- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
snPATHO-seq: A Detailed Protocol for Single Nucleus RNA Sequencing From FFPE
(*Contributed equally to this work, §Contributed equally to this work) Published: Vol 15, Iss 9, May 5, 2025 DOI: 10.21769/BioProtoc.5291 Views: 1935
Reviewed by: Alessandro DidonnaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Protocol for Laser-Assisted Microdissection and tRF & tiRNA Sequencing in Lung Adenocarcinoma
Zi Wang [...] Feng Jiang
Apr 5, 2025 2992 Views

A Guide to Basic RNA Sequencing Data Processing and Transcriptomic Analysis
Rowayna Shouib [...] Rineke Steenbergen
May 5, 2025 7749 Views

RACE-Nano-Seq: Profiling Transcriptome Diversity of a Genomic Locus
Lu Tang [...] Philipp Kapranov
Jul 5, 2025 2521 Views
Abstract
Formalin-fixed paraffin-embedded (FFPE) samples remain an underutilized resource in single-cell omics due to RNA degradation from formalin fixation. Here, we present snPATHO-seq, a robust and adaptable approach that enables the generation of high-quality single-nucleus (sn) transcriptomic data from FFPE tissues, utilizing advancements in single-cell genomic techniques. The snPATHO-seq workflow integrates optimized nuclei isolation with the 10× Genomics Flex assay, targeting short RNA fragments to mitigate FFPE-related RNA degradation. Benchmarking against standard 10× 3' and Flex assays for fresh/frozen tissues confirmed robust detection of transcriptomic signatures and cell types. snPATHO-seq demonstrated high performance across diverse FFPE samples, including diseased tissues like breast cancer. It seamlessly integrates with FFPE spatial transcriptomics (e.g., FFPE Visium) for multi-modal spatial and single-nucleus profiling. Compared to workflows like 10× Genomics’ snFFPE, snPATHO-seq delivers superior data quality by reducing tissue debris and preserving RNA integrity via nuclei isolation. This cost-effective workflow enables high-resolution transcriptomics of archival FFPE samples, advancing single-cell omics in translational and clinical research.
Key features
• Optimized nuclei isolation from FFPE tissues enables high-quality single-nucleus transcriptomics by minimizing debris and maximizing intact nuclear yield.
• Compatible with 10× Genomics Flex, leveraging short RNA probes to overcome FFPE RNA fragmentation challenges.
• Outperforms existing FFPE workflows in cell type detection sensitivity across archival, degraded, or aged samples.
• Low-cost, accessible protocol using off-the-shelf reagents, suitable for broad translational and archival tissue applications.
Keywords: snRNA-seqGraphical overview
Background
Clinical tissue specimens are predominantly preserved using the formalin-fixed paraffin-embedded (FFPE) technique, a universally established method for the long-term conservation of biological tissues. In the United States alone, pathology laboratories process between 10,000 and 100,000 FFPE blocks annually, underscoring the immense scale and significance of this resource for clinical diagnostics and translational research [1]. These FFPE samples serve as a cornerstone for retrospective studies, enabling molecular and histopathological analyses that drive advancements in precision medicine and biomedical discovery [1]. Advances in molecular technologies, including genomics, transcriptomics, and proteomics, have expanded the utility of FFPE samples for molecular characterization [2,3]. However, these applications are traditionally conducted at the bulk tissue level, which fails to resolve the intricate molecular heterogeneity inherent in cancers and other complex biological settings. Technologies enabling single-cell transcriptomic profiling of FFPE tissues thus hold immense potential for advancing human health research.
Previously, our team successfully conducted single-nucleus (sn) transcriptomic profiling using clinical human FFPE tissue samples, demonstrating the feasibility of isolating intact nuclei from FFPE samples for molecular characterization [4]. However, extending this technology to transcriptomics poses a significant challenge due to RNA fragmentation caused by formalin fixation, heat exposure, and paraffin embedding [2]. RNA degradation poses a significant challenge to the performance of widely used single-cell/nucleus RNA sequencing (sc/sn RNA-seq) methods, such as the 10× Genomics 3' assay and SMART-seq, which rely on poly(dT) probes to selectively capture and reverse-transcribe intact mRNA molecules [5,6]. RNA-binding probes enable gene expression profiling in FFPE samples and are used in spatial methods like MERFISH, 10× Visium, and Xenium. While resilient to RNA fragmentation, these methods lack transcriptome-wide or single-cell resolution [7–9]. To address this limitation, 10× Genomics introduced Flex chemistry, which employs RNA-targeting probes capable of capturing short RNA fragments, significantly improving compatibility with FFPE-derived material [10].
