- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Rapid Miniprep of Intact Chloroplasts from Arabidopsis thaliana Leaves
(*contributed equally to this work) Published: Vol 15, Iss 10, May 20, 2025 DOI: 10.21769/BioProtoc.5313 Views: 2627
Reviewed by: Shweta PanchalYu LiuDheeraj Singh RathoreAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Sorghum bicolor Extracellular Vesicle Isolation, Labeling, and Correlative Light and Electron Microscopy
Deji Adekanye [...] Jeffrey L. Caplan
Oct 5, 2024 2105 Views
Optimized Isolation of Lysosome-Related Organelles from Stationary Phase and Iron-Overloaded Chlamydomonas reinhardtii Cells
Jiling Li and Huan Long
Nov 20, 2024 1754 Views

Isolation and Biophysical Characterization of Extracellular Vesicles From Hairy Root Cultures
Marisa Conte [...] Alfredo Ambrosone
Mar 5, 2025 2197 Views
Abstract
Cell subfractionation is a common technique employed in many research laboratories to isolate organelles or intracellular compartments for the study of metabolism or biomolecule purification. While numerous protocols exist for isolating organelles, few are specifically designed for starting materials in the milligram range. Here, we present a detailed milligram-scale miniprep protocol for purifying intact chloroplasts from Arabidopsis thaliana leaves. This chloroplast miniprep procedure is suitable for applications such as confocal microscopy, western blotting, enzymatic assays, and other downstream analyses.
Key features
• This protocol condenses key aspects of existing methods and adapts them to the miniprep scale.
• Researchers can isolate purified chloroplasts within 1.5 h.
• This protocol requires standard laboratory equipment, such as microcentrifuges (ultracentrifuges are not required).
Keywords: ChloroplastsGraphical overview
Background
Differential centrifugation has substantially enriched available toolkits in cell biology and biochemistry. For example, decades ago, Bensley and Hoerr successfully isolated mitochondria from guinea pig and rabbit liver tissue [1]; later, Chen and Lehninger refined such procedures in higher plants [2]. Around the same time, Walker developed a method for efficiently isolating chloroplasts using sorbitol as osmotic support, enabling successful carbon fixation studies [3]. Later, isopycnic centrifugation protocols allowed for the recovery of highly pure organelle preparations with good yields, albeit requiring substantial quantities of starting material (~100 g) [4]. While these experiments sparked research on isolated organelles, most protocols required starting material quantities in the gram range [5].
During the 1990s, the successful introduction of optimized procedures to isolate DNA, known as minipreps, standardized DNA isolation and manipulation in thousands of laboratories around the world. This allowed the rapid isolation of DNA from bacteria, filamentous microorganisms, and even plants [6–9]. These preparations are typically purchased as kits offering different isolation scales (i.e., minipreps, midipreps, and even maxipreps). Kits specifically designed to isolate various biological materials, including mitochondria, nuclei, or chloroplasts, are also available at different scales and costs. However, most of these kits are either expensive or difficult to obtain in several countries. An effective chloroplast isolation protocol should require minimal biological and laboratory materials. In addition, organelle isolation should be completed as quickly as possible to ensure functional chloroplasts for downstream applications.
The purpose of this protocol is to provide easy-to-follow instructions for isolating intact chloroplasts from leaves in an inexpensive manner, using standard equipment and materials. It requires only small amounts of starting biological material and has the potential for portability (i.e., in-field isolations). The isolated chloroplasts are suitable for western blotting, microscopy, and omics analyses.
