- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Scalable Alkaline Extraction Protocol for Microbial DNA Screening by PCR
(*contributed equally to this work) Published: Vol 15, Iss 8, Apr 20, 2025 DOI: 10.21769/BioProtoc.5290 Views: 1709
Reviewed by: Alba BlesaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimized Protocol for DNA Extraction in Three Theobroma Species
Angie F. Riascos-España [...] Pedro A. Velasquez-Vasconez
May 5, 2025 2043 Views
A Comparative Protocol for Preserving Deep-Water Marine Invertebrate Tissues: DNA/RNA Shield vs. Liquid Nitrogen for Dual Extraction of High-Quality Nucleic Acids
Ana S. Gomes [...] Olivier Laroche
Nov 20, 2025 1319 Views
Plasmid DNA Purification Using Filterprep With an Optional Endotoxin Removal Step
Yu-Qian Lin [...] Chung-Te Chang
Dec 20, 2025 1055 Views
Abstract
In molecular diagnosis, DNA extraction kits are sample-specific and proprietary, preventing lateral distribution among similar facilities from different sectors to alleviate supply shortages during a crisis. Previous fast extraction protocols such as detergent-based ones allow fast DNA extraction for nucleic acid amplification tests (NAAT), mainly polymerase chain reaction (PCR). The use of NaOH (dense alkali) to rupture cells and nuclei and destabilize the conformation of DNases might alleviate shortages and costs while retaining enough robustness to treat complicated samples with minimal environmental and logistical footprint. Biological samples are hand-crushed using a pestle in 1.5 mL tubes with 360 μL of 0.2 M NaOH for 3–5 min and incubated at 75 °C for 10 min. For immediate use, 115.2 μL of 1 M Tris (pH 8) and 364.8 μL nuclease-free water are added, and the sample is vortexed for 10 s and spun at 10,000× g for 3 min; then, 700 μL is transferred to a clean microtube. Two serial dilutions follow, and all concentrations are used as templates for PCR. A refined, storable extract can be produced by adding 70 μL of HCl 1 M (instead of Tris-HCl) and one volume of cold isopropanol to the extract for standard precipitation. This method can increase throughput in emergencies by field deployment in resource-limited settings (RLS) or allow benchtop backup in cases of acquisition disruption or sample surge in established facilities. The crude extract can be used for immediate PCR in both benchtop and portable thermocyclers, thus allowing NAAT in resource-limited settings with low costs and waste footprint or during prolonged crises, where supply chain failures may occur. The refined version produces alcohol-precipitated nucleic acids, suitable for both immediate use and for storage or dispatch for spatiotemporally separate analysis while offering much better amplification quality with a small increase in time and minimal increase in expendables/chemicals needed.
Key features
• DNA extraction from different sample types using only boiling water and occasional mechanical assistance.
• Crude extract serially diluted to bypass purification and quantification steps.
• Refined extract is partly purified, more enriched, storable, and transportable and contributes to higher sensitivity.
• Both versions decrease costs and the overall footprint of testing to increase sustainability in field operations and in standard lab environments under supply chain derailment.
