- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Cartilaginous Organoid System Derived From Human Expanded Pluripotent Stem Cells (hEPSCs)
(*contributed equally to this work) Published: Vol 15, Iss 9, May 5, 2025 DOI: 10.21769/BioProtoc.5304 Views: 2228
Reviewed by: Xiaokang WuKrishna Murthy NakuluriWilliam C. W. ChenPhilipp Wörsdörfer

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
The Establishment of 3D Polarity-Reversed Organoids From Human Endometrial Tissue as a Model for Infection-Induced Endometritis
Xin Zhang [...] Zhaohui Liu
Jun 20, 2025 1746 Views

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3751 Views
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1249 Views
Abstract
The development of human organotypic models of cartilage provides essential insights into chondrogenesis and chondrocyte hypertrophy while enabling advanced applications in drug discovery, gene editing, and tissue regeneration. Here, we present a robust and efficient protocol for differentiating human expanded pluripotent stem cells (hEPSCs) into hypertrophic chondrocytes through a sclerotome intermediate. The protocol involves initial sclerotome induction, followed by 3D chondrogenic culture and subsequent hypertrophic maturation induced by bone morphogenetic protein-4 (BMP4), thyroid hormone (T3), and β-glycerophosphate. This protocol also allows for sensitive testing of the effects of various compounds on hypertrophic differentiation during the maturation process. Furthermore, we identify an α-adrenergic receptor antagonist, phentolamine, as an inhibitor of hypertrophic differentiation. This organoid system provides a practical platform for exploring cartilage hypertrophy mechanisms and testing therapeutic strategies for cartilage regeneration.
Key features
• This differentiation protocol generates hypertrophic chondrocytes from hEPSCs through a sclerotome intermediate.
• This protocol facilitates sensitive testing of compounds during the hypertrophic maturation stage, enabling the study of molecular mechanisms and therapeutic interventions for cartilage hypertrophy.
• This protocol identifies the α-adrenergic receptor antagonist phentolamine as a modulator of hypertrophic differentiation.
Keywords: Human expanded pluripotent stem cellsGraphical overview
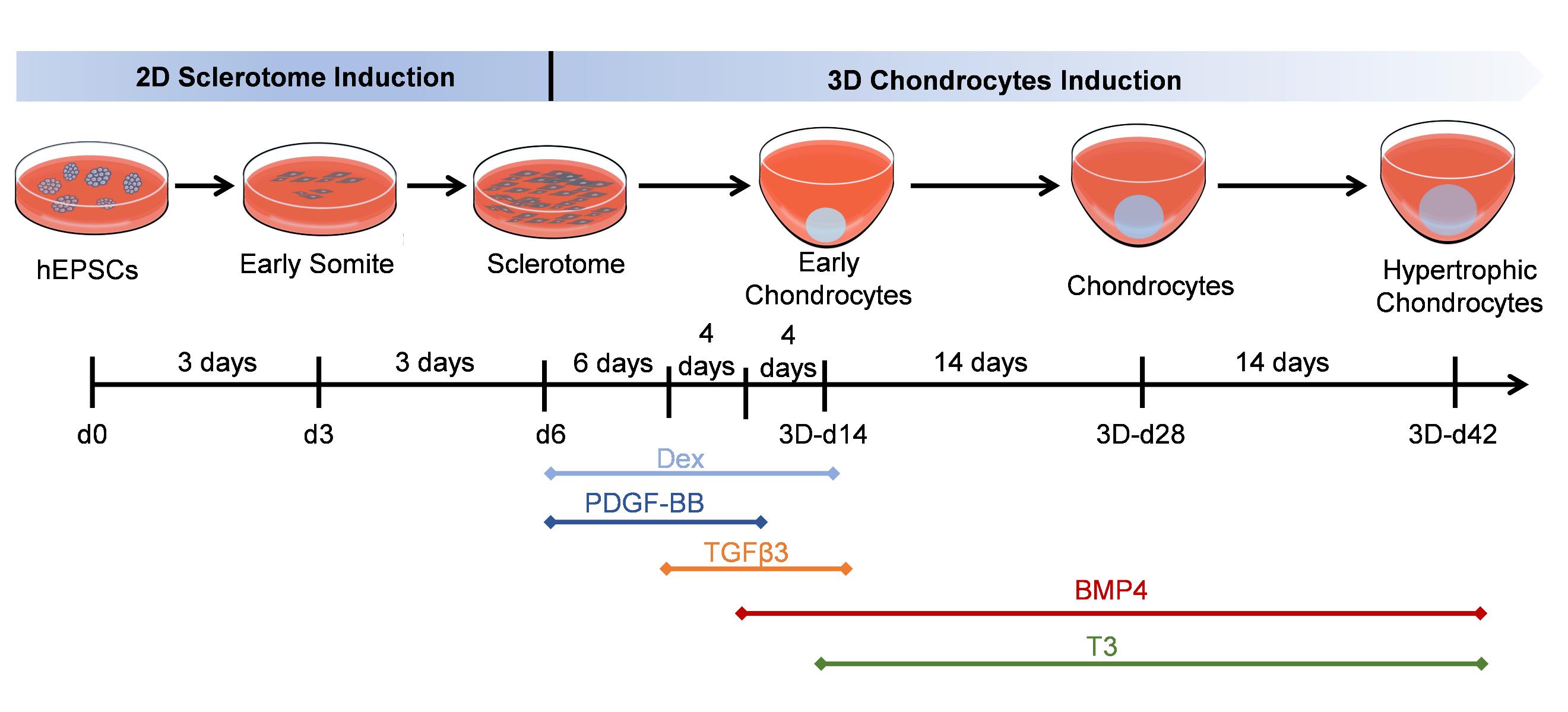
Generate hypertrophic chondrocytes from human expanded pluripotent stem cells (hEPSCs) through a sclerotome intermediate.
Background
Cartilage repair and regeneration remain significant challenges in regenerative medicine due to the limited regenerative capacity of cartilage tissues [1] and the complex biology underlying chondrogenesis and chondrocyte hypertrophy [2,3]. Human organotypic models derived from human pluripotent stem cells (hPSCs) offer a promising approach for advancing cartilage research [4,5]. Several protocols for generating hPSC-derived chondrocytes exist, including differentiation protocols based on mesodermal or sclerotomal induction [6,7], but these protocols often lack the complexity of 3D architecture and do not fully recapitulate the hypertrophic maturation stage.