Materials and reagents
Biological materials
1. Arabidopsis thaliana natural accession Col-0 (Columbia) (ABRC, CS22625)
Reagents
1. Agar (Sigma-Aldrich, catalog number: A1296-500G)
2. Tween 20 (Sigma-Aldrich, catalog number: P9416-100ML)
3. Commercial bleach solution containing 6% NaOCl
4. D-Mannitol (Sigma-Aldrich, catalog number: M1902-5KG)
5. EDTA (J. T. Baker, catalog number: 8993-01)
6. Hoagland solution (Sigma-Aldrich, catalog number: H2395-10L); use full-strength solution
7. KCl (1 M stock) (J. T. Baker, catalog number: 3040)
8. K2HPO4 (J. T. Baker, catalog number: 3252-01)
9. MgCl2 (2 M stock) (J. T. Baker, catalog number: 2444-01)
10. Miracle-Gro 8 and Miracle Gro perlite (The Scotts Miracle-Gro Company, model: 70752300)
11. Murashige and Skoog (MS) basal medium (Merck, catalog number: M5519)
12. Percoll (GE Healthcare, catalog number: 17-0891-02)
13. Sucrose (Sigma-Aldrich, catalog number: S7903-5KG)
14. Trizma base pH = 7.5 (1 M stock) (Sigma-Aldrich, catalog number: RDD008-1KG)
15. Sunshine Mix 3 substrate (Sungro Horticulture)
16. Anti-PsaB (Agrisera, catalog number: AS10 695)
17. Anti-Actin (Sigma-Aldrich, catalog number: A0480)
Solutions
1. Chloroplast isolation (CI) buffer (see Recipes)
Recipes
1. Chloroplast isolation (CI) buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 400 mM | 27.4 g |
| MgCl2 | 5 mM | 0.5 mL |
| KCl | 10 mM | 2 mL |
| Trizma pH 7.5 | 20 mM | 4 mL |
| Milli-Q Water | Make up to 200 mL |
Laboratory supplies
1. Eppendorf tubes (Sigma-Aldrich, catalog number: EP022431021)
2. Pellet pestles (Sigma-Aldrich, catalog number: Z359947)
3. Microscope slides (Sigma-Aldrich, catalog number: CLS294875X25)
4. Pipettes (2–20 μL, 20–200 μL, and 100–1,000 μL)