Keywords: Alkaline extractionGraphical overview
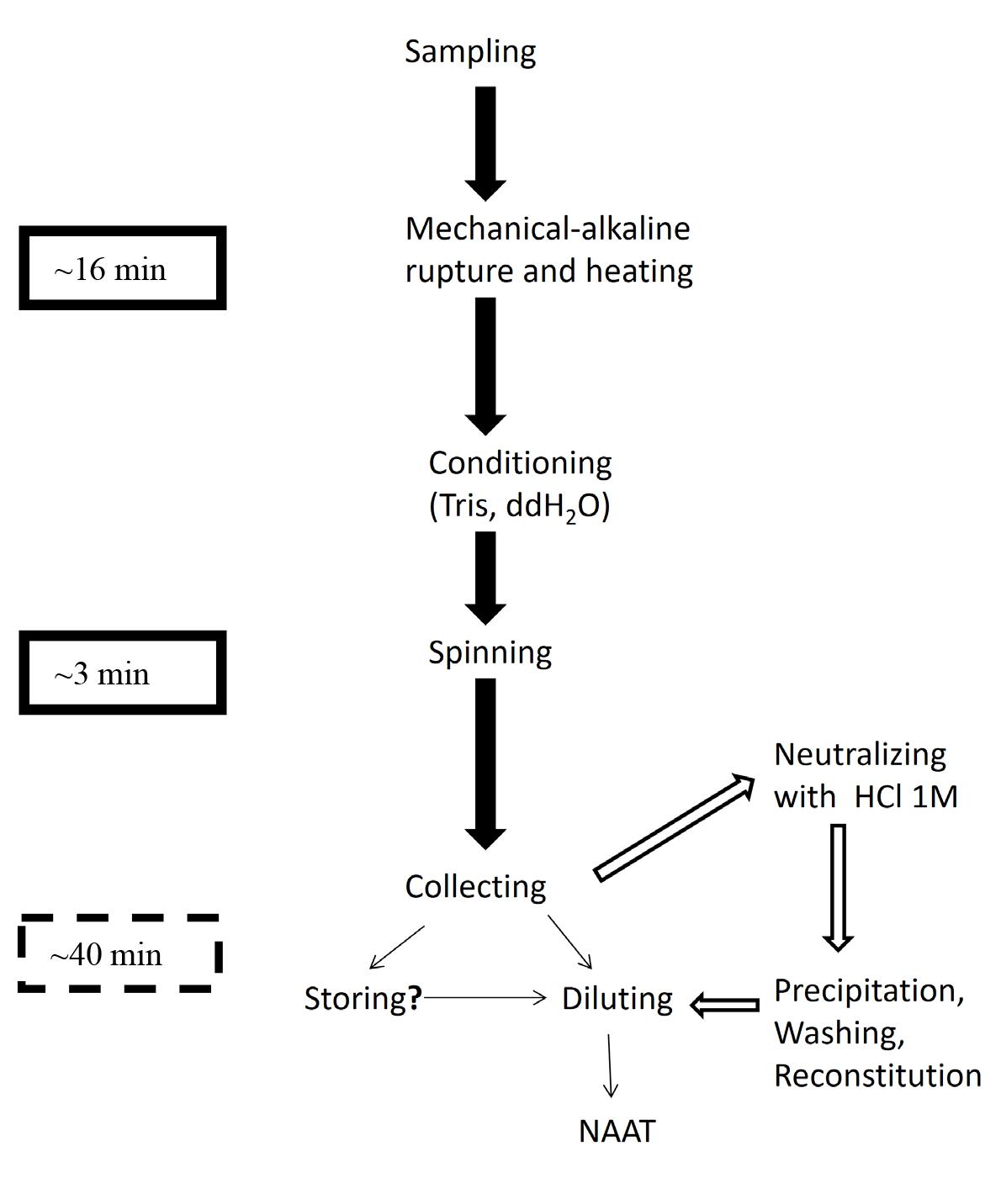
Successive steps of the scalable NaOH-based extraction protocol. Solid arrows and text box: Short protocol, crude extract. Hollow arrows and dashed text box: Refinements. Line arrows: Post-extraction procedures: Procedure not tested.
Background
In routine conditions or in crisis, surveillance and screening require fast and massive DNA extraction pipelines for nucleic acid amplification tests (NAAT), especially PCR, both in field conditions [usually in resource-limited settings (RLS)] and in proper lab setups. Although current technology for DNA extraction achieves significant yields from complex and/or small samples, their use is expensive and subject to market manipulation and practices and supply chain integrity [1,2]. The alkaline approach has been suggested for contingency [3] and field conditions [4]. It is a straightforward and inexpensive method, less susceptible to supply chain and market fluctuations as it uses only widely available, inexpensive chemicals and expendables. In particular, NaOH—the main lysis factor—is much more efficient than ultra-short, detergent-based approaches, either as kits or as household formulations [5] or boiling water [6]. Due to its scalability, which is its novelty, the protocol described herein may result in a template for immediate use in NAAT (mainly PCR) or in an extract that is storable, transportable, much more enriched, and amplification-friendly. In both cases, the serial dilutions just before use overcome the lack of a spectrophotometer in RLS to manage concentration uncertainties [7].
