosphorylation of only the amplified gene product and not the vector largely eliminates instances of vector self-ligation and, hence, empty vector cloning.
Validation of protocol
The part of the protocol related to the creation of the deletion mutant has been validated in the following research article:
Rana et al. [10]. Comparative genomics-based insights into Xanthomonas indica, a non-pathogenic species of healthy rice microbiome with bioprotection function. Appl Environ Microbiol. (Figure 6C, Supplementary Figure 8).
Acknowledgments
RR planned and carried out all the experiments and drafted the manuscript. A.S. helped with the screening of clones and gene deletion mutants. A.S. and A.D. carried out gene complementation. P.B.P. conceived and participated in designing the study and finalized the manuscript. This work was funded by the grant CRG/2022/009447 (GAP238) titled “Genetic and transcriptomics insights into anti-pathogenic activity and ecology of Xanthomonas sontii, a non-pathogenic species of rice microbiome” by the Science and Engineering Research Board, Department of Science and Technology (DST-SERB), Government of India. We also acknowledge the funding provided by the CSIR through the Microbial Type Culture Collection and Gene Bank (MTCC) project MLP-065.
This protocol integrates flow cytometry with t-SNE for high-dimensional data analysis, enabling detailed characterization of cellular populations.
The following figures were created using BioRender:
Graphical overview: BioRender.com/igf3kjm; Figure 1: BioRender.com/a34g618; Figure 2: BioRender.com/v19q851; Figure 3: BioRender.com/olmds0v; Figure 4: BioRender.com/2y52epf; Figure 5: BioRender.com/bw6cdin
Competing interests
The authors declare no competing interests.
References
- Pradhan, B. B., Ranjan, M. and Chatterjee, S. (2012). XadM, a Novel Adhesin of Xanthomonas oryzae pv. oryzae, Exhibits Similarity to Rhs Family Proteins and Is Required for Optimum Attachment, Biofilm Formation, and Virulence. Mol Plant Microbe Interact. 25(9): 1157–1170. https://doi.org/10.1094/mpmi-02-12-0049-r
- Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G. and Pühler, A. (1994). Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 145(1): 69–73. https://doi.org/10.1016/0378-1119(94)90324-7
- Gay, P., Le Coq, D., Steinmetz, M., Berkelman, T. and Kado, C. I. (1985). Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 164(2): 918–921. https://doi.org/10.1128/jb.164.2.918-921.1985
- Pelicic, V., Reyrat, J. M. and Gicquel, B. (1996). Expression of the Bacillus subtilis sacB gene confers sucrose sensitivity on mycobacteria. J Bacteriol. 178(4): 1197–1199. https://doi.org/10.1128/jb.178.4.1197-1199.1996
- Schuster, C. F., Howard, S. A. and Gründling, A. (2019). Use of the counter selectable marker PheS* for genome engineering in Staphylococcus aureus. Microbiology (N Y). 165(5): 572–584. https://doi.org/10.1099/mic.0.000791
- Zhang, X. Z., Yan, X., Cui, Z. L., Hong, Q. and Li, S. P. (2006). mazF, a novel counter-selectable marker for unmarked chromosomal manipulation in Bacillus subtilis. Nucleic Acids Res. 34(9): e71–e71. https://doi.org/10.1093/nar/gkl358
- Bansal, K., Kaur, A., Midha, S., Kumar, S., Korpole, S. and Patil, P. B. (2021). Xanthomonas sontii sp. nov., a non-pathogenic bacterium isolated from healthy basmati rice (Oryza sativa) seeds from India. Antonie van Leeuwenhoek. 114(11): 1935–1947. https://doi.org/10.1007/s10482-021-01652-1
- Jullien, N. AmplifX v2.0.7. Aix-Marseille Univ, CNRS, INP, Inst Neurophysiopathol, Marseille, France, Available online: https://inp.univ-amu.fr/en/amplifx-manage-test-and-design-your-primers-for-pcr
- Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 35(6): 1547–1549. https://doi.org/10.1093/molbev/msy096
- Rana, R., Nayak, P. K., Madhavan, V. N., Sonti, R. V., Patel, H. K. and Patil, P. B. (2024). Comparative genomics-based insights into Xanthomonas indica, a non-pathogenic species of healthy rice microbiome with bioprotection function. Appl Environ Microbiol. 90(9): e00848–24. https://doi.org/10.1128/aem.00848-24
- Chang, A. Y., Chau, V., Landas, J. A. and Pang, Y. (2017). Preparation of calcium competent Escherichia coli and heat-shock transformation. JEMI methods. 1(22–25). https://ujemi.microbiology.ubc.ca/node/127
- Choi, K. H., Kumar, A. and Schweizer, H. P. (2006). A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: Application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods. 64(3): 391–397. https://doi.org/10.1016/j.mimet.2005.06.001
- Krueger, F. (2015). Trim Galore!: A wrapper around Cutadapt and FastQC to consistently apply adapter and quality trimming to FastQ files, with extra functionality for RRBS data. Babraham Institute.
- Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. and Korobeynikov, A. (2020). Using SPAdes De Novo Assembler. Curr Protoc Bioinformatics. 70(1): e102. https://doi.org/10.1002/cpbi.102
- Gurevich, A., Saveliev, V., Vyahhi, N. and Tesler, G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics. 29(8): 1072–1075. https://doi.org/10.1093/bioinformatics/btt086
- Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. and Tyson, G. W. (2015). CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25(7): 1043–1055. https://doi.org/10.1101/gr.186072.114
Article Information
Publication history
Received: Jan 2, 2025
Accepted: Apr 15, 2025
Available online: May 6, 2025
Published: May 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Rana, R., Sharma, A., Dutta, A. and Patil, P. B. (2025). I-PREFR: Inverse PCR-Based Restriction Enzyme FRee Unidirectional Strategy for Rapid Markerless Chromosomal Gene Deletion and Reconstitution in Bacteria Using Suicide Vectors. Bio-protocol 15(10): e5314. DOI: 10.21769/BioProtoc.5314.
Category
Microbiology > Microbial genetics > Genome editing
Molecular Biology > DNA > Chromosome engineering
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