Here, we provide a reproducible, efficient method for differentiating human expanded pluripotent stem cells (hEPSCs) into hypertrophic chondrocytes. EPSCs have enriched molecular signatures of blastomeres and possess developmental potency for all embryonic and extra-embryonic cell lineages. Our protocol is based on a 6-day-long sclerotome induction [8] followed by a 6+ week 3D chondrogenic culture [9]. Hypertrophic maturation is based on stage-specific administration of transforming growth factors (TGFs) and bone morphogenetic proteins (BMPs), controlling the induction of the sclerotome cells into chondrocyte differentiation and subsequent hypertrophy. After four weeks of maturation, the majority of hEPSC-derived chondrocytes express the typical chondrocyte marker type II collagen (COL2A1). Furthermore, we identify the α-adrenergic receptor antagonist phentolamine as an inhibitor of the hypertrophic chondrocyte marker type X collagen (COL10A1), demonstrating its potency in suppressing hypertrophic differentiation. This protocol systematically replicates the progression of cartilage development, from mesodermal commitment to sclerotome specification, chondroprogenitor differentiation, extracellular matrix maturation, and terminal hypertrophy, faithfully recapitulating the hierarchical stages of chondrogenesis. Additionally, this protocol enables the testing of various compounds for their effects on hypertrophic differentiation, providing a platform for drug discovery and therapeutic development.
Materials and reagents
Biological materials
1. M1 human expanded pluripotent stem cells (hEPSCs) carrying a COL2A1mCherry and COL10A1eGFP double reporter (generated in-house [10])
Reagents
1. Gelatin (Macklin, catalog number: G821281-250g)
2. Phosphate buffer saline (PBS) (Thermo Fisher Scientific, Gibco, catalog number: C10010500BT)
3. DMEM, high glucose, pyruvate (Thermo Fisher Scientific, Gibco, catalog number: C11995500BT)
4. Fetal bovine serum, qualified, Australia (FBS) (Thermo Fisher Scientific, Gibco, catalog number: 10099141C)
5. Penicillin-streptomycin (pen/strep) (5,000 U/mL) (Thermo Fisher Scientific, Gibco, catalog number: 15070063)
6. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650)
7. DMEM/F12 (Thermo Fisher Scientific, Gibco, catalog number: 21331020)
8. N-2 Supplement (100×) (N2) (Thermo Fisher Scientific, Gibco, catalog number: 17502048)
9. B-27 serum-free supplement (50×) (B27) (Thermo Fisher Scientific, Gibco, catalog number: 17504044)
10. GlutaMAX (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
11. MEM non-essential amino acids solution (NEAA) (100×) (Thermo Fisher Scientific, Gibco, catalog number: 11140050)
12. L-ascorbic acid (Sigma-Aldrich, catalog number: 49752-10G, product format: 100 mg/mL in PBS)
13. XAV939 (Sigma-Aldrich, catalog number: X3004, product format: 50 mM in DMSO)
14. CHIR99021 (TOCRIS, catalog number: 4423, product format: 10 mM in DMSO)
15. A419259 (TOCRIS, catalog number 3914, product format: 5 mM in sterile H2O)
16. Human leukemia inhibitory factor (LIF) (Sigma-Aldrich, catalog number: LIF1050)
17. 2-mercaptoethanol (Sigma-Aldrich, catalog number: M6250)
18. Y-27632 (APExBIO, catalog number: A3008, 100 mM in PBS)
19. 0.25% trypsin/EDTA (Thermo Fisher Scientific, Gibco, catalog number: 25200072)
20. Matrigel (Corning, catalog number: 356230)
21. IMDM, GlutaMAXTM supplement (IMDM+Glu) (Thermo Fisher Scientific, Gibco, catalog number: 31980030)
22. Ham’s F12, GlutaMAXTM supplement (Ham’s F12+Glu) (Thermo Fisher Scientific, Gibco, catalog number: 31765035)
23. 7.5% BSA solution (Solarbio, catalog number: H1130)
24. Chemically defined lipid concentrate (CD lipid) (Thermo Fisher Scientific, Gibco, catalog number: 11905031)
25. Insulin, transferrin, selenium, ethanolamine solution (ITS-X) (Thermo Fisher Scientific, Gibco, catalog number: 51500056)
26. Monothioglycerol (Sigma-Aldrich, catalog number: M6145)
27. Activin A (R&D Systems, catalog number: 338-AC-050, product format: 100 μg/mL in sterile 4 mM HCl)
28. Recombinant human FGF basic (FGF2) (R&D Systems, catalog number: 233-FB-025, product format: 250 μg/mL in PBS containing 0.1% BSA)
29. A8301 (Sigma-Aldrich, catalog number: SML0788, product format: 10 mM in DMSO)
30. LDN193189 (Stemgent, catalog number: 04-0074, product format: 10 mM in DMSO)
31. C59 (Cellagentech, catalog number: C7641-2s, product format: 10 mM in DMSO)
32. PD173074 (TOCRIS, catalog number: 3044, product format: 10 mM in DMSO)
33. SAG (Sigma-Aldrich, catalog number: SML1314-1MG, product format: 1 mM in H2O)
34. DMEM/F-12, GlutaMAXTM supplement (DMEM/F12+Glu) (Thermo Fisher Scientific, Gibco, catalog number: 10565018)
35. Proline (Sigma-Aldrich, catalog number: P5607, product format: 50 mg/mL in PBS)
36. D -(+)-Glucose solution (glucose) (Sigma-Aldrich, catalog number: G8769, product format: 45% in H2O)
37. Sodium pyruvate (Sigma-Aldrich, catalog number: S8636)
38. Dexamethasone (Dex) (Wako, catalog number: 047-18863, product format: 1 mM in ethanol)
39. Human PDGF-BB proteins (PDGF-BB) (R&D Systems, catalog number: 220-BB-010, product format: 100 μg/mL in sterile 4 mM HCl)
40. Recombinant human TGF-beta 3 protein (TGFβ3) (R&D Systems, catalog number: 243-B3-002, product format: 20 μg/mL in sterile 4 mM HCl containing 0.1% BSA)
41. Recombinant human BMP4 protein (BMP4) (R&D Systems, catalog number: 314-BP-010, product format: 100 μg/mL in sterile 4 mM HCl containing 0.1% BSA)