In a health or ecological crisis, massive spatial dispersion and increased processing output of incoming samples may overload and overrun standard public health or agronomic facilities and infrastructures. Thus, ease of procurement and use by personnel moderately trained in molecular diagnosis but experienced in other sectors of biosciences may increase the processing rate in point-of-collection (PoC) settings, thus neutralizing infectious potential and offloading work from dedicated infrastructures; the latter would tackle unresolved or suspect samples and provide confirmatory analyses as needed. The choice for in situ refinement allows safe dispatching of samples to distant facilities [8], which is otherwise expensive, creates biosafety and biosecurity risks, and may degrade the samples, thus devaluing the actual diagnostic procedure.
The present protocol may be used in RLS, ideally combined with portable instrumentation [9] in its crude, fast version for immediate testing in time-sensitive scenarios, and/or severe RLS. In more permissive scenarios and conditions, its refined version may be implemented, producing much better results [3]. This protocol may be used to improve the turnaround time and volume throughput in established, indoor facilities, especially in its refined version, to increase sensitivity. This comes at the expense of other performance metrics, including speed and simplicity. This method may be used for public health and environmental and agronomic emergencies [10], thus qualifying for implementing the One-Health framework. Processing uncomplicated human/animal samples for genotyping genomic biomarkers by basic PCR protocols or other NAAT should be relatively straightforward and requires no modification, as such samples are easier than the ones tested in the validation publication. Blood is always an unknown quantity regarding extraction efficiency and robustness; less demanding samples, such as mouth swabs, are easy to process and sufficient for targeted biomarker analyses since they are within the capability limits of the less robust thermo-osmotic protocol [6]. Additionally, simplex, single-locus PCR results may be further analyzed downstream by restriction digestion [11,12], single-locus sequencing [13], or any other approach.
Prospective operators should be aware of some limitations of this protocol. Namely, the extract is impure, and thus quantification by standard methods is inapplicable. For the same reason, DNA may be degraded due to a number of factors directly affecting its duration (and thus some of them alleviated in the refined version); the same stands for inhibitor molecules that may hamper the NAAT step. The use of different mechanical rupture techniques, such as sonication, is always a possibility but is not included in this protocol. Enzymatic cell lysis may also be tried; however, it is possible that the chemical environment, due to the NaOH, may degrade or nullify the enzymes used, and thus extensive modification may be required. In any case, the use of enzymes clearly undermines the lean, minimal footprint, supply-chain resilient, and affordable nature of this protocol as presented herein and has not been tested.
Materials and reagents
Note: The provisions below do not include expendables and instrumentation necessary for PCR and other NAAT nor for downstream procedures, including but not limited to gel electrophoresis.
Biological materials
1. Klebsiella pneumoniae and Staphylococcus aureus (kindly supplied as anonymous cultures by Assoc Prof. Kolonitsiou Fevronia from the Department of Microbiology, Medical School, University of Patras)
2. Fungal strains of Cryptococcus neoformans, Candida albicans, Aspergillus fumigatus, and Malassezia pachydermatis (all on solid culture on Petri dishes upon identification by NCPF/UoA)
3. Household fruits (tomatoes) showing observable mycelial development, collected by the authors and kept unidentified
4. Petioles excised using a sterile scalpel blade from leaves of olive trees suspect for Xylella spp. (especially X. fastidiosa) infection, collected by the authors (PPDP)
Reagents
1. NaOH, pellets (Riedel-de-Haen, catalog number: 30620)
2. Tris-HCl, powder (Invitrogen, catalog number: 15504-020)
3. Hydrochloric acid (HCl) 37% (PanReac AppliChem, catalog number: 131020.1212)
4. Isopropanol/2-propanol (Sigma-Aldrich, catalog number: 34863)
5. Ethanol (absolute) (Fisher Scientific, catalog number: E/0650DF/17)
6. Water, molecular biology (PanReac AppliChem, catalog number: A7398.100)
Solutions
1. Ethanol 70% v/v (see Recipes)
2. HCl 1 M (see Recipes)
3. Tris-HCl 1 M, pH 8 (see Recipes)
4. NaOH 0.2 M (see Recipes)
Recipes
1. Ethanol 70% v/v
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol (absolute) | 70% | 700 mL |
| Distilled H2O | 30% | 300 mL |
| Total | n/a | 1,000 mL |
2. HCl 1 M (pH 8)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| HCl 37% | 1 M | 5 mL |
| Distilled H2O | n/a | 45 mL |
| Total | n/a | 50 mL |
Caution: Diluting the commercially available HCl stock solution to working concentrations requires proper precautions due to its vapors and extreme corrosiveness. Use only rated glassware, add the acid slowly in water (not the opposite), and work with gloves (latex is the absolute minimum, but chemical-resistant gloves are the best choice) and other available personal protective equipment (PPE) including but not limited to eye protection and face mask (even standard surgical ones are better than nothing). Also, work only in designated spaces/fume hoods.