5. Precision tweezers (Sigma-Aldrich, catalog number: Z680273)
6. Coverslips
7. Dissecting scissors (Merck, catalog number: Z265969)
8. Miracloth (Merck, catalog number: 475855)
Equipment
1. Autoclave
2. Epifluorescence or confocal microscope
3. Laminar flow hood for MS medium preparation
4. pH potentiometer
5. Vacuum pump
6. Plant incubation chamber (HERATherm, Thermo, catalog number: 50125590)
7. Lordem LED Grow Light (Lordem, catalog number: HL-0026)
8. Mini ceramic mortar and pestle (Sigma-Aldrich, catalog number: Z247464)
9. MC-24R refrigerated microcentrifuge (Benchmark Scientific, catalog number: C2417-R)
Procedure
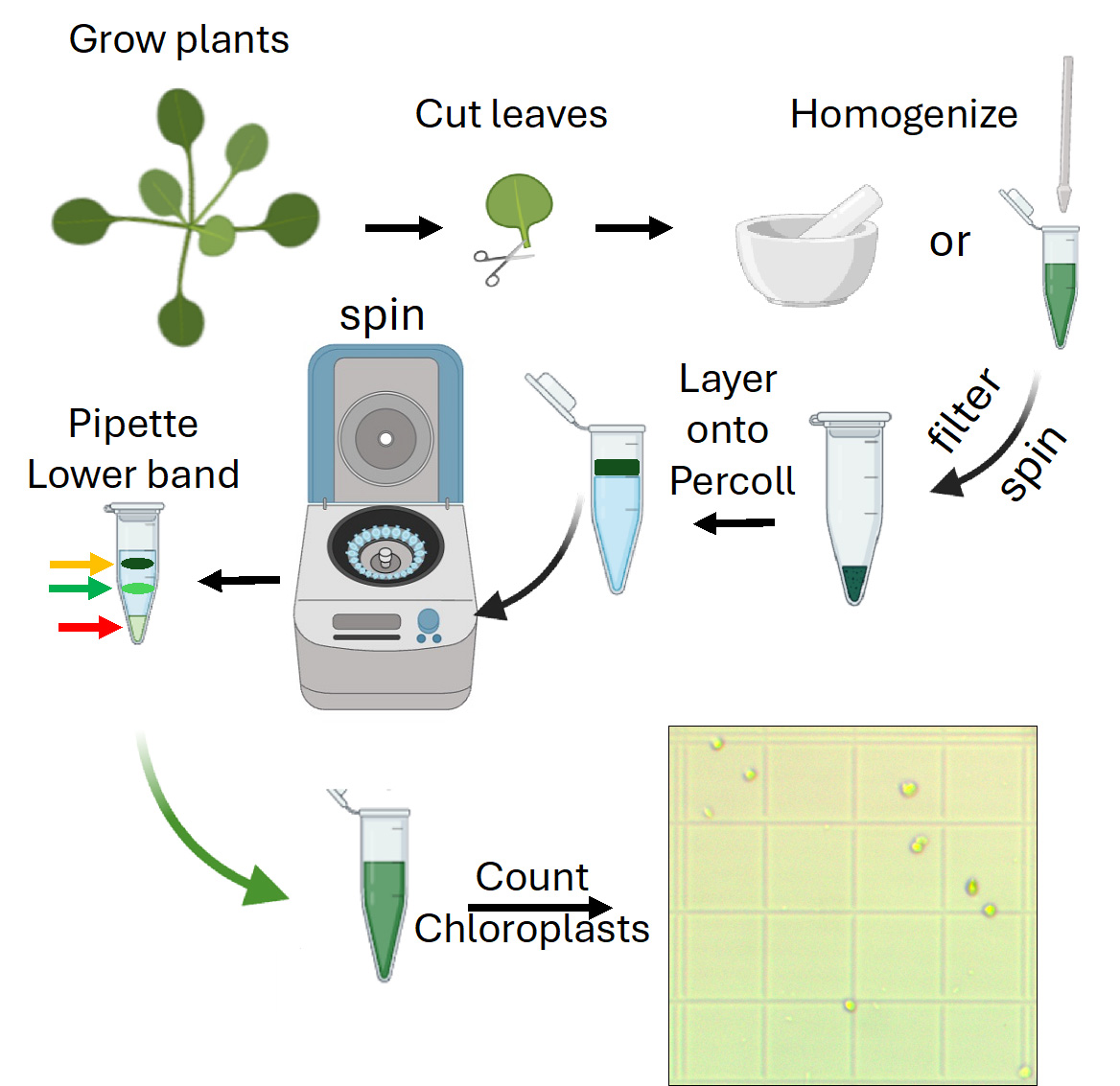
Isolated chloroplasts can be obtained from either cotyledons or true leaves. However, to achieve the desired yields, sufficient initial biological material is required [10]. Reagents such as agar, MS medium, bleach, Tween 20, Hoagland solution, and substrate components (e.g., Sunshine Mix 3, perlite) are included in the materials list as they are used for seed sterilization, stratification, and plant cultivation steps prior to chloroplast isolation, following previously described procedures [11]. This protocol is designed to extract chloroplasts from a minimum of 0.3 g of starting material, making it more practical to use 4-week-old plants grown under standard conditions (16/8 h light/dark, 22 °C, ~100–150 μmol·m-2·s-1 light intensity) rather than younger seedlings. If seedlings are chosen, it will be necessary to germinate many seeds to obtain the minimal mass required for this method to be effective. Conversely, working with leaves from mature plants will simplify this protocol as it will result in higher yields. We recommend 0.5 g of starting material to achieve good yields.