42. L-3,3,5-Triiodothyronine, free acid (T3) (Sigma-Aldrich, catalog number: 642511, product format: 0.1 mM in sterile 0.1% NaOH)
43. β-glycerophosphate (Sigma-Aldrich, catalog number: 50020, product format: 1 M in DMEM/F12+Glu)
44. Phentolamine (TargetMol, catalog number: T1275, product format: 10 mM in DMSO)
Solutions
1. Gelatin coating (see Recipes)
2. Feeder medium (M10) (see Recipes)
3. hEPSC medium (hEPSCM) (see Recipes)
4. hEPSC subculture medium (see Recipes)
5. Matrigel coating (see Recipes)
6. Chemically-defined basal medium (CDBM) (see Recipes)
7. Anterior primitive streak induction medium (APSIM) (see Recipes)
8. Paraxial mesoderm induction medium (PMIM): 2D-d1 (see Recipes)
9. Early somite induction medium (ESIM): 2D-d2 (see Recipes)
10. Sclerotome induction medium (SCLIM): 2D-d3–d6 (see Recipes)
11. Chondrogenic basal medium (CBM) (see Recipes)
12. Early chondrocyte induction medium (ECIM): 3D-d0–d6 (see Recipes)
13. Chondrocyte differentiation medium 1 (CDIM1): 3D-d6–d10 (see Recipes)
14. Chondrocyte differentiation medium 2 (CDIM2): 3D-d10–d14 (see Recipes)
15. Chondrocyte maturation medium (CMM): 3D-d14–d28 (see Recipes)
16. Chondrocyte hypertrophy medium (CHM): 3D-d28–d42 (see Recipes)
Recipes
1. Gelatin coating
First, prepare a stock concentration (20×): Dilute 2 g of gelatin granules in 100 mL of H2O and autoclave. Aliquot the 20×solution in 10 mL/aliquot. Next, prepare the working concentration (1×): Dilute 2 mL of the stock concentration (20×) with 38 mL of sterile H2O to make a 1× solution. Both 20× and 1× aliquots can be stored at 4 °C.
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| H2O | 100% | 100 mL |
| Gel (granules) | 2% | 2 g |
| Reagent | Working concentration | Quantity or Volume |
|---|---|---|
| H2O | 99.9% | 38 mL |
| Gel (20×) | 0.1% | 2 mL |
2. Feeder medium (M10)
Prepare ~500 mL of bulk solution of DMEM-HG, FBS, and pen/strep and filter sterilize. Thaw the frozen FBS at 2–8 °C overnight. Mix the thawed FBS and pen/strep by gently inverting the vial a couple of times and then aseptically transfer it to the bottle of DMEM-HG. Swirl the bottle to mix. M10 can be stored at 2–8 °C for up to two weeks.
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM-HG | 89% | 445 mL |
| FBS | 10% | 50 mL |
| Pen/strep | 1% | 5 mL |
| Total | 100% |
3. hEPSC medium (hEPSCM)
Prepare ~500 mL of the bulk basic medium (DMEM/F12, N2, B27, GlutaMAX, pen/strep, NEAA, L-ascorbic acid) and filter sterilize. Dissolve the small molecule compounds in advance and dispense the required amounts needed to prepare 500 mL of the medium. Add fresh small molecule compounds each time the medium is prepared, avoiding repeated freezing and thawing. Combine all required ingredients into the base medium and swirl gently to mix. Dispense the medium promptly and store any unused portion at -20 °C. If needed, thaw the unused medium overnight at 2–8 °C. hEPSCM can be stored at 2–8 °C for two weeks. Filter sterilize and bring hEPSCM to room temperature before use.
| Reagent | Final concentration | Volume (for 500 mL) |
|---|---|---|
| DMEM/F12 | 95.5% | 477.5 mL |
| N2 | 0.5% | 2.5 mL |
| B27 | 1% | 5 mL |
| GlutaMAX | 1% | 5 mL |
| Pen/strep | 1% | 5 mL |
| NEAA | 1% | 5 mL |
| L-ascorbic acid | 50 μg/mL | 250 μL |
| XAV939 | 5 μM | 50 μL |
| CHIR99021 | 1 μM | 50 μL |
| A-419259 | 0.1 μM | 10 μL |
| LIF | 10 ng/mL | 50 μL |
| 2-mercaptoethanol | 10 μM | 5 μL |
4. hEPSC subculture medium
Prepare ~50 mL of bulk solution of basic medium (hEPSCM and FBS) and filter sterilize. Mix the thawed FBS by gently inverting the vial a couple of times and then aseptically transfer it to the bottle of hEPSCM. To prepare the hEPSC subculture medium, make an aliquot of the required volume for the day of hEPSCM+FBS and add Y-27632 fresh on the day of use. Filter sterilize and bring the hEPSC subculture medium solution to room temperature before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| hEPSCM | 95% | 47.5 mL |
| FBS | 5% | 2.5 mL |
| Y-27632 | 10 μM | 5 μL |
5. Matrigel coating
Place the entire bottle of Matrigel in a crushed ice box, then store the ice box in a 4 °C refrigerator overnight to thaw. Ensure there is enough crushed ice to surround the bottle. After the Matrigel has melted, gently rotate the bottle to check that it has fully thawed, with no remaining ice crystals or solid clumps. Pre-cool all consumables that will come into contact with the Matrigel, such as pipette tips and tubes, and perform all procedures aseptically on ice. Dispense the Matrigel into 400 μL aliquots per tube and store at -80 °C. When needed, place the dispensed Matrigel in a crushed ice box to thaw, then transfer it to a 4 °C refrigerator overnight. Once melted, quickly transfer the Matrigel into pre-cooled sterile PBS and mix thoroughly. Matrigel-coated surfaces can be stored at 2–8 °C for up to one week.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| PBS | 99% | 39.6 mL |
| Matrigel | 1% | 400 μL |
6. Chemically-defined basal medium (CDBM)
Prepare ~200 mL of bulk solution of basic medium (IMDM+Glu, Ham’s F12+Glu, BSA, CD lipid, ITS-X, and pen/strep) and filter sterilize. Add all ingredients to the base medium as required. Swirl the bottle to mix. CDBM can be stored at 2–8 °C for two weeks. Filter sterilize and bring the CDBM to room temperature before use.