To address challenges in analyzing FFPE samples, we developed snPATHO-seq, a nuclei isolation protocol optimized for FFPE tissues. This workflow integrates rehydration, enzyme-based dissociation, and nuclei isolation (an example of good-quality nuclei is shown in Figure 1), followed by gene expression analysis using the 10× Genomics Flex assay. We validated snPATHO-seq on three human breast cancer FFPE samples, demonstrating strong agreement with standard 10× 3' and Flex chemistries, previously restricted to fresh or frozen tissues.
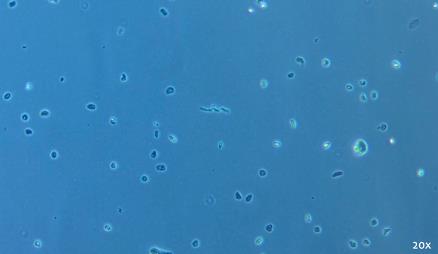
Figure 1. Representative example of good-quality nuclei preparation. Brightfield image was taken with a 20× objective on the ZEISS Primovert.
In parallel, 10× Genomics introduced a single-cell fixation protocol for single-cell RNA sequencing [11]. 10× Genomics’ scFFPE protocol does not include a defined nuclei isolation step, leading to the isolation of a mixture of intact cells and nuclei from FFPE samples. This incomplete separation can compromise data quality by introducing variability in transcript capture efficiency and downstream interpretation. In contrast, snPATHO-seq incorporates dedicated membrane lysis and nuclei isolation steps, enabling consistent enrichment for nuclei and minimizing contamination from partially digested cells. Our direct comparison of the two workflows demonstrated that snPATHO-seq delivers more robust and reproducible gene expression profiles, better suited for high-quality single-nucleus transcriptomics from FFPE tissues [12]. This technical advancement strengthens snPATHO-seq as a more mature and reliable protocol for FFPE single-nucleus studies.
In summary, snPATHO-seq unlocks the potential of archival FFPE samples, offering a robust and mature protocol for single-nucleus transcriptomic analysis. By overcoming the technical challenges of RNA degradation and enabling single-cell resolution profiling, snPATHO-seq bridges the gap between traditional pathology and molecular research, fostering new opportunities for clinical and translational studies.
Materials and reagents
Biological materials
1. FFPE tissue archive blocks
Reagents
1. RNAse Away surface decontaminant (Thermo Fisher Scientific, catalog number: 7002PK)
2. Xylene (Sigma, catalog number: 214736)
3. Ethanol (Sigma, catalog number: E7023)
