- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Workflow for a Functional Assay of Candidate Effectors From Phytopathogens Using a TMV-GFP-based System
Published: Vol 15, Iss 8, Apr 20, 2025 DOI: 10.21769/BioProtoc.5287 Views: 1706
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth
Zeinab Fotoohiyan and Ali Salehi Sardoei
Oct 5, 2025 1340 Views
Reproducible Emu-Based Workflow for High-Fidelity Soil and Plant Microbiome Profiling on HPC Clusters
Henrique M. Dias [...] Christopher Graham
Jan 20, 2026 373 Views
Abstract
The ability to efficiently screen plant pathogen effectors is crucial for understanding plant–pathogen interactions and developing disease-resistant crops. Traditional methods are often labor-intensive and time-consuming. Here, we present a robust, high-throughput screening assay using the tobacco mosaic virus–green fluorescent protein (TMV-GFP) vector system. The screening system combines the TMV-GFP vector and Agrobacterium-mediated transient expression in the model plant Nicotiana benthamiana. This system enables the rapid identification of effectors that interfere with plant immunity (both activation and suppression). The biological function of these effectors can be easily evaluated within six days by observing the GFP fluorescence signal using a UV lamp. This protocol significantly reduces the time required for screening and increases the throughput, making it suitable for large-scale studies. The method is versatile, cost-effective, and can be adapted to effectors with immune interference activity from various pathogens.
Key features
• A robust, cost-effective, and high-throughput functional screening system for plant pathogen effectors.
• Utilizes the TMV-GFP vector for rapid monitoring of effector activity.
• Evaluates the function of effectors within a few days using just a UV lamp.
• Adaptable to both apoplastic and cytoplasmic effectors from various phytopathogens.
Keywords: VirulenceBackground
Understanding the molecular mechanisms of plant–pathogen interactions is essential for developing disease-resistant crops [1–3]. Traditional screening methods for plant pathogen effectors are often laborious and time-consuming, limiting their application in large-scale studies [4–8]. The tobacco mosaic virus (TMV) is a well-known positive-sense single-stranded RNA virus that can infect numerous plant species. Researchers have developed a TMV-based vector that expresses green fluorescent protein (GFP) to visualize viral movement. Under UV light, GFP fluorescence allows for easy tracking of the virus's spread [9]. When the TMV RNA is transcribed from the T-DNA, the vector begins to self-replicate and produces GFP. Consequently, the GFP fluorescence intensity provides a dependable measure of the TMV population size. The TMV-GFP vector system offers a robust approach, allowing for high-throughput screening of pathogen effectors. In this study, we took advantage of the TMV-GFP vector, established a TMV-GFP system, and demonstrated that it can be used to identify both functional cytoplasmic and apoplastic effectors from different plant pathogens based on TMV multiplication [9–11]. In our system, it is unnecessary to insert the effector gene into the TMV-GFP vector, overcoming size constraints about inserting foreign fragments when using a virus-based expression vector. Agrobacteria harboring the effector binary vector and the TMV-GFP vector were solely mixed before infiltration, and TMV infection was easily evaluated by observing the GFP fluorescence signal using a UV lamp within only a few days [12]. As such, as long as you already have an effector’s binary vector library, this functional screening assay can be easily initiated. The protocol described here is optimized for Nicotiana benthamiana but can be adapted to other plant species and pathogens. This method streamlines the screening process, significantly reducing the time and labor required, and provides a versatile tool for plant pathology research.