| Reagent | Final concentration | Volume (for 200 mL) |
|---|---|---|
| IMDM+Glu | 45.25% | 90.5 mL |
| Ham’s F12+Glu | 45.25% | 90.5 mL |
| BSA | 5 mg/mL | 13.35 mL |
| CD lipid | 1% | 2 mL |
| ITS-X | 1% | 2 mL |
| Pen/strep | 1% | 2 mL |
| Monothioglycerol | 450 μM | 7.8 μL |
7. Anterior primitive streak induction medium (APSIM): 2D-d0
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare APSIM, make an aliquot of the required volume for the day of basic medium solution and add Activin A, CHIR99021, and FGF2 fresh on the day of use. Filter sterilize and bring the APSIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| Activin A | 50 ng/mL | 20 μL |
| CHIR99021 | 10 μM | 40 μL |
| FGF2 | 20 ng/mL | 3.2 μL |
8. Paraxial mesoderm induction medium (PMIM): 2D-d1
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare PMIM, make an aliquot of the required volume for the day of basic medium solution and add A8301, LDN193189, CHIR99021, and FGF2 fresh on the day of use. Filter sterilize and bring the PMIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| A8301 | 1 μM | 4 μL |
| LDN193189 | 250 nM | 1 μL |
| CHIR99021 | 3 μM | 12 μL |
| FGF2 | 20 ng/mL | 3.2 μL |
9. Early somite induction medium (ESIM): 2D-d2
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare ESIM, make an aliquot of the required volume for the day of basic medium solution and add C59 and PD173074 fresh on the day of use. Filter sterilize and bring the ESIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| C59 | 1 μM | 4 μL |
| PD173074 | 100 nM | 0.4 μL |
10. Sclerotome induction medium (SCLIM): 2D-d3–d6
Prepare >40 mL of CDBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare SCLIM, make an aliquot of the required volume for the day of basic medium solution and add LDN193189 and SAG fresh on the day of use. Filter sterilize and bring the SCLIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 40 mL) |
|---|---|---|
| CDBM | 100% | 40 mL |
| LDN193189 | 600 nM | 2.4 μL |
| SAG | 100 nM | 4 μL |
11. Chondrogenic basal medium (CBM)
Prepare >500 mL of the bulk solution of basic medium (DMEM/F12+Glu, ITS-X, L-ascorbic acid 2-phosphate, proline, glucose, sodium pyruvate, and pen/strep) and filter sterilize. Add all ingredients to the base medium as required. Swirl the bottle to mix. CBM can be stored at 2–8 °C for two weeks. Filter sterilize and bring the CBM to room temperature before use.
| Reagent | Final concentration | Volume (for 500 mL) |
|---|---|---|
| DMEM/F12+Glu | 95.6% | 478 mL |
| ITS-X | 1% | 5 mL |
| L-ascorbic acid 2-phosphate | 0.17 mM | 250 μL |
| Proline | 0.35 mM | 400 μL |
| Glucose | 0.15% | 1.67 mL |
| Sodium pyruvate | 1% | 5 mL |
| GlutaMAX | 1% | 5 mL |
| Pen/strep | 1% | 5 mL |
12. Early chondrocyte induction medium (ECIM): 3D-d0–d6
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare ECIM, make an aliquot of the required volume for the day of basic medium solution and add Dex and PDGF-BB fresh on the day of use. Filter sterilize and bring the ECIM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| Dex | 0.1 μM | 5 μL |
| PDGF-BB | 40 ng/mL | 20 μL |
13. Chondrocyte differentiation medium 1 (CDIM1): 3D-d6–d10
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CDIM1, make an aliquot of the required volume for the day of basic medium solution and add Dex, PDGF-BB, and TGFβ3 fresh on the day of use. Filter sterilize and bring the CDIM1 solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| Dex | 0.1 μM | 5 μL |
| PDGF-BB | 40 ng/mL | 20 μL |
| TGFβ3 | 10 ng/mL | 25 μL |
14. Chondrocyte differentiation medium 2 (CDIM2): 3D-d10–d14
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CDIM2, make an aliquot of the required volume for the day of basic medium solution and add Dex, TGFβ3, and BMP4 fresh on the day of use. Filter sterilize and bring the CDIM2 solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| Dex | 0.1 μM | 5 μL |
| TGFβ3 | 10 ng/mL | 25 μL |
| BMP4 | 50 ng/mL | 25 μL |
15. Chondrocyte maturation medium (CMM): 3D-d14–d28
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CMM, make an aliquot of the required volume for the day of basic medium solution and add Dex, BMP4, and T3 fresh on the day of use. Filter sterilize and bring the CMM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| BMP4 | 50 ng/mL | 25 μL |
| T3 | 10 nM | 5 μL |
16. Chondrocyte hypertrophy medium (CHM): 3D-d28–d42
Prepare >50 mL of CBM and filter sterilize. The basic medium can be stored at 2–8 °C for two weeks. To prepare CHM, make an aliquot of the required volume for the day of basic medium solution and add Dex, TGFβ3, BMP4, and β-glycerophosphate fresh on the day of use. Filter sterilize and bring the CHM solution to 37 °C before use.