3. Tris-HCl 1 M, pH 8
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-HCl | 1 M | 12.114 g |
| Distilled H2O | n/a | 100 mL |
| Total | n/a | 100 mL |
4. NaOH 0.2 M
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaOH | 0.2 M | 0.8 g |
| Distilled H2O | n/a | 100 mL |
| Total | n/a | 100 mL |
See Troubleshooting 2.
Caution: Solubilizing the commercially available NaOH form (pellets) to working concentrations requires precautions due to the extremely caustic nature of the pellets. Do not make contact with exposed skin and add to the solvent gradually, some pellets at a time. Use only properly rated glassware. Work with gloves (latex is the absolute minimum, but chemical-resistant gloves are preferable) and other available PPE including but not limited to eye protection and face mask (even standard surgical ones are better than nothing). Also, work only in designated spaces/fume hoods.
Laboratory supplies
1. Plastic pellet pestles, blue polypropylene autoclavable (Sigma-Aldrich, catalog number: Z359947-100EA)
2. Scalpel blades (no. 22 stainless disposable; FEATHER, lot 03067430) or box cutter (e.g., Q-Connect, catalog number: 233471)
3. Beaker, 1,000 mL (Hamed, catalog number: 303173)
4. Falcon-shaped plastic tubes, 50 mL (KIMA Vacutest, catalog number: KIMA-17102-50)
5. Plastic microtubes w/cap, 1.5 mL (PierceTM, catalog number: 69715)
6. Pipette tips, 0–200 μL (KIMA Vacutest, catalog number: KIMA-18260)
7. Pipette tips, 1,000 μL (KIMA Vacutest, catalog number: KIMA-18172)
8. Disposable inoculation loops 10 μL, sterile (LABSOLUTE®, catalog number: 7696430)
9. Rack (50-position for 18 mm diameter microtubes) (Fruugo)
Equipment
1. Thermo shaker (Kisker Biotech GmbH & Co, model: TS-100)
2. Pipette P20, 0–20 μL (Gilson PIPETMAN®, catalog number: F123600)
3. Pipette P200, 20–200 μL (Gilson PIPETMAN®, catalog number: F123601)
4. Pipette P1000, 200–1,000 mL (Gilson PIPETMAN®, catalog number: F123602)
Procedure
A. Sample collection and preparation
1. When testing pure bacterial and fungal colonies in solid media, collect one loopful of yeast or bacterium from the Petri dish using an inoculation loop (preferably disposable, pre-sterilized, and plastic) and transfer it into a sterile, capped 1.5 mL microtube.
2. In the case of solid cultures of molds, scrap the surface of the agar to collect mycelial tissue using any bladed instrument such as scalpels, razor blades, or box cutters (see General note 1) or an inoculation loop. Place it into a sterile, capped 1.5 mL microtube.
3. For infected fruits, excise thin slices of infected tissue (mostly skin) with as little flesh/host tissue as possible, and place it into a sterile, capped 1.5 mL microtube. A sterile scalpel or other bladed instruments properly sterilized may be used to cut off a piece (3–5 mm in diameter) of the mycelium grown on the plant tissue.