4. RPMI-1640 media (Gibco, catalog number: 11875093)
5. Liberase TH 100 mg (Sigma, catalog number: 5401135001)
6. RiboLock RNase inhibitor (40 U/μL) (Thermo Fisher Scientific, catalog number: EO0382)
7. Nuclei Isolation kit Nuclei EZ Prep (Sigma, catalog number: NUC101)
8. Bovine serum albumin (BSA) solution, sterile filtered (Sigma-Aldrich, catalog number: A1595)
9. LiberaseTM research grade, 10 mg (Roche, catalog number: 5401119001)
10. Collagenase D (Roche, catalog number: 11088858001)
11. 10× PBS buffer, pH 7.4 (Invitrogen, catalog number: AM9624 or AM9625)
12. Ambion nuclease-free water (Invitrogen, catalog number: AM9932)
13. Acridine Orange/Propidium Iodide (AO/PI) Viability kit (Logos, catalog number: F23011)
Solutions
1. 70% Ethanol (see Recipes)
2. 50% Ethanol (see Recipes)
3. 30% Ethanol (see Recipes)
4. Digestion solution (see Recipes)
5. Lysis solution (see Recipes)
6. Wash solution 1 (see Recipes)
7. Wash solution 2 (see Recipes)
Recipes
1. 70% Ethanol
| Reagent | Stock | Final | 1× + 10% (μL) | 2× + 10% (μL) |
|---|---|---|---|---|
| Ethanol | 100% | 70% | 770 | 1540 |
| Nuclease-free water | - | - | 330 | 660 |
| Total | 1,100 | 2,200 |
2. 50% Ethanol
| Reagent | Stock | Final | 1× + 10% (μL) | 2× + 10% (μL) |
|---|---|---|---|---|
| Ethanol | 100% | 50% | 550 | 1,100 |
| Nuclease-free water | - | - | 550 | 1,100 |
| Total | 1,100 | 2,200 |
3. 30% Ethanol
| Reagent | Stock | Final | 1× + 10% (μL) | 2× + 10% (μL) |
|---|---|---|---|---|
| Ethanol | 100% | 30% | 330 | 660 |
| Nuclease-free water | - | - | 770 | 1,540 |
| Total | 1,100 | 2,200 |
4. Digestion solution
| Reagent | Stock | Final | 1× + 10% (μL) | 2× + 10% (μL) |
|---|---|---|---|---|
| Liberase TH | 1 mg/mL | 1 mg/mL | 1072.5 | 2,145 |
| RNAse inhibitor | 40 U/μL | 1 U/μL | 27.5 | 55 |
| Total | 1,100 | 2,200 |
Note: 1 mg/mL of Liberase TH works well for most tissues we tested, but testing different concentrations up to 5 mg/mL for your tissue type is recommended.
5. Lysis solution
| Reagent | Stock | Final | 1× + 10% (μL) | 2× + 10% (μL) |
|---|---|---|---|---|
| Nuclei EZ lysis buffer | 1× | 1× | 825 | 1,650 |
| BSA | 10% | 2% | 220 | 440 |
| RNAse inhibitor | 40 U/μL | 1 U/μL | 55 | 110 |
| Total | 1,100 | 2,200 |
6. Wash solution 1
| Reagent | Stock | Final | 1× + 10% (μL) | 2× + 10% (μL) |
|---|---|---|---|---|