| Reagent | Final concentration | Volume (for 50 mL) |
|---|---|---|
| CBM | 100% | 50 mL |
| BMP4 | 50 ng/mL | 25 μL |
| T3 | 10 nM | 5 μL |
| β-glycerophosphate | 10 mM | 500 μL |
Laboratory supplies
1. 1.5 mL MaxyClear Snaplock (Axygen, catalog number: MCT-150-C)
2. Cell culture multi-well plates (12-well plate) (Corning, catalog number: 3513)
3. Cell culture multi-well plates (6-well plate) (Corning, catalog number: 3471)
4. 15 mL conical tubes (Labselect, catalog number: CT-012-15)
5. 50 mL conical tubes (Labselect, catalog number: CT-012-50)
6. 2 mL cryovials (NEST, catalog number: 607101)
7. 10 mL serological pipettes (Corning, catalog number: 4488)
8. 25 mL serological pipettes (Corning, catalog number: 4489)
9. Millex-GP syringe filter unit, pore 0.22 µm (Millipore, catalog number: SLGPR33RB)
10. 20 mL disposable sterilized syringe (Jetway, catalog number: ZSQ-20mL)
11. 10 μL pipette tips (Kirgen, catalog number: KG1011)
12. 200 µL pipette tips (Kirgen, catalog number: KG1212)
13. 1,000 µL pipette tips (Kirgen, catalog number: KG1313)
14. CountessTM cell counting chamber slides (Thermo Fisher Scientific, Invitrogen, catalog number: C10283)
15. 96-well clear round bottom ultra-low attachment microplate (Corning, catalog number: 7007)
16. 50 mL reagent reservoirs (Biosharp, catalog number: BS-PS50-RR)
Equipment
1. NordicSafe® Class II biological safety cabinet (ESCO, catalog number: NC2-L)
2. HeracellTM Vios 160i CR CO2 incubator (Thermo Fisher Scientific, catalog number: 51033773)
3. Olympus inverted fluorescence microscope (objectives: 4×, 10×, 20×) (Olympus, catalog number: IX73)
4. Laboratory centrifuge with rotors for 15 and 50 mL conical tubes and plates (Thermo Fisher Scientific, catalog number: 75007200)
5. Block M heating metal bath (FOUR E’S, catalog number: TC0401002)
6. 0.1–2.5 μL pipetter (Eppendorf, catalog number: 3123000217)
7. 0.5–10 μL pipetter (Eppendorf, catalog number: 3123000225)
8. 10–100 μL pipetter (Eppendorf, catalog number: 3123000241)
9. 20–200 μL pipetter (Eppendorf, catalog number: 3123000250)
10. 100–1000 μL pipetter (Eppendorf, catalog number: 3123000268)
11. 30–300 μL 8-channel pipette (Eppendorf, catalog number: 3123000101)
12. Mr. FrostyTM freezing container (Thermo Fisher Scientific, catalog number: 5100-0001)
13. CountessTM II FL automated cell counter (Thermo Fisher Scientific, catalog number: AMQAF1000)
14. Racks
15. Liquid nitrogen (N2) tank
16. Freezer (-20 °C)
17. Refrigerator (2–8 °C)
Procedure
A. Preparation of hEPSCs for differentiation
1. Prepare SNL76/7 STO feeders according to standard protocol [11]. Plate feeders (7 × 105 cells/well) on gelatin-coated 6-well plates in 2 mL/well of feeder medium until they reach confluency. hEPSCs are seeded on confluent feeders in hEPSC subculture medium at a seeding density of 1.2 × 104 cells/well. The medium is changed 24 h later to hEPSCM for cell expansion and replenished daily (see Recipes 1–4).
2. Cell lines are prepared for differentiation at approximately 80% confluency. Passage cells twice to ensure complete removal of feeder cells.
3. For each passage, remove the hEPSCM, wash cells once with 1 mL/well of PBS, and incubate with 1 mL/well of room temperature 0.05% trypsin/EDTA for 1–2 min at 37 °C with 5% CO2 to dissociate the feeders.
4. After incubation, gently tap the plate. The feeders will detach first. Remove the dissociated feeders with the spent trypsin/EDTA.
5. hEPSC colonies may require additional incubation time. Add fresh room temperature 0.05% trypsin/EDTA and incubate for 1–2 min at 37 °C with 5% CO2 to dissociate the colonies.
6. Neutralize the trypsin with 2 mL of M10 (see Recipe 2).
7. Centrifuge the cells at 1,000× g for 5 min, discard the supernatant, and resuspend the cells in fresh hEPSC subculture medium.
8. Prepare Matrigel-coated 6-well plates and incubate them at 37 °C with 5% CO2 for at least 1 h (see Recipe 5). Ensure the plates remain hydrated at all times.
9. Discarded the Matrigel solution. Plate cells at a 1:2 ratio on Matrigel-coated 6-well plates in hEPSC subculture medium, ensuring even distribution. Incubate the plate at 37 °C with 5% CO2.
Note: During the feeder removal process, hEPSCs were plated at a relatively high density to ensure optimal clonal growth.
10. The following morning, aspirate the medium and wash cells gently with 2 mL of PBS per well to remove residual serum. Add 2 mL of hEPSCM to each well.
11. Passage cells again when they reach approximately 80% confluency following the same procedure to ensure complete removal of feeder cells.
B. Sclerotome induction
1. Dissociate undifferentiated hEPSCs using 1 mL of 0.05% trypsin/EDTA for 1–2 min. Neutralize trypsin with 2 mL of M10. Gently pipette down the loosened colonies and transfer the cell suspension to individual 15 mL conical tubes.
Critical: Avoid excess pipetting to sustain clumps of colonies. Gently scrape the loosened colonies with a cell scraper if necessary.
2. Centrifuge at 1,000× g for 5 min, discard the supernatant, and gently resuspend cell clumps in fresh hEPSC subculture medium containing 10 μM Y-27632.
3. Sparsely passage cell suspension at 1:6 onto new Matrigel-coated cell culture plates in hEPSC subculture medium. Incubate the plate at 37 °C with 5% CO2.
Note: To promote complete differentiation, cells should be seeded as sparse clumps to maintain a single-cell state during differentiation. The cell clumps are necessary for cell–cell contact to survive d1–d6 of the differentiation protocol.
4. The following morning, aspirate the medium and briefly wash the cells in PBS. Add anterior primitive streak induction medium (see Recipe 7).
Note: Mark as day 0 (d0). Sparse, round cell clusters can be observed. See Figure 1.
5. After 24 h incubation, aspirate the medium and briefly wash the cells in PBS. Add paraxial mesoderm induction medium (see Recipe 8).
Note: Mark as d1.
6. After 24 h incubation, aspirate the medium and briefly wash the cells in PBS. Add early somite induction medium (see Recipe 9).
Note: Mark as d2. Cells radiate outward from the cell clusters. See Figure 1.
7. After 24 h incubation, aspirate the medium and briefly wash the cells in PBS. Add sclerotome induction medium (see Recipe 10).
Note: Mark as d3. Cells grow outward in a radial pattern and gradually merge with neighboring cells. See Figure 1.
8. Incubate the cells in sclerotome induction medium for 48–72 h until they fully merge and form a spiral structure. Replace the sclerotome induction medium every 24 h.
Note: Mark as d4–d6. Cells are usually 100% confluent on d6 and form a spiral structure. See Figure 1.
C. Chondrocytes induction
1. Wash sclerotome cells twice with PBS to remove suspended dead cells.
2. Incubate with 1 mL/well of room temperature 0.05% trypsin/EDTA for 5–8 min at 37 °C with 5% CO2.
3. Neutralize the trypsin with 2 mL of M10 and carefully pipette up and down to dissociate sclerotome cells into signal cells.