A. Percoll gradient generation and chloroplast isolation (time for completion: 1.5 h)
1. Ensure the leaves, buffers, and materials are kept below 4 °C during the process.
2. Generate a Percoll gradient by centrifuging 0.9 mL of Percoll and 0.1 mL of 2.5 M sucrose in an Eppendorf tube at 16,000× g for 30 min. Use soft braking, if possible, to avoid disturbing the gradient.
3. Cut and weigh ~0.3–0.5 g of true leaves out of the desired plants.
4. Grind leaves in 1 mL of ice-cold CI buffer per 0.1 g of leaf tissue using an ice-cold mini ceramic mortar.
Critical: Make sure leaf tissue is thoroughly homogenized (15–20 pestle passes should suffice for 0.5 g). On the other hand, excessive homogenization can yield broken chloroplasts.
Optional: If the samples require gentler homogenization, Teflon pellet pestles can be used to lyse leaf cells within Eppendorf tubes.
5. Filter the homogenate through a funnel using a 3 cm × 3 cm piece of Miracloth premoistened with CI buffer and distribute evenly into clean, prechilled Eppendorf tubes (Figure 1).
Optional: If Miracloth is not available, filtering can be achieved with Muslin cloth, nylon mesh filters, or four layers of cheesecloth.
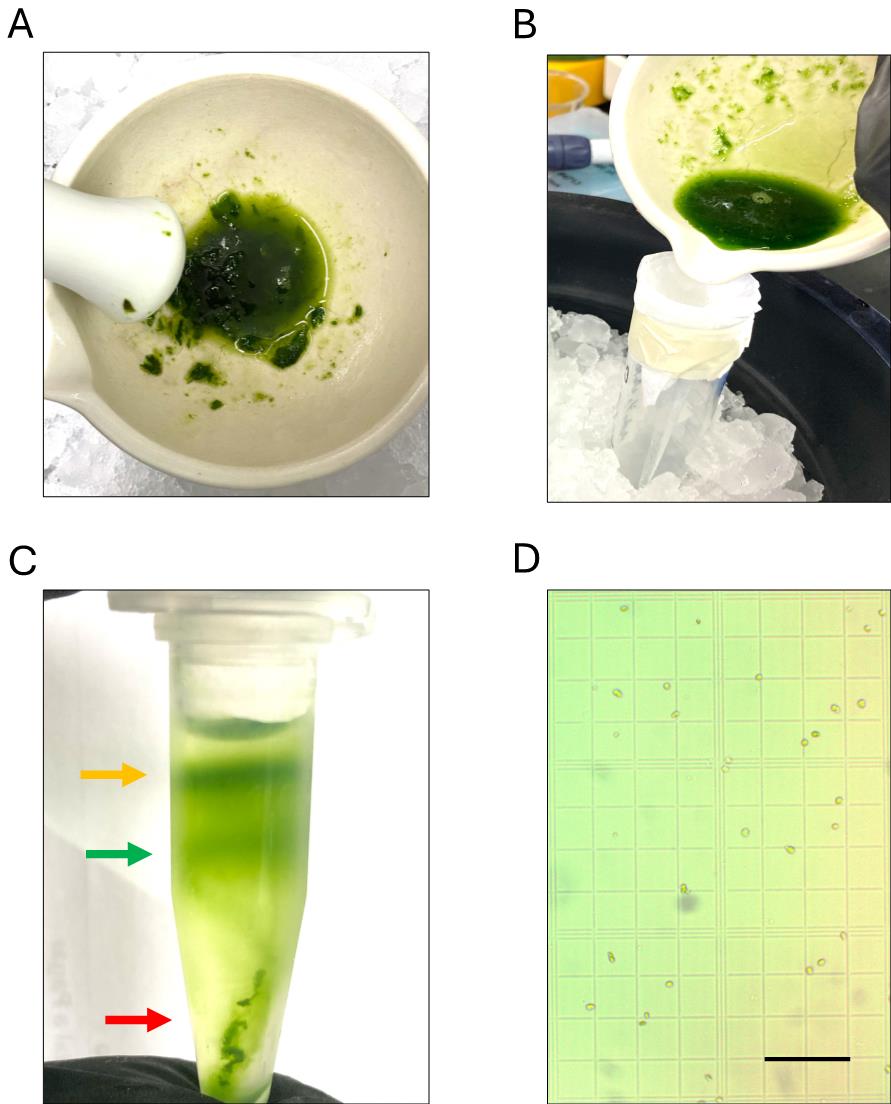
Figure 1. Chloroplast homogenization, filtering, Percoll gradient separation, and yield determination. (A) Suggested extent of homogenization. (B) Homogenate filtration using a prechilled Miracloth with buffer to prevent organelle degradation. (C) After centrifugation in a tube with a preformed Percoll gradient, two green bands should form, with cell debris collected in the pellet. The band in the middle indicates intact chloroplasts (green arrow), the upper band corresponds to broken chloroplasts (yellow arrow), and cell debris is collected at the bottom of the tube (red arrow). (D) Once intact chloroplasts are resuspended and washed, the isolation yield can be determined using an improved Neubauer chamber. Scale bar represents 100 μm.