| Nuclease-free water | - | - | 1,760 | 3,520 |
| PBS | 10× | 1× | 220 | 440 |
| BSA | 10% | 1% | 220 | 440 |
| Total | 2,200 | 4,400 |
7. Wash solution 2
| Reagent | Stock | Final | 1× + 10% (μL) | 2× + 10% (μL) |
|---|---|---|---|---|
| Nuclease-free water | - | - | 2,085.6 | 4,171.2 |
| PBS | 10× | 0.5× | 110 | 220 |
| BSA | 10% | 0.02% | 4.4 | 8.8 |
| Total | 2,200 | 4,400 |
Laboratory supplies
1. Desiccant silica gel packets (Root Lab, catalog number: DES100)
2. Bel-Art disposable pestles (Sigma, catalog number: BAF199230001)
3. Precision glide needle 25 G (BD, catalog number: 962776)
4. 1 mL syringe (Sigma, catalog number: Z683531-100EA)
5. DNA LoBind tubes 1.5 mL (Eppendorf, catalog number: 022431021)
6. DNA LoBind tubes 2.0 mL (Eppendorf, catalog number: 022431048)
7. pluriStrainer mini 70 μm (pluriSelect, catalog number: 43-10070-40)
8. pluriStrainer mini 40 μm (pluriSelect, catalog number: 43-50040-51)
9. Falcon conical centrifuge tubes (Corning, catalog numbers: 352070 for 50 mL, 352095 for 15 mL)
10. Sterile serological pipettes with pipettor
11. Luna Photon low fluorescent counting chambers (Logos Biosystems, catalog number: L12005)
12. Rainin Pipet-Lite LTS filtered pipette tips (Rainin, catalog numbers: 30389240, 30389213, 30389226)
Equipment
1. HistoCore BIOCUT, manual rotary microtome (Leica, catalog number: 149BIO000C1)
2. Digital dry bath/block heater (Thermo Fisher, catalog number: 88870001)
3. ThermoMixer C (Eppendorf, catalog number: 5382000023)
4. Centrifuge 5810/5810R (Eppendorf, catalog number: EP022628188) with rotor S-4-104
5. Rotor F-35-6-30 (Eppendorf, catalog number: EP5427716009 or similar)
6. S-4-104 rotor adapters for 50× 1.5/2 mL tubes (Eppendorf, catalog number: 58-257-40009)
7. Standard heavy-duty vortex mixer (VWR or Fisherbrand, catalog number: 97043-562)
8. Ice bucket with cool blocks or cool rack CFT30 (Corning, catalog number: CLS432052)
9. Luna-FX7 automated cell counter (Logos Biosystems, catalog number: L700002)
Software and datasets
1. BioRender (https://www.biorender.com/). The following figures were created using BioRender: Graphical overview, https://BioRender.com/j4ty88o
Procedure
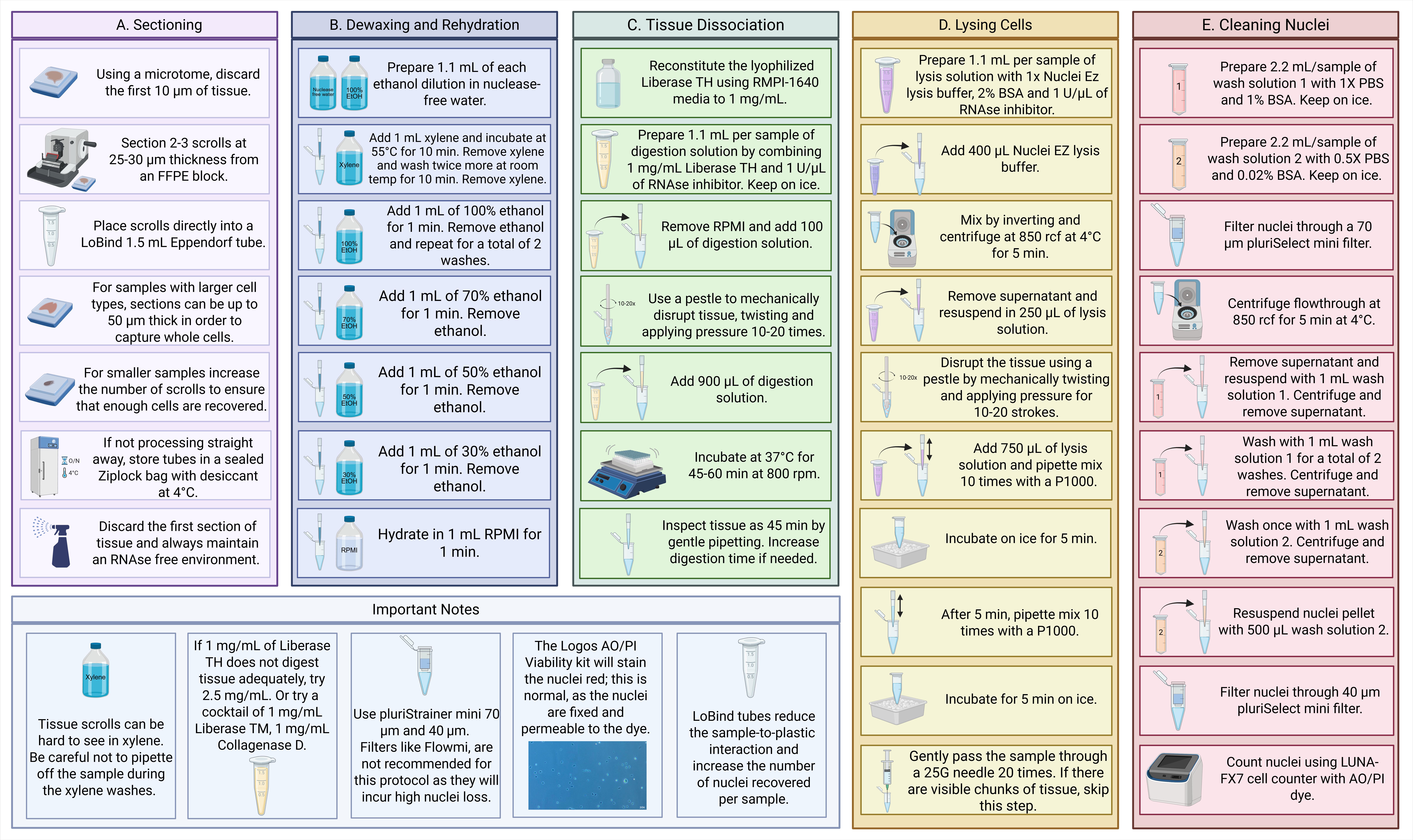
A. Sectioning
1. Discard the first 10 µm of tissue from an FFPE block as the RNA quality will be reduced if the tissue has been exposed to oxygen.