Materials and reagents
Biological materials
1. Nicotiana benthamiana seeds
2. Agrobacterium tumefaciens strain GV3101 (pMP90)
3. Escherichia coli strain DH5a
Reagents
1. Sterile deionized water
2. Sodium chloride (NaCl) (Sangon Biotech, catalog number: A501218-0001)
3. Tryptone (OXOID, catalog number: LP0042)
4. Yeast extract (Sangon Biotech, catalog number: A515245-0500)
5. Agar (Solarbio, catalog number: A8190)
6. MES free acid monohydrate (MES) (Solarbio, catalog number: M8010)
7. Magnesium chloride hexahydrate (MgCl2·6H2O) (Sangon Biotech, catalog number: A601336-0500)
8. 3’,5’-Dimethoxy-4’-hydroxyacetophenone (acetosyringone; AS) (Sigma-Aldrich, catalog number: 2478-38-8)
9. Rifampicin (Sangon Biotech, catalog number: A600812-0025)
10. Gentamycin (Sangon Biotech, catalog number: A620217-0005)
11. Spectinomycin dihydrochloride pentahydrate (Sangon Biotech, catalog number: A600901-0005)
12. Kanamycin sulfate (Sangon Biotech, catalog number: A600286-0005)
13. Dimethyl sulfoxide (DMSO) (Solarbio, catalog number: D8370)
14. Potassium hydroxide (KOH) (Sangon Biotech, catalog number: A610441-0500)
Solutions
1. Antibiotic solution (see Recipes)
2. LB medium (see Recipes)
3. Agrobacterium infiltration buffer (see Recipes)
Recipes
1. Antibiotic solution
Prepare a stock solution of gentamycin (50 mg/mL), spectinomycin dihydrochloride pentahydrate (50 mg/mL), and kanamycin sulfate (50 mg/mL) in sterile distilled water and rifampicin (25 mg/mL) in DMSO. Store at -20 °C. Dilute 1,000× and add to the medium.
Note: Depends on the construct and Agrobacterium strain. In this experiment, the GV3101 (pMP90) strain was used, and the nuclear gene contained the screening tag, rifampicin resistance gene, and the Ti plasmid pMP90, which conferred gentamycin resistance to the GV3101 strain. Also, different constructs have different screening tags; for example, the main construct used in this experiment is pGWB505, which has a spectinomycin dihydrochloride pentahydrate resistance. Therefore, media containing different antibiotics was used to grow the Agrobacterium needed for the experiment.
2. LB medium
Tryptone 10 g
NaCl 5 g
Yeast extract 5 g
Adjust pH to 7.5 with KOH and add sterile deionized water to 1 L. To prepare solid medium, add 15 g of agar to 1 L of medium and autoclave.
3. Agrobacterium infiltration buffer
1 M MES 100 μL
1 M MgCl2 100 μL
150 mM AS 10 μL
Add 9.790 mL of sterile deionized water to reach 10 mL.
Adjust MES to pH 5.7 with KOH.
Note: Dissolve MES and MgCl2 in sterile water and filter the solutions with a 0.22 μm syringe filter to avoid contamination. Store the solution at 4 °C. Dissolve AS in DMSO and store the stock solution at -20 °C.
Laboratory supplies
1. Soil mix or substrate (potting soil and vermiculite)
2. Plastic cups (8 cm diameter and 10 cm height)
3. Plant trays
4. Syringes (1 mL)
5. Tissue paper
6. Petri dishes (60 mm diameter)
7. 1.5 and 2.0 mL tubes (BBI, catalog number: F600620-0001)
8. Pipette tips (AIBIO, catalog number: T1040000)
9. Medical rubber gloves
10. Metal beads (3 mm)
Equipment
1. Plant growth chamber at 24 °C under a light intensity of 150 μmol·m-2·s-1 and a 16/8 h light/dark photoperiod
2. Spectrophotometer capable of OD600 measurements (e.g., Shimadzu, catalog number: 206-67001-34)
3. Petri dish incubator at 28tri (Panasonic, model: MIR-262-PC)
4. Handheld UV lamp (BLAK-RAY B-100AP LAMP, UVP, USA)
5. Digital camera (Canon, model: EOS 200D II)
Software and datasets
1. ImageJ (https://fiji.sc/)
2. GraphPad Prism 8 (http://www.graphpad.com)
Procedure
A. Plant growth conditions (Figure 1)
1. Sow N. benthamiana seeds in a 2:5 mix of potting soil and vermiculite and place them in a growth chamber at 24 °C under a 16/8 h light/dark photoperiod. Water plants every 3–4 days (2 L per tray with 18 cups).
2. After 7 days, keep a well-growing seedling in each plastic cup and allow it to grow under the same growth conditions until it is 4–5 weeks old.

Figure 1. Growth of N. benthamiana seedlings in soil. (A) Germination of N. benthamiana seeds in a 2:5 mixture of standard potting soil and vermiculite. (B) Four-week-old N. benthamiana plant is ready for assay. White arrows indicate leaves suitable for infiltration.
B. Preparation of Agrobacterium cultures (Figure 2)
1. Separately culture the A. tumefaciens GV3101 (pMP90) carrying the TMV-GFP empty vector, the effectors to be tested (we use an effector that activates plant immunity from Cao et al. [12] as an example), or the mock (LTI6b, a non-immune-related control protein as negative control) on the plates containing the corresponding antibiotics at 28 °C.