4. Centrifuge at 1,000× g for 5 min, discard the supernatant, and resuspend cells in sclerotome induction medium + 10 μM Y-27632.
5. Plate the cells in a non-adhesive round-bottom 96-well plate at 2.5–5×105 cells per well with 100 μL/well of sclerotome induction medium + 10 μM Y-27632.
Note: Adjusting the number of seeded cells allows the formation of spheroids in various sizes.
6. Centrifuge the plate at 300× g for 5 min. Incubate the plate overnight at 37 °C with 5% CO2.
Note: After centrifugation, the cells gather at the center of each well.
7. The following morning, carefully aspirate the medium with a narrow-tipped pipette. Add 100 μL of early chondrocyte induction medium per well (see Recipe 12). Incubate the plate for 6 days. Change the medium once during this stage with fresh chondroprogenitor induction medium.
Notes:
a. The cells aggregate toward the center, forming spherical structures after 6 days of incubation. Mark as 3D-d7.
b. It is not necessary to wash the spheroids before changing the medium. Ensure the culture medium is removed as thoroughly as possible.
c. For large-scale operations, use a multi-channel pipette to replace the culture medium.
8. Carefully aspirate the medium. Add chondrocyte differentiation medium 1. Incubate the plate for 4 days (see Recipe 13).
Note: It is not necessary to change medium during this stage.
9. Carefully aspirate the medium. Add chondrocyte differentiation medium 2. Incubate the plate for 4 days (see Recipe 14).
Note: It is not necessary to change medium during this stage. The size of the spheroids expands noticeably by the end of this stage. Mark as 3D-d14.
10. Carefully aspirate the medium. Add chondrocyte maturation medium. Incubate the plate for 14 days (see Recipe 15). Change the medium every 3 days.
Note: The spheroids exhibit intense COL2A1 expression by the end of this stage. Mark as 3D-d28.
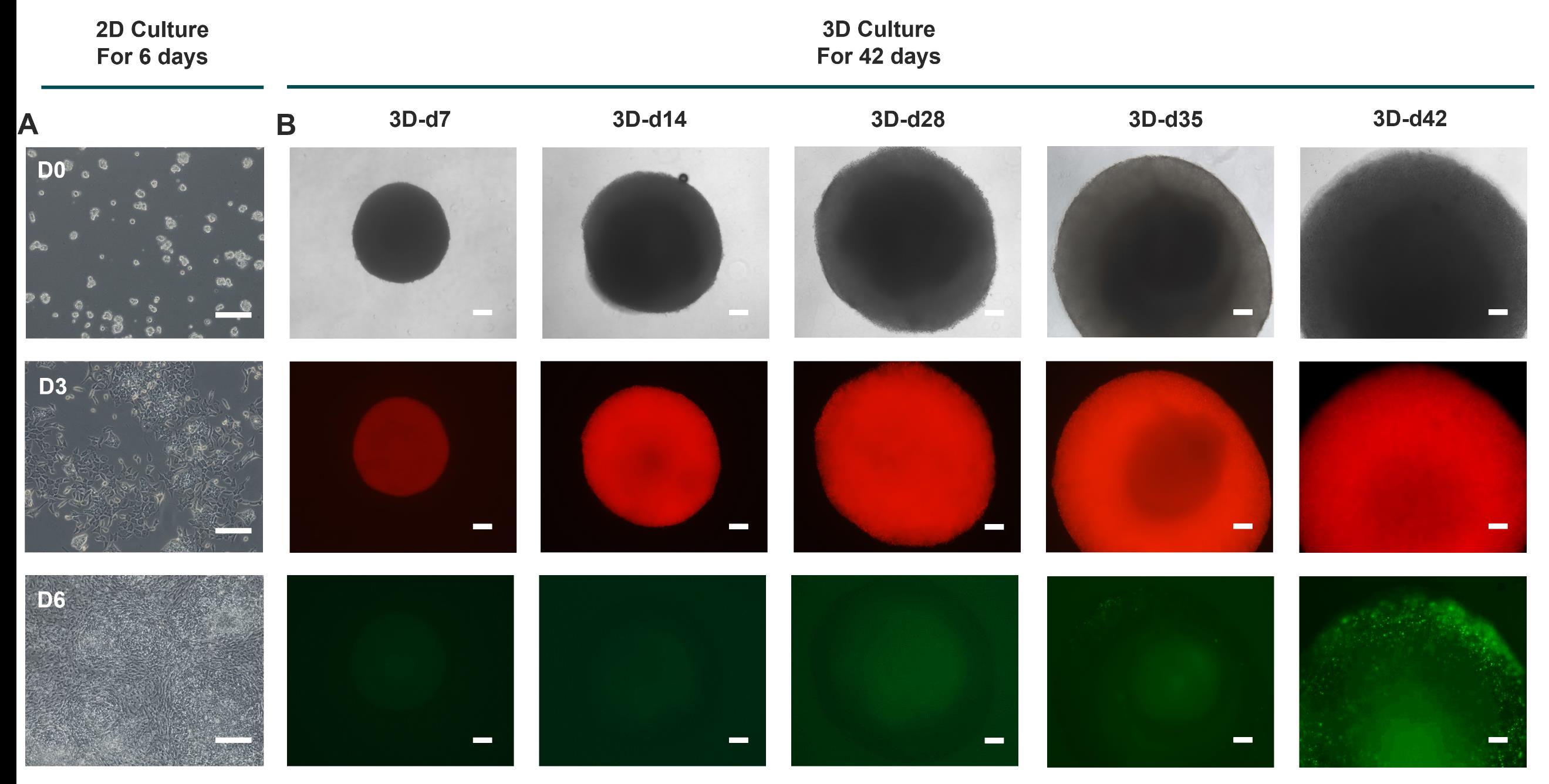
Figure 1. Cell morphology during the hypertrophic chondrocyte differentiation protocol. (A) Brightfield images of cells during sclerotome differentiation protocol. Scale bar: 200 μm. (B) Top, brightfield images of spheroids at representative time points. Middle, representative fluorescent (red) images at representative time points during the 3D culture process. Bottom, representative fluorescent (green) images at representative time points during the 3D culture process. Scale bars: 200 μm. This figure is adapted from Xiaocui Wei et al. [10].