2. Section 2–3 scrolls at 25–30 μm thickness from an FFPE tissue block using a microtome with a fresh blade.
3. Place scrolls directly into a LoBind 1.5 mL Eppendorf LoBind tube.
Critical: Maintain RNAse-free conditions using RNAse Away and
Note: The number and thickness of the scrolls depend on the size of the sample in the block. You want to get whole cells; so, if your sample type has very large cells, then section up to 50 μm scrolls. If your sample is very small, increase the number of sections to ensure you recover enough cells for downstream applications.
4. If not processing straight away, store tubes in a sealed Ziplock bag with desiccant at 4 °C.
B. Dewaxing and rehydration
1. Prepare ethanol dilutions (70%, 50%, 30%; see Recipes). Vortex to mix and maintain at room temperature.
2. Dewax samples by adding 1 mL of xylene and incubating at 55 °C for 10 min on a heat block. Remove xylene with a P1000 pipette and wash twice more with 1 mL of xylene for 10 min each at room temperature.
Note: Be careful when removing the solution, as samples are hard to see in xylene and will become opaque with ethanol addition.
3. Rehydrate in 1 mL of ethanol dilutions for 1 min each. Twice in 100% ethanol, once in 70%, once in 50%, and once in 30%.
4. Finally, hydrate in 1 mL of RPMI-1640 media for 1 min.
C. Tissue dissociation
1. Reconstitute the lyophilized liberase TH using RMPI-1640 media to 1 mg/mL.
2. Prepare digestion solution (see Recipes). Pipette mix 15× and keep on ice until use.
3. Remove RPMI-1640 media from the sample and add 100 μL of digestion solution.
4. Mechanically disrupt tissue using a pestle by twisting and applying pressure for 10–20 strokes until visibly dissociated.
5. Add a further 900 μL of digestion solution.
6. Incubate at 37 °C for 45–60 min at 800 rpm.
Note: Some tissues may require extended digestion times. At 45 min, gently mix the sample by pipetting with a P1000 pipette. If the tissue appears partially digested, increase the digestion time as needed. Complete digestion is not necessary; the goal is to sufficiently loosen the tissue matrix to facilitate efficient nuclei release.
D. Cell lysis
1. Prepare lysis solution (see Recipes). Pipette mix 15× and keep on ice until use.
2. After digestion, add 400 μL of nuclei EZ lysis buffer.
3. Mix by inverting, and centrifuge at 850 rcf for 5 min at 4 °C.
4. Remove supernatant and resuspend in 250 μL of lysis solution.
5. Mechanically disrupt the tissue again using a pestle by twisting and applying pressure for 10–20 strokes.
6. Add 750 μL of lysis solution and pipette mix 10 times with a P1000 pipette.
7. Incubate on ice for 5 min.
8. After 5 min, pipette mix 10 times again.
9. Incubate for a further 5 min on ice.
10. If there are no large chunks of tissue in solution, gently pass the sample through a 25 G needle 20 times (avoid foaming). If there are visible chunks of tissue, skip this step as the needle will clog and you will lose sample.
E. Nuclei washing
1. Prepare wash solution 1 (see Recipes). Pipette mix 15× and keep on ice until use.
2. Prepare wash solution 2 (see Recipes). Pipette mix 15× and keep on ice until use.
3. Filter nuclei through a 70 μm pluriStrainer mini filter into a 1.5 mL LoBind tube.
4. Centrifuge the flowthrough at 850 rcf for 5 min at 4 °C.
Note: All subsequent centrifuging steps are performed at 850 rcf for 5 min at 4 °C.
5. Remove supernatant and resuspend nuclei in 1 mL of wash solution 1. Centrifuge and remove supernatant.
6. Resuspend in 1 mL of wash solution 1. Centrifuge and remove supernatant.
7. Resuspend nuclei pellet in 1 mL of wash solution 2. Centrifuge and remove supernatant.
8. Resuspend nuclei pellet in wash solution 2 (the volume depends on the size of the pellet; if unsure, resuspend in 500 μL).