2. Two days later, scrape the fresh Agrobacterium cells from the plates (or use a singly colony liquid culture), suspend, and adjust to specific concentrations (OD600 = 0.4 for TMV-GFP and OD600 = 0.8 for LTI6b or effectors).
3. Mix the TMV-GFP suspension with LTI6b or effector suspension in equal volumes and incubate them in a 28 °C incubator for 2–4 h.
Note: The total volume of the mix suspension is determined by the needs of the experiment; just pay attention to the accuracy of OD600 before mixing.
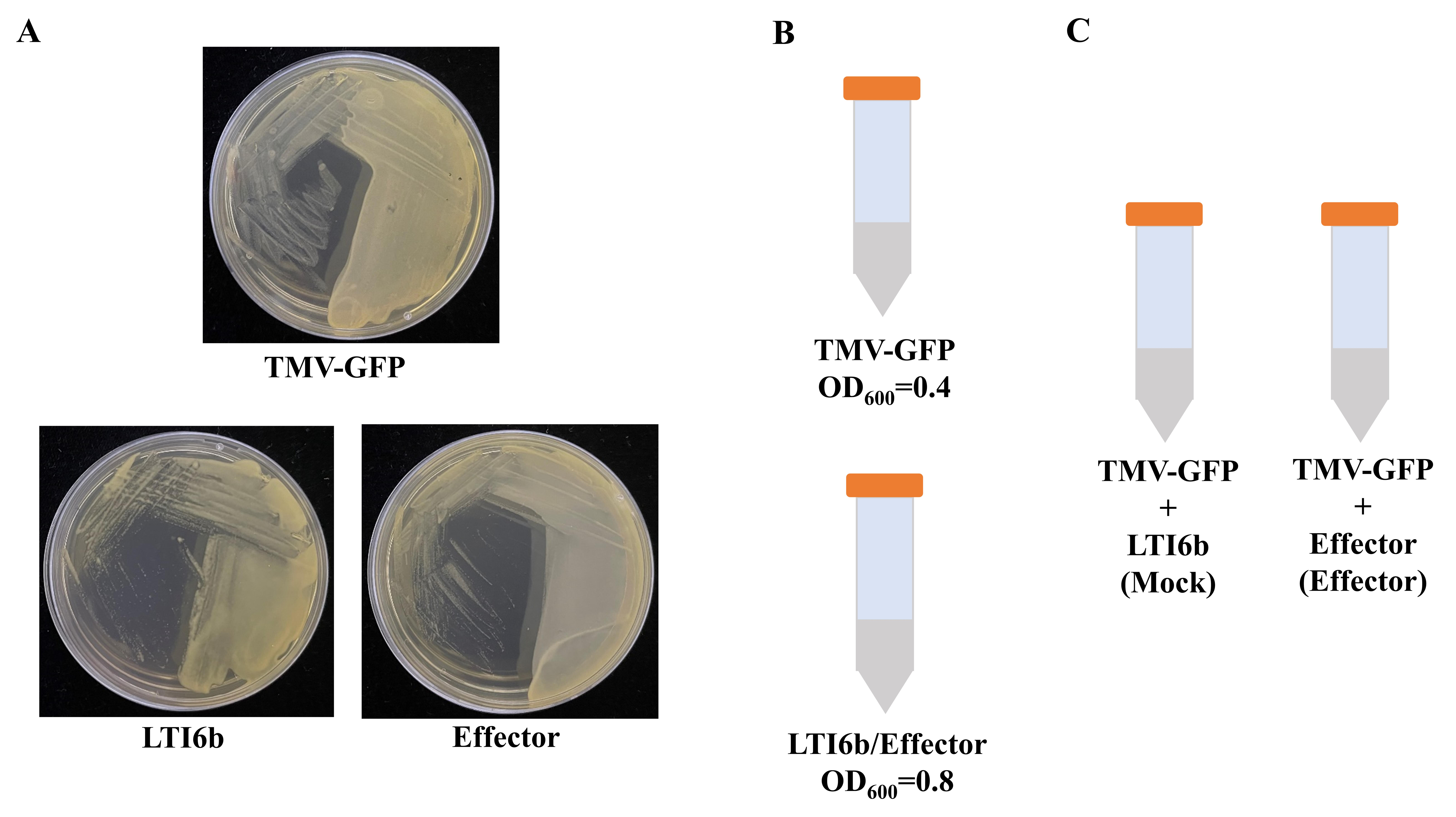
Figure 2. Agrobacterium culture and collection. (A) Agrobacterium growing on solid LB medium. (B) Agrobacterium suspension in infiltration buffer. (C) Mix the final Agrobacterium suspension to OD600 = 0.2 for TMV-GFP and OD600 = 0.4 for LTI6b or effectors.