D. Hypertrophy chondrocytes induction and compound testing
1. For hypertrophy chondrocytes induction:
a. Carefully aspirate the medium of 3D-d28 spheroids and add chondrocyte hypertrophy medium (CHM) (see Recipe 16).
b. Incubate in chondrocyte hypertrophy medium for up to 14–28 days. Change the medium every 3 days.
Note: The size of the spheroids increases significantly during this stage. The spheroids exhibit intense COL10A1 expression after 14 days of incubation. Mark as 3D-d42. The spheroids can reach a size of up to 2 mm by 3D-d42.
2. For testing the effects of compounds on hypertrophic differentiation:
a. Prepare each compound in CHM. Include equal amounts of DMSO in CHM as a control.
b. Carefully aspirate the medium from 3D-d28 spheroids and add CHM + compound or CHM + DMSO. Change the medium every 3 days.
c. Analyze the expression of markers after at least 7 days of incubation.
Note: CHM + 1 μM phentolamine shows decreased COL10A1 expression compared to CHM + DMSO after 14 days of incubation. See Figure 2.
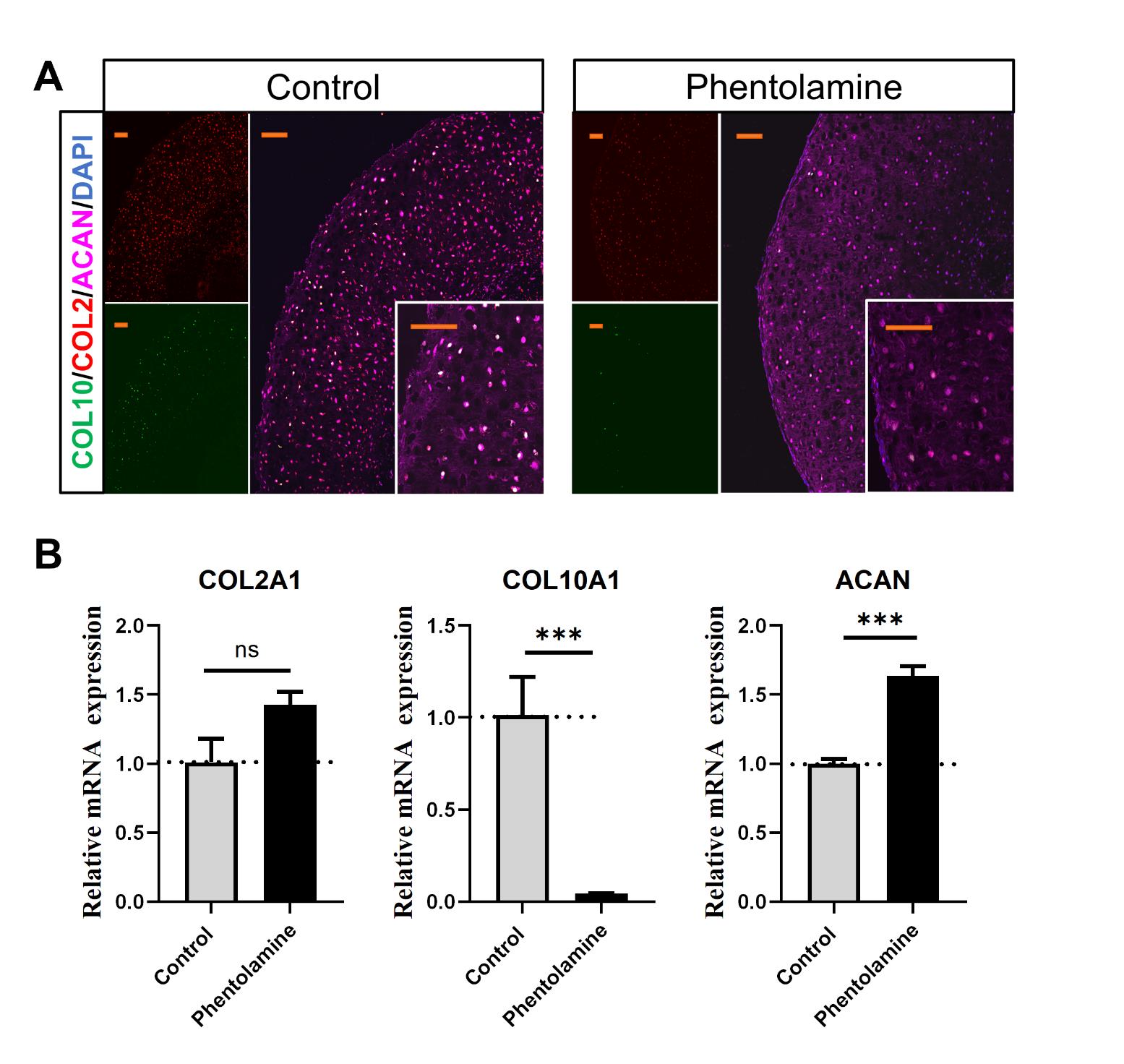
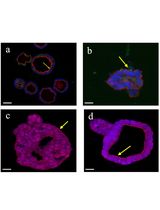
Figure 2. Phentolamine inhibits chondrocyte hypertrophy verified with immunofluorescence and quantitative real-time PCR (qPCR). (A) Representative confocal images of human expanded pluripotent stem cell (hEPSC)-derived chondrocytes treated with chondrocyte hypertrophy medium (CHM; control) and CHM plus phentolamine for 14 days, stained with the chondrocyte-specific marker ACAN. Nuclei are stained with DAPI (blue). Scale bar: 100 μm. (B) Relative mRNA expression of COL2A1, COL10A1, and ACAN in pellets treated with CHM (control) and CHM plus phentolamine for 14 days measured by qPCR. Data are shown as the mean ± SD (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001; ns, non-significant.
Validation of protocol
This protocol has been used and validated in the following research article:
Wei et al. [10]. A human organoid drug screen identifies α2-adrenergic receptor signaling as a therapeutic target for cartilage regeneration. Cell Stem Cell. (Figures 1, 3, and 5 and Supplemental Figures 2–4).
The protocol is an optimized version of a previous hiPSC-derived chondrocyte protocol, which was used and validated in the following research article:
Pretemer et al. [9] Differentiation of hypertrophic chondrocytes from human iPSCs for the in vitro modeling of chondrodysplasias. Stem Cell Rep. (Figure 1).