Caution: The use of scalpels and other bladed instruments should be implemented with due caution; a proper first aid kit and decontamination supplies should be available nearby.
4. Store the sample in the 1.5 mL microtube until step B1. If needed, use some guidance/assistance to put it in (see General note 2).
B. Cell lysis
1. Add 180 μL of the 0.2 M NaOH solution to the extraction microtube containing the sample from step A4.
2. Crush with a spiral motion of the pellet pestle for 3–5 min.
3. Use an equal volume of 0.2 M NaOH solution (180 μL, as suggested in step B1) to wash the cellular debris of the pellet pestle within the microtube.
4. Close the lid of the microtube and vortex or shake vigorously by hand for 1 min.
5. Incubate in a thermo shaker set to 75 °C for 10 min (see General note 3).
6. Add 115.2 μL of Tris-HCl 1 M pH 8 and 364.8 μL of water for molecular biology.
7. Vortex for 10 s and then spin at 10,000× g for 3 min.
8. Collect 700 μL using a mechanical pipette with a proper tip and transfer it to a clean microtube.
Critical: If cellular debris is aspirated, the inhibition for NAAT would be considerable and unpredictable, not following any linearity to the concentration of the debris.
Pause point: Although not advised, the protocol may be paused here to freeze the sample. Results have been obtained after thawing after keeping the sample frozen for a maximum of one week.
9. (I) Use immediately for serial dilutions by adding 5 μL of the extract in a microtube containing 45 μL of water for molecular biology; mix by repeated pipetting, proceed to the next tube containing 45 μL of water, and then to the third tube. Use dilutes for PCR or other NAAT. Alternatively, (II) go straight to step C1.
C. Refinement
1. In the microtube with the 700 μL of crude extract, add 70 μL of HCl 1 M to neutralize the NaOH (see General note 4 and Troubleshooting 2) and mix by repeated pipetting.
2. Count the volume by pipetting in a 1,000 μL Gilson or similar mechanical pipette with a proper tip and add an equal volume of isopropanol, preferably cold.
Note: If step B8 was properly executed and followed by C1, the volume is 770 μL and needs no verification; an equal volume of isopropanol may be added without delay.
3. After mixing by repeatedly inverting the tube for 2–5 min, let it stay at -20 °C for 20 min.
Note: Isopropanol is used preferably over ethanol, as the former is expected to work in ambient temperature (either outdoor air temperature or room temperature), a requirement for RLS/PoC settings and especially for field use.
Pause point: At this point, the sample may be stored long-term at -20 °C (practically indefinitely), although the absence of a true purification step in the procedure makes it more susceptible to spontaneous degradation than other properly purified extracts [14].
4. Spin at 2,000× g for 5 min and discard the supernatant.
5. Add 50 μL of 70% v/v ethanol and mix gently by inversion 2–3 times.
6. Spin at 2,000× g for 5 min and discard the supernatant.
7. Inverse and let it dry for as long as necessary.
Note: Use a heat block or thermo shaker, not a water bath, to prevent water droplets from contaminating the sample and vice versa (crossover contamination).
8. Reconstitute/solubilize in 50 μL of water for molecular biology or proper buffer, as per the SOPs and protocols of the lab/institution (see General notes 5 and 6).
9. Go to step B9 (I) for serial dilutions and PCR or other NAAT either upon the completion of the protocol for immediate use or after due storage.
Note: Storage at 4 °C for up to a week has been tried, and the extract produces amplicon. Still, as there is no purification step to thoroughly remove agents of spontaneous deterioration, storage at this temperature should be as brief as possible.
D. Dilutions
1. Transfer 5 μL of the 10 μL to the first dilution tube and mix with 45 μL of distilled water by softly pipetting in and out 5–10 times, to have a 1/10 dilution. Use a 20 μL pipette for the whole procedure.