6. Spin samples at 3,000× g for 8 min at 4 °C.
7. Remove the supernatant using a pipette or by applying vacuum through a vacuum line connected to a pipette tip.
8. Resuspend the pellet (crude chloroplasts) in 0.3 mL of cold CI buffer.
9. Carefully pipette the crude preparation and layer it onto the Percoll gradient.
10. Spin samples at 3,000× g for 15 min at 4 °C.
11. Two green bands should form afterward (Figure 1B). The lower band corresponds to the intact chloroplast samples (Figure 1B, green arrow).
12. Carefully remove the lower band with the aid of a pipette.
13. Place the samples into a new Eppendorf tube.
14. Add CI buffer to a final volume of 1 mL.
15. Spin samples at 3,000× g for 10 min at 4 °C.
16. Remove supernatant using a pipette or by applying vacuum through a vacuum line connected to a pipette tip.
17. Resuspend the pellet in ice-cold CI buffer to a final volume of 1mL.
18. Count chloroplasts with a Newbauer chamber to determine isolation yield.
Pause point: Samples can be either stored at 4 °C for a few hours or used for downstream applications (see troubleshooting).
19. Purified chloroplasts can be readily used for microscopy, western blotting, enzyme assays, or purification, among other biochemical or molecular procedures.
Validation of protocol
Purified chloroplasts can be readily assessed under the microscope at 20–40×. These organelles appear as green disk-shaped organelles and can be counted with the aid of a Neubauer chamber (Figure 1D). In our hands, a typical yield of 1–2 × 106 chloroplasts per gram of fresh leaf tissue is attained with this protocol, depending on the extent of homogenization and the recovery of intact chloroplasts from the Percoll gradient. Alternatively, organellar protein concentration for western blotting or other applications can be assessed spectrophotometrically at 280 nm. Typical sample purity assessments include either western blotting against organellar or cytoplasmic proteins (Figure 2A) or assessing chloroplast structure and morphology using confocal microscopy approaches (Figure 2B).