9. Filter through a 40 μm pluriStrainer mini filter into a 1.5 mL LoBind tube.
10. Count nuclei using acridine orange/propidium iodide (AO/PI) on the Luna-FX7 cell counter.
Validation of protocol
The snPATHO-seq workflow has been rigorously validated through comprehensive benchmarking against existing single-nucleus RNA sequencing (snRNA-seq) assays, including analyses of matched fresh-frozen samples and methods for profiling sn (scRandom-seq [14]) and sc ([15] and 10× Genomics) from FFPE samples. These methods became available while the snPATHO-seq manuscript was under revision, as detailed in Wang et al. [13] (see Figures 2 and 4 and Supplementary Figure 3). Testing on FFPE breast cancer tissues and their frozen counterparts demonstrated that snPATHO-seq accurately resolves cell types and preserves gene expression profiles, despite the RNA degradation commonly associated with FFPE processing. Importantly, snPATHO-seq outperformed the 10× Genomics FFPE workflow, particularly in older FFPE samples where the 10× protocol exhibited incomplete tissue dissociation and reduced gene detection sensitivity. The robustness of snPATHO-seq in challenging archival material, coupled with its protocol availability and supporting datasets, provides a valuable resource to the field and is poised to accelerate broader adoption of FFPE-based single-cell transcriptomic research.
General notes and troubleshooting
General notes
Tip 1: Maintain RNAse-free environments
Always maintain RNAse-free environments, especially when sectioning, as histology cores do not typically maintain RNAse-free standards. Ensure that new microtome blades are used and changed between samples. Discard the first 10 μm section as tissue exposed to air oxidizes and damages RNA.
Tip 2: Pay attention to the sample when dewaxing
In xylene, tissue scrolls can be difficult to see and appear translucent. Make sure you do not pipette off the sample during the xylene washes. Invert the first 100% ethanol incubation to ensure xylene is completely removed from the sample and nothing is trapped on the lid. If xylene remains, digestion will be suboptimal.
Tip 3: Different tissues can require optimizing digestion enzymes
If 1 mg/mL of liberase TH does not digest tissue adequately, try to increase the concentration to 2.5 mg/mL. Alternatively, use a cocktail of 1 mg/mL Liberase TM (from Roche, see Reagents list), 1 mg/mL Collagenase D, and 1 U/μL of RiboLock RNAse Inhibitor in RPMI-1640 media.
Tip 4: Do not filter out your nuclei with the debris
Make sure to use pluriStrainer mini 70 μm and 40 μm (pluriSelect).
Note: Other filters like Flowmi, commonly used in 10× Genomics workflows, are not recommended for this protocol as they will incur high nuclei loss.
Tip 5: Accurate nuclei counting is critical for downstream applications
The Logos AO/PI Viability kit will stain the nuclei red; this is normal, as the nuclei are fixed and permeable to the dye.
Tip 6: Use LoBind tubes to maximize sample recovery
LoBind tubes reduce the sample-to-plastic interaction and increase the number of nuclei recovered per sample.
Acknowledgments
This project has received funding from the Commonwealth Standard Grant Agreement 4-F26M8TZ (L.M., K.W.). The South Australian immunoGENomics Cancer Institute (SAiGENCI) received grant funding from the Australian Government. J.P. is funded by grants from the Ovarian Cancer Research Alliance (ECIG-2022-3-1143) and the Chan Zuckerburg Foundation (OS00001235 and 2024-345901). This protocol was originally described and validated in Wang et al. [13].
Competing interests
There are no conflicts of interest or competing interests.