C. Infiltration of N. benthamiana leaves (Figure 3)
1. Using a sterilized needle, gently score a minuscule wound on either side (left and right) of the leaf’s lower epidermis without penetrating through the entire leaf.
2. On the upper surface of the leaf, label “Mock” on the left side and “Effector” on the right side using a non-toxic, water-resistant marker.
3. Prepare syringes without needles for infiltrating the Agrobacterium suspension.
4. Inject 100 μL of the respective Agrobacterium suspensions—Mock and Effector—into the corresponding micro-wounds of the marked areas from the lower leaf surface.
5. Allow the plants to grow under the same growth conditions for 72 h.
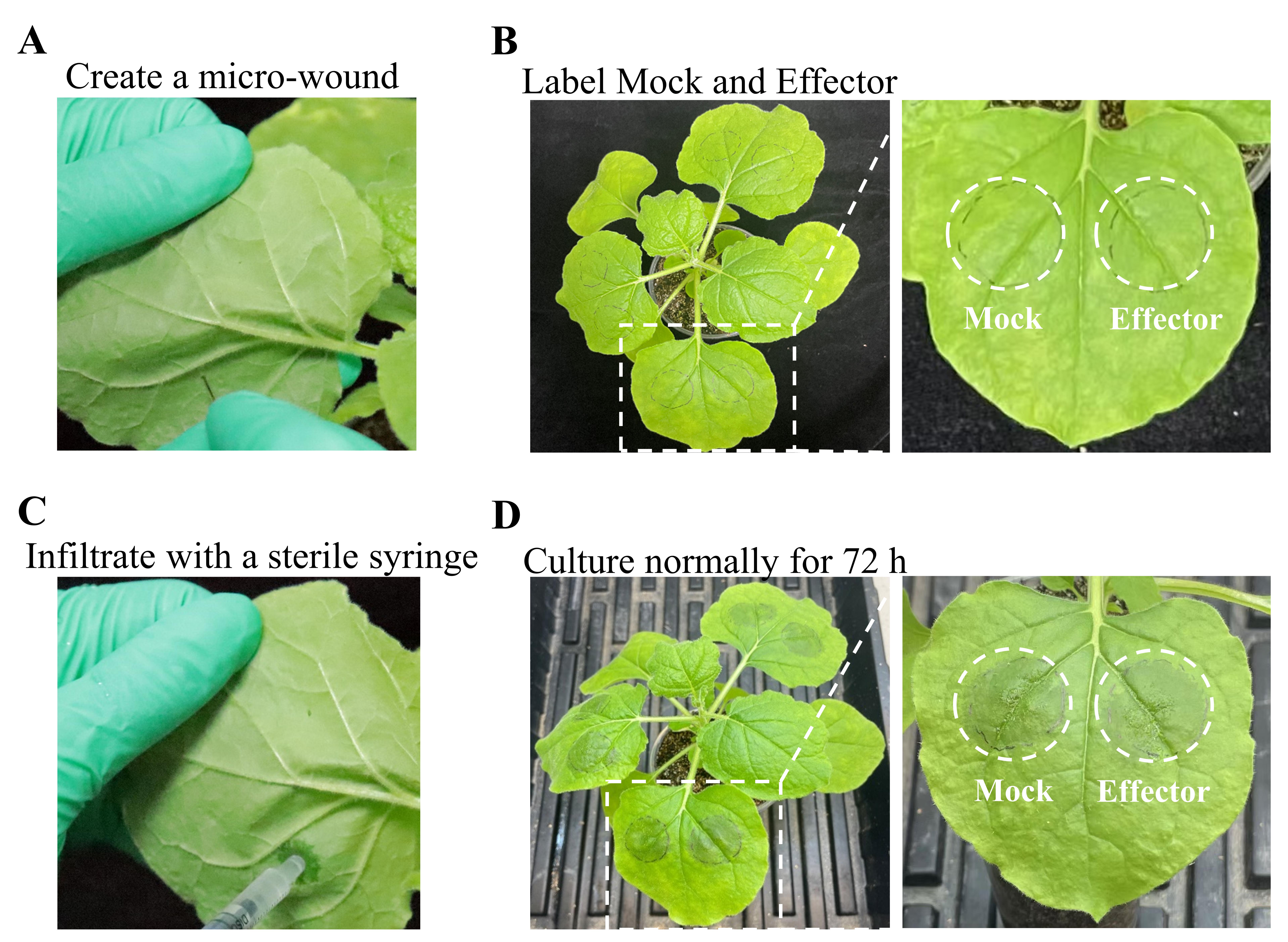
Figure 3. Illustration of the infiltration step. (A) On the abaxial side of the leaf, prick softly with a needle. (B) Using a marker pen, draw a circle on each half of the leaf to control the area for infiltration. (C) Infiltrate the “Mock” and the “Effector” Agrobacterium suspension into the marked area correspondingly. (D) Allow the plants to grow for 72 h.