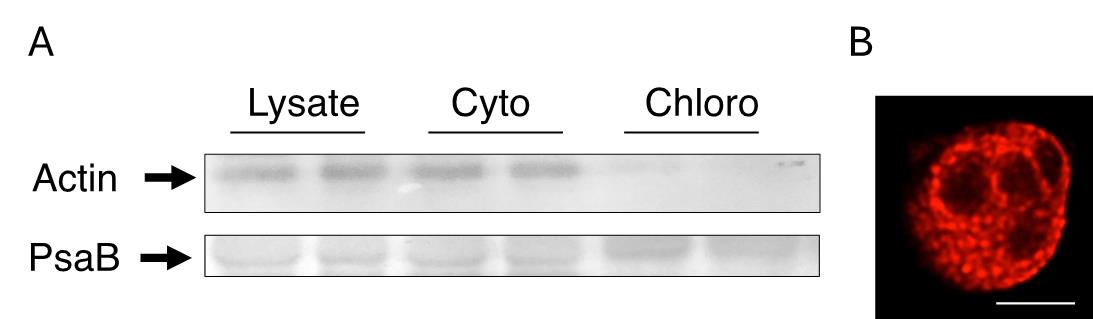
Figure 2. Validation of chloroplast fraction purity and structure. (A) The fractions obtained during chloroplast purification, including whole lysate, cytoplasmic (Cyto), and chloroplast (Chloro) fractions, can be assessed for protein markers such as Actin or PsaB to detect cytoplasmic or chloroplast-specific markers, respectively. (B) Chlorophyll autofluorescence of an isolated chloroplast Z-slice as viewed under the confocal microscope at 63× using light at 400nmex/685nmem. Starch granules appear as dark spots. Scale bar represents 5 μm. Representative experiments n = 3.
Under these conditions, it is possible to determine the lack of contaminating actin on fractions containing purified chloroplasts, while the core subunit of photosystem I (PsaB) is enriched in the same fractions (Figure 2A). Contamination from other organelles (i.e., mitochondria) is typically absent.
General notes and troubleshooting
General notes
This protocol is designed to isolate chloroplasts at a miniprep scale using standard laboratory equipment. The yield of each preparation depends on several factors, including plant incubator light intensity, and can be quantified by standard methods such as chlorophyll content, total protein measurement, or even organelle counting using an improved Neubauer chamber under appropriate dilution conditions. Although chloroplast preparations can be refrigerated, enzymatic activity may degrade over time. Therefore, experiments should be performed within a few hours of isolation to ensure optimal functionality.
Troubleshooting
Problem 1: Western blotting shows degraded proteins.
Possible cause: The presence of contaminating proteases.
Solution: Add protease inhibitors to the CI buffer before tissue homogenization.
Problem 2: Low yield of isolated chloroplasts.
Possible cause 1: Incomplete homogenization of leaves.
Solution: Homogenize leaves in a cold mortar with 5–10 more pestle passes (20–30 passes in total) to ensure efficient breakage while maintaining chloroplast integrity.
Possible cause 2: Inadequate light conditions.
Solution: Ensure light conditions are set to 16 h light/8h dark, 22 °C, and ~100–150 μmol·m-2·s-1 light intensity.
Problem 3: Contamination with proteins from other organelles.
Possible cause: Samples were not retrieved adequately from the Percoll gradient.
Solution: Perform an additional wash step by spinning samples at 3,000× g for 10 min at 4 °C and resuspending in CI buffer.
Acknowledgments
Author contributions: Conceptualization, M.G.-A; methodology, B.A.C-C., K. M. D.-C.; validation, M.G.-A., B.A.C-C., K. M. D.-C.; writing—original draft preparation, M.G.-A.; writing—review and editing, M.G.-A., B.A.C-C., K. M. D.-C.; supervision, M.G-A.; project administration, M.G.-A.; funding acquisition, M.G.-A. All authors have read and agreed to the published version of the manuscript.
This research was funded by Dirección General de Asuntos del Personal Académico (DGAPA) PAPIIT, grant number IN217624, and Facultad de Química, UNAM, PAIP 50009115 to M.G.-A.
The technical support of Jesus Vázquez-Maldonado, Raúl Rodríguez-Ruiz, Leonor G. Mendoza-Vázquez, Montserrat Silva Cruz, Ana Paola Velazquez Arenas, Maria Fernanda Morin Miranda, and Blanca Patricia Robles Hernandez is acknowledged. Marina Gavilanes-Ruiz and Laura Carmona-Salazar kindly provided critical reagents for the completion of this protocol. Natalia Chiquete-Félix and Salvador Uribe-Carvajal provided critical training and resources.