2. Remove 5 μL of the 1/10 dilution to a second PCR tube (of 200 μL volume or as used per standard practice).
Remove another 5 μL of the 1/10 dilution to the second dilution tube and mix with 45 μL of distilled water by softly pipetting in and out 5–10 times, to have a 1/100 dilution.
3. Remove 5 μL of the 1/100 dilution to a third PCR tube and repeat.
4. Use a specific volume (5 μL is a good starting point) as target for PCR or other NAAT (see General note 6).
Table 1. Indicative results of the two versions of the protocol by sample type. Simplex PCR tests crude and refined extracts from different sample types, tested with different primers and primer concentrations. M13 is a primer for fungal RAPD typing (random amplification of polymorphic DNA). ITS series are generic fungal primers (internal transcribed spacers); 27F/1492R are a pair of generic bacterial primers. This table is a reduced version of Table 1 from the original publication [3]).
| Primers ID | Template | Crude extract dilutions | Refined extract dilutions | ||||
| 1 | 0.1 | 0.01 | 1 | 0.1 | 0.01 | ||
| ITS-3/4 | M. pachydermatis | + | NA | NA | NA | NA | NA |
| M13 | M. pachydermatis | + | NA | NA | NA | NA | NA |
| ITS-5/4 | A. fumigatus | 0 | + | + | 0 | + | + |
| ITS-5/4 | C. albicans | 0 | + | + | 0 | + | + |
| ITS-5/4 | C. neoformans | 0 | + | 0 | 0 | + | + |
| 27F/1492R | S. aureus | 0 | + | + | + | + | + |
| 27F/1492R | K. pneumoniae | 0 | + | + | + | + | + |
| ITS-5/4 | Tomato mycelial extract | 0 | + | + | + | + | + |
| 27F/1492R | Olive leaf extract | 0 | + | 0 | 0 | 0 | + |
| 27F/1492R | Olive leaf extract | 0 | + | 0 | 0 | 0 | + |
| ITS-5/4 | Tomato mycelial extract | + | + | + | NA | NA | NA |
Validation of protocol
This protocol has been used and validated in the following research article:
Goudoudaki et al. [3]. Fast, Scalable, and Practical: An Alkaline DNA Extraction Pipeline for Emergency Microbiomics Biosurveillance. OMICS- J Integr Biol (Figures 2–3 and Table 1).
General notes and troubleshooting
General notes
1. Samples of target cells as pure as possible must be collected (“pure” is understood as containing as few admixtures and as homogenous a cellular population as possible). The quantity of the sample must be limited, as there is no purification step to dispose of cell debris and consequent background noise and inhibitors, including but not limited to proteases and nucleases (step A2).
2. If needed, help/guide the sample of the collection instrument into the microtube using a similarly sterilized microbiology needle or a similar piece (i.e., a second glass slide) or a plastic tip (step A4).
3. Thermoblocks and thermo shakers build up temperature quickly and accurately; the latter allows shaking to mix the contents. In terms of logistics, there are portable versions to deploy in crisis for field/expeditionary use, but these may not be readily available in numbers nor in plentiful supply to acquire on short notice. Thus, water baths are always an option for incubations with caps/lids closed (step B5).
4. The initial concentration is 0.2 M NaOH in 360 μL, added in two steps of 180 μL each (steps B1 and B3). The addition of Tris-HCl and H2O (480 μL) more than doubles the volume, bringing the concentration down to 0.1 M (specifically 0.085 M), which consequently is the concentration of NaOH in the collected 700 μL (step B8). Adding 70 μL of HCl 1 M, roughly one-tenth of the volume and ten times the concentration, achieves proper neutralization, keeping the total volume low enough for the addition of the same volume of isopropanol in the same microtube (step C2). If different volumes are collected, corrections must be factored in for the volume of HCl 1 M.
5. Use a quantity of solvent according to your own judgment of the pellet size (step C8).
6. The protocol is set for PoC in RLS, and thus quantification of nucleic acids is not included; also, the lack of purification, even for the refined version, makes assessment by spectrophotometry unreliable. It rests with the operators to determine, by pilot experiments, the reconstitution volume and the template volume (steps C8 and D4).