References
- Yuan, Y., Van Dyke, A. L., Petkov, V. I., Hussey, S., Moravec, R., Altekruse, S. F., Sandoval, M., Cress, R. D., Mueller, L. M., Mogi, A., et al. (2021). Pathology Laboratory Policies and Procedures for Releasing Diagnostic Tissue for Cancer Research. Arch Pathol Lab Med. 145(2): 222–226. https://doi.org/10.5858/arpa.2019-0474-oa
- Greytak, S. R., Engel, K. B., Bass, B. P. and Moore, H. M. (2015). Accuracy of Molecular Data Generated with FFPE Biospecimens: Lessons from the Literature. Cancer Res. 75(8): 1541–1547. https://doi.org/10.1158/0008-5472.can-14-2378
- Friedrich, C., Schallenberg, S., Kirchner, M., Ziehm, M., Niquet, S., Haji, M., Beier, C., Neudecker, J., Klauschen, F., Mertins, P., et al. (2021). Comprehensive micro-scaled proteome and phosphoproteome characterization of archived retrospective cancer repositories. Nat Commun. 12(1): 3576. https://doi.org/10.1038/s41467-021-23855-w
- Martelotto, L. G., Baslan, T., Kendall, J., Geyer, F. C., Burke, K. A., Spraggon, L., Piscuoglio, S., Chadalavada, K., Nanjangud, G., Ng, C. K. Y., et al. (2017). Whole-genome single-cell copy number profiling from formalin-fixed paraffin-embedded samples. Nat Med. 23(3): 376–385. https://doi.org/10.1038/nm.4279
- Picelli, S., Faridani, O. R., Björklund, Ã. K., Winberg, G., Sagasser, S. and Sandberg, R. (2014). Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc. 9(1): 171–181. https://doi.org/10.1038/nprot.2014.006
- 10× Genomics. (2024). Chromium GEM-X Single Cell 3’ Reagent Kits v4. Document Number CG000731 Rev A.
- 10× Genomics. (2024). Visium CytAssist Spatial Gene Expression Reagent Kits. Document Number CG000495 Rev F.
- 10× Genomics. (2024). Xenium In Situ Gene Expression. Document Number CG000582 Rev F.
- Xia, C., Babcock, H. P., Moffitt, J. R. and Zhuang, X. (2019). Multiplexed detection of RNA using MERFISH and branched DNA amplification. Sci Rep. 9(1): 7721. https://doi.org/10.1038/s41598-019-43943-8
- 10× Genomics. (2022). Chromium Fixed RNA Profiling Reagent Kits. Document Number CG000477 Rev C.
- Janesick, A., Shelansky, R., Gottscho, A. D., Wagner, F., Williams, S. R., Rouault, M., Beliakoff, G., Morrison, C. A., Oliveira, M. F., Sicherman, J. T., et al. (2023). High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat Commun. 14(1): 8353. https://doi.org/10.1038/s41467-023-43458-x
- 10× Genomics. (2024). Demonstrated Protocol: Sample Preparation from FFPE Tissue Sections for Chromium Fixed RNA Profiling.
- Wang, T., Roach, M. J., Harvey, K., Morlanes, J. E., Kiedik, B., Al-Eryani, G., Greenwald, A., Kalavros, N., Dezem, F. S., Ma, Y., et al. (2024). snPATHO-seq, a versatile FFPE single-nucleus RNA sequencing method to unlock pathology archives. Commun Biol. 7(1): 1340. https://doi.org/10.1038/s42003-024-07043-2
- Xu, Z., Zhang, T., Chen, H., Zhu, Y., Lv, Y., Zhang, S., Chen, J., Chen, H., Yang, L., Jiang, W., et al. (2023). High-throughput single nucleus total RNA sequencing of formalin-fixed paraffin-embedded tissues by snRandom-seq. Nat Commun. 14(1): 2734. https://doi.org/10.1038/s41467-023-38409-5
- Chung, H., Melnikov, A., McCabe, C., Drokhlyansky, E., Van Wittenberghe, N., Magee, E. M., Waldman, J., Spira, A., Chen, F., Mazzilli, S., et al. (2022). SnFFPE-Seq: towards scalable single nucleus RNA-Seq of formalin-fixed paraffin-embedded (FFPE) tissue. bioRxiv. e505257. https://doi.org/10.1101/2022.08.25.505257
Article Information
Publication history
Received: Feb 3, 2025
Accepted: Mar 31, 2025
Available online: Apr 11, 2025
Published: May 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Arjumand, W., Wise, K., DuBose, H., Plummer, J. and Martelotto, L. G. (2025). snPATHO-seq: A Detailed Protocol for Single Nucleus RNA Sequencing From FFPE. Bio-protocol 15(9): e5291. DOI: 10.21769/BioProtoc.5291.
Category
Cell Biology > Single cell analysis
Systems Biology > Transcriptomics > RNA-seq
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