Competing interests
The authors declare no conflicts of interest.
References
- Bensley, R. R. and Hoerr, N. L. (1934). Studies on cell structure by the freezing‐drying method VI. The preparation and properties of mitochondria. Anat Rec. 60(4): 449–455. https://doi.org/10.1002/ar.1090600408
- Chen, C. H. and Lehninger, A. L. (1973). Ca2+ transport activity in mitochondria from some plant tissues. Arch Biochem Biophys. 157(1): 183–196. https://doi.org/10.1016/0003-9861(73)90404-9
- Fiore, M. F., Moon, D. H., Tsai, S. M., Lee, H. and Trevors, J. T. (2000). Miniprep DNA isolation from unicellular and filamentous cyanobacteria. J Microbiol Methods. 39(2): 159–169. https://doi.org/10.1016/s0167-7012(99)00110-4
- Khan, I. A., Awan, F. S., Ahmad, A. and Khan, A. A. (2004). A modified mini-prep method for economical and rapid extraction of genomic DNA in plants. Plant Mol Biol Rep. 22(1): 89–89. https://doi.org/10.1007/bf02773355
- Lu, Y., Wei, L. and Wang, T. (2015). Methods to isolate a large amount of generative cells, sperm cells and vegetative nuclei from tomato pollen for “omics” analysis. Front Plant Sci. 6: e00391. https://doi.org/10.3389/fpls.2015.00391
- O'Sullivan, D. J. and Klaenhammer, T. R. (1993). Rapid Mini-Prep Isolation of High-Quality Plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol. 59(8): 2730–2733. https://doi.org/10.1128/aem.59.8.2730-2733.1993
- Saunders, S. E. and Burke, J. F. (1990). Rapid isolation of miniprep DNA for double strand sequencing. Nucleic Acids Res. 18(16): 4948–4948. https://doi.org/10.1093/nar/18.16.4948
- Seigneurin-Berny, D., Salvi, D., Dorne, A. J., Joyard, J. and Rolland, N. (2008). Percoll-purified and photosynthetically active chloroplasts from Arabidopsis thaliana leaves. Plant Physiol Biochem. 46(11): 951–955. https://doi.org/10.1016/j.plaphy.2008.06.009
- Veiga, T. A. M., King-Díaz, B., Marques, A. S. F., Sampaio, O. M., Vieira, P. C., da Silva, M. F. D. G. F. and Lotina-Hennsen, B. (2013). Furoquinoline alkaloids isolated from Balfourodendron riedelianum as photosynthetic inhibitors in spinach chloroplasts. J Photochem Photobiol B. 120: 36–43. https://doi.org/10.1016/j.jphotobiol.2013.01.006
- Walker, D. A. (1964). Improved Rates of Carbon Dioxide Fixation by Illuminated Chloroplasts. Biochem J. 92(3): 22C–23C. https://doi.org/10.1042/bj0920022c
- Gutiérrez-Mireles, E. R., Páez-Franco, J. C., Rodríguez-Ruíz, R., Germán-Acacio, J. M., López-Aquino, M. C. and Gutiérrez-Aguilar, M. (2023). An Arabidopsis mutant line lacking the mitochondrial calcium transport regulator MICU shows an altered metabolite profile. Plant Signal Behav. 18(1): 2271799. https://doi.org/10.1080/15592324.2023.2271799
Article Information
Publication history
Received: Feb 20, 2025
Accepted: Apr 8, 2025
Available online: Apr 30, 2025
Published: May 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Carranza-Correa, B. A., Dahlman-Cabrera, K. M. and Gutiérrez-Aguilar, M. (2025). Rapid Miniprep of Intact Chloroplasts from Arabidopsis thaliana Leaves. Bio-protocol 15(10): e5313. DOI: 10.21769/BioProtoc.5313.
Category
Plant Science > Plant cell biology > Organelle isolation
Cell Biology > Organelle isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