Troubleshooting
Problem 1: Inability to accurately weigh the desired NaOH quantity.
Possible cause: The nature of the reagent in pellets.
Solution: Weigh as close as possible to the desired mass and then adjust the volume of the water you add; remember that 0.2 M NaOH is by definition 8 g of NaOH pellets per liter of water, and the mass and volume are proportional (refers to Recipe 1).
Problem 2: The condition of the extract does not allow collecting 700 μL to proceed to step B9.
Possible cause: Excessive or highly complex sample with massive debris floating around.
Solution: Collect as much as possible but try to avoid visual contaminants/debris by starting to pipette liquid from the surface and progressing deeper into the volume of the extract. Then, quantify it using a mechanical pipette and add HCl 1 M to 1/10 of the volume of the extract (see General note 4) (refers to steps B8 and C1).
Acknowledgments
Specific contributions of each author: Conceptualization, M.K. and G.P.; Investigation, S.G. and M.K. Writing—Original Draft, M.K., S.G., A.M., and S.K.; Writing—Review & Editing, A.V. and Y.M; Funding acquisition, Y.M., A.V., and G.P.; Supervision, A.V., Y.M., and G.P.
This work has been partially supported by the project ‘‘Synthetic Biology: From omics technologies to genomic engineering (OMIC-ENGINE)’’ (MIS: 5002636), which is implemented under the Action ‘‘Reinforcement of the Research and Innovation Infrastructure,’’ which is funded by the Operational Program ‘‘Competitiveness, Entrepreneurship and Innovation’’ (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).
This protocol has been used and validated in the research article [3]. This protocol has been inspired by a paper describing field detection of a mosquito-borne parasite [4].
Competing interests
The authors declare no conflicts of interest.
References
- Benda, A., Zerajic, L., Ankita, A., Cleary, E., Park, Y. and Pandey, S. (2021). COVID-19 Testing and Diagnostics: A Review of Commercialized Technologies for Cost, Convenience and Quality of Tests. Sensors. 21(19): 6581. https://doi.org/10.3390/s21196581
- Fomsgaard, A. S. and Rosenstierne, M. W. (2020). An alternative workflow for molecular detection of SARS-CoV-2 – escape from the NA extraction kit-shortage, Copenhagen, Denmark, March 2020. Eurosurveillance. 25(14): e2000398. https://doi.org/10.2807/1560-7917.es.2020.25.14.2000398
- Goudoudaki, S., Milioni, A., Kritikou, S., Velegraki, A., Patrinos, G. P., Gioula, G., Manoussopoulos, Y. and Kambouris, M. E. (2021). Fast, Scalable, and Practical: An Alkaline DNA Extraction Pipeline for Emergency Microbiomics Biosurveillance. OMICS: J Integr Biol. 25(8): 484–494. https://doi.org/10.1089/omi.2021.0090
- Zaky, W. I., Tomaino, F. R., Pilotte, N., Laney, S. J. and Williams, S. A. (2018). Backpack PCR: A point-of-collection diagnostic platform for the rapid detection of Brugia parasites in mosquitoes. PLoS NeglTrop Dis. 12(11): e0006962. https://doi.org/10.1371/journal.pntd.0006962
- Kambouris, M. (1999). Sequences from the aspergillopepsin PEP gene of Aspergillus fumigatus: evidence on their use in selective PCR identification of Aspergillus species in infected clinical samples. FEMS Immunol Med Microbiol. 25(3): 255–264. https://doi.org/10.1016/s0928-8244(99)00095-4
- Goudoudaki, S., Kambouris, M. E., Siamoglou, S., Gioula, G., Kantzanou, M., Manoussopoulou, M., Patrinos, G. P. and Manoussopoulos, Y. (2023). Can Water-Only DNA Extraction Reduce the Logistical Footprint of Biosurveillance and Planetary Health Diagnostics? Toward a New Method. OMICS: J Integr Biol. 27(3): 116–126. https://doi.org/10.1089/omi.2022.0168