General notes and troubleshooting
General notes
1. Always change the medium daily during hEPSC culture.
2. The medium can be changed every 2–4 days during 3D culture.
3. Seed the hEPSCs as fine clumps for differentiation.
Troubleshooting
Problem 1: A significant number of cells fail to survive during the first four days of 2D differentiation.
Possible cause: Undifferentiated hEPSCs may have been dissociated for too long, leading to a failure to maintain clumps of colonies.
Solution: Avoid dissociating hEPSCs for an extended period. If necessary, gently scrape the loosened colonies with a cell scraper.
Problem 2: Cells fail to aggregate into spheroids during 3D differentiation.
Possible cause: Sclerotome induction medium is incubated for too long, or cell density at day 6 is too high.
Solution: During sclerotome induction, transition to 3D differentiation when cells reach 100% confluency and form a spiral structure. Do not strictly adhere to the 72 h incubation period if conditions are optimal earlier.
Acknowledgments
Conceptualization, X.W. and H.W.; Investigation, X.W.; Writing – original draft, H.W., Y.L., and J.Q.; Writing – Review & Editing, X.W. and X.B.; Funding acquisition, X.B.; Supervision, X.B. and X.W.
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81991510).
This protocol is adapted from Pretemer et al. [9], which has been modified from Matsuda et al. [12] and Umeda et al. [13].
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
The study was approved by the Clinical Research Ethics Committee of Southern Medical University. All experiments were performed in accordance with relevant guidelines and regulations.
References
- Huey, D. J., Hu, J. C. and Athanasiou, K. A. (2012). Unlike Bone, Cartilage Regeneration Remains Elusive. Science. 338(6109): 917–921. https://doi.org/10.1126/science.1222454
- Armiento, A. R., Alini, M. and Stoddart, M. J. (2019). Articular fibrocartilage - Why does hyaline cartilage fail to repair? Adv Drug Delivery Rev. 146: 289–305. https://doi.org/10.1016/j.addr.2018.12.015
- Bertrand, J., Kräft, T., Gronau, T., Sherwood, J., Rutsch, F., Lioté, F., Dell'Accio, F., Lohmann, C. H., Bollmann, M., Held, A., et al. (2020). BCP crystals promote chondrocyte hypertrophic differentiation in OA cartilage by sequestering Wnt3a. Ann Rheum Dis. 79(7): 975–984. https://doi.org/10.1136/annrheumdis-2019-216648
- Vandana, J. J., Manrique, C., Lacko, L. A. and Chen, S. (2023). Human pluripotent-stem-cell-derived organoids for drug discovery and evaluation. Cell Stem Cell. 30(5): 571–591. https://doi.org/10.1016/j.stem.2023.04.011
- Marsee, A., Roos, F. J., Verstegen, M. M., Gehart, H., de Koning, E., Lemaigre, F., Forbes, S. J., Peng, W. C., Huch, M., Takebe, T., et al. (2021). Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell. 28(5): 816–832. https://doi.org/10.1016/j.stem.2021.04.005
- Khan, N. M., Diaz-Hernandez, M. E., Chihab, S., Priyadarshani, P., Bhattaram, P., Mortensen, L. J., Guzzo, R. M. and Drissi, H. (2022). Differential chondrogenic differentiation between iPSC-derived from healthy and OA cartilage is associated with changes in epigenetic regulation and metabolic transcriptomic signatures. eLife: e512213. https://doi.org/10.1101/2022.10.14.512213
- Wu, C. L., Dicks, A., Steward, N., Tang, R., Katz, D. B., Choi, Y. R. and Guilak, F. (2021). Single cell transcriptomic analysis of human pluripotent stem cell chondrogenesis. Nat Commun. 12(1): e1038/s41467–020–20598–y. https://doi.org/10.1038/s41467-020-20598-y
- Loh, K. M., Chen, A., Koh, P. W., Deng, T. Z., Sinha, R., Tsai, J. M., Barkal, A. A., Shen, K. Y., Jain, R., Morganti, R. M., et al. (2016). Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell. 166(2): 451–467. https://doi.org/10.1016/j.cell.2016.06.011
- Pretemer, Y., Kawai, S., Nagata, S., Nishio, M., Watanabe, M., Tamaki, S., Alev, C., Yamanaka, Y., Xue, J. Y., Wang, Z., et al. (2021). Differentiation of Hypertrophic Chondrocytes from Human iPSCs for the In Vitro Modeling of Chondrodysplasias. Stem Cell Rep. 16(3): 610–625. https://doi.org/10.1016/j.stemcr.2021.01.014
- Wei, X., Qiu, J., Lai, R., Wei, T., Lin, Z., Huang, S., Jiang, Y., Kuang, Z., Zeng, H., Gong, Y., et al. (2024). A human organoid drug screen identifies α2-adrenergic receptor signaling as a therapeutic target for cartilage regeneration. Cell Stem Cell. 31(12): 1813–1830.e8. https://doi.org/10.1016/j.stem.2024.09.001
- Gao, X., Nowak-Imialek, M., Chen, X., Chen, D., Herrmann, D., Ruan, D., Chen, A. C. H., Eckersley-Maslin, M. A., Ahmad, S., Lee, Y. L., et al. (2019). Establishment of porcine and human expanded potential stem cells. Nat Cell Biol. 21(6): 687-699. https://doi.org/10.1038/s41556-019-0333-2
- Matsuda, M., Yamanaka, Y., Uemura, M., Osawa, M., Saito, M. K., Nagahashi, A., Nishio, M., Guo, L., Ikegawa, S., Sakurai, S., et al. (2020). Recapitulating the human segmentation clock with pluripotent stem cells. Nature. 580(7801): 124–129. https://doi.org/10.1038/s41586-020-2144-9
- Umeda, K., Zhao, J., Simmons, P., Stanley, E., Elefanty, A. and Nakayama, N. (2012). Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Sci Rep. 2(1): 455. https://doi.org/10.1038/srep00455
Article Information
Publication history
Received: Jan 14, 2025
Accepted: Apr 7, 2025
Available online: Apr 24, 2025
Published: May 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Wang, H., Qiu, J., Lin, Y., Bai, X. and Wei, X. (2025). A Cartilaginous Organoid System Derived From Human Expanded Pluripotent Stem Cells (hEPSCs). Bio-protocol 15(9): e5304. DOI: 10.21769/BioProtoc.5304.
Category
Stem Cell > Embryonic stem cell > Cell differentiation
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