- Goudoudaki, S., Kambouris, M., Manoussopoulou, M., Patrinos, G., Velegraki, A. and Manoussopoulos, Y. (2023). Fast and Sustainable Thermo-osmotic DNA Extraction Protocol for Trans-spectrum Contingency and Field Use. Bio Protoc. 13(17): e4796. https://doi.org/10.21769/bioprotoc.4796
- Priye, A., Wong, S., Bi, Y., Carpio, M., Chang, J., Coen, M., Cope, D., Harris, J., Johnson, J., Keller, A., et al. (2016). Lab-on-a-Drone: Toward Pinpoint Deployment of Smartphone-Enabled Nucleic Acid-Based Diagnostics for Mobile Health Care. Anal Chem. 88(9): 4651–4660. https://doi.org/10.1021/acs.analchem.5b04153
- Kambouris, M. E., Siamoglou, S., Kordou, Z., Milioni, A., Vassilakis, S., Goudoudaki, S., Kritikou, S., Manoussopoulos, Y., Velegraki, A., Patrinos, G. P., et al. (2020). Point-of-need molecular processing of biosamples using portable instrumentation to reduce turnaround time. Biosaf Health. 2(3): 177–182. https://doi.org/10.1016/j.bsheal.2020.06.001
- Kambouris, M. E., Manoussopoulos, Y., Kritikou, S., Milioni, A., Mantzoukas, S. and Velegraki, A. (2018). Toward Decentralized Agrigenomic Surveillance? A Polymerase Chain Reaction–Restriction Fragment Length Polymorphism Approach for Adaptable and Rapid Detection of User-Defined Fungal Pathogens in Potato Crops. OMICS: J Integr Biol. 22(4): 264–273. https://doi.org/10.1089/omi.2018.0012
- Kambouris, M. E., Manoussopoulos, Y., Kritikou, S., Milioni, A., Mantzoukas, S. and Velegraki, A. (2018). Toward Decentralized Agrigenomic Surveillance? A Polymerase Chain Reaction–Restriction Fragment Length Polymorphism Approach for Adaptable and Rapid Detection of User-Defined Fungal Pathogens in Potato Crops. OMICS: J Integr Biol. 22(4): 264–273. https://doi.org/10.1089/omi.2018.0012
- Arabatzis, M., Kollia, K., Menounos, P., Logotheti, M. and Velegraki, A. (2004). Delineation of Clavispora lusitaniae clinical isolates by polymerase chain reaction-single strand conformation polymorphism analysis of the ITS1 region: a retrospective study comparing five typing methods. Med Mycol. 42(1): 27–34. https://doi.org/10.1080/1369378032000141444
- Velegraki, A., Kambouris, M., Kostourou, A., Chalevelakis, G. and Legakis, N. (1999). Rapid extraction of fungal DNA from clinical samples for PCR amplification. Med. Mycol. 37(1), 69–73. https://doi.org/10.1046/j.1365-280X.1999.00193.x
- Irinyi, L., Serena, C., Garcia-Hermoso, D., Arabatzis, M., Desnos-Ollivier, M., Vu, D., Cardinali, G., Arthur, I., Normand, A. C., Giraldo, A., et al. (2015). International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database—the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med Mycol. 53(4): 313–337. https://doi.org/10.1093/mmy/myv008
- Velegraki, A. (1999). Identification of medically significant fungal genera by polymerase chain reaction followed by restriction enzyme analysis. FEMS Immunol Med Microbiol. 23(4): 303–312. https://doi.org/10.1016/s0928-8244(98)00150-3
Article Information
Publication history
Received: Feb 5, 2025
Accepted: Mar 30, 2025
Available online: Apr 10, 2025
Published: Apr 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Goudoudaki, S., Kambouris, M. E., Kritikou, S., Milioni, A., Velegraki, A., Manoussopoulos, Y. and Patrinos, G. P. (2025). Scalable Alkaline Extraction Protocol for Microbial DNA Screening by PCR. Bio-protocol 15(8): e5290. DOI: 10.21769/BioProtoc.5290.
Category
Microbiology > Pathogen detection > PCR
Molecular Biology > DNA > DNA extraction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