D. Image capture (Figure 4)
1. Warm up the handheld UV lamp for 2 min to reach its peak luminosity.
2. Adjust the camera settings (Canon EOS 200D II) to optimize the photographic capture.
a. Set the focal length to 25 mm with an aperture range of F1.6 to F4.0.
b. Set the shutter speed to 1/15 s and adjust the ISO sensitivity between 3,200 and 6,400 to accommodate low-light conditions.
Note: These settings were carefully chosen to maximize light capture in the dark room while balancing exposure and maintaining image sharpness.
3. Excise the leaf sample approximately 1–2 cm from the petiole, gently tap it to remove any debris, and then lay it flat on a desk with a black background cloth.
4. Position the UV light source 10 cm above the leaf at a 45° angle laterally oblique to the leaf, ensuring that the light is precisely focused on the center of the leaf area.
5. Align the camera lens perpendicularly to the leaf, maintaining a distance of 10 cm, and take the photo.
Note: Minor adjustments to the parameters mentioned above should be made as necessary to achieve a clear and detailed image of the leaf.
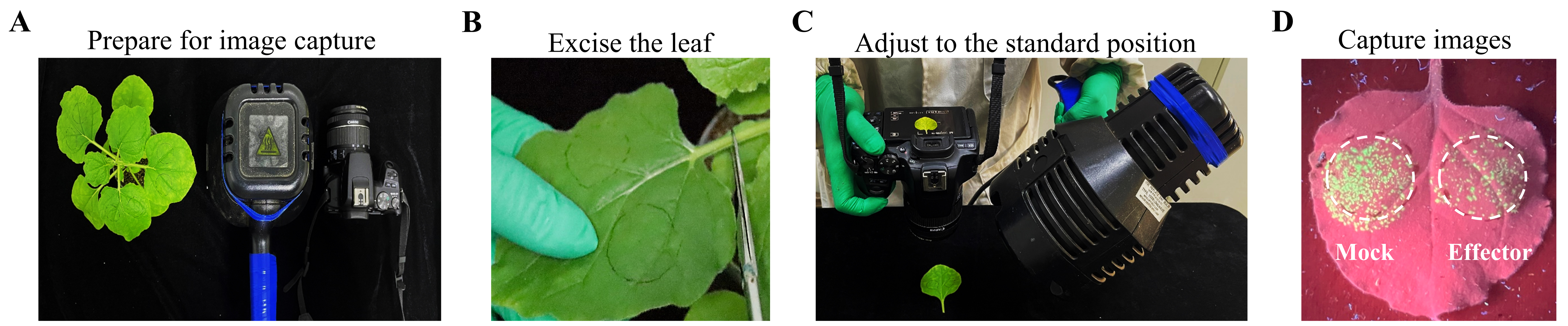
Figure 4. Images are captured after 72 h under a UV lamp in a dark room to monitor GFP expression. (A) Prepare to capture images after 72 h. (B) Excise the leaf sample from the petiole. (C) Move the camera or UV lamp to a position suitable for image capture. (D) Capture images under UV light. All of the above steps are performed in a dark room.
Data analysis
Proceed with the following steps for quantification of GFP intensity in images obtained from the above experiments.
1. Open the image in ImageJ, navigate to Image, and then Color. Select Channels Tool and check the Channel 1 checkbox.
2. Click on Image, then Color, and select Split Channels to separate the image into red, green, and blue channels. Choose the green-channel image for GFP intensity quantification.
3. Click on Image, Adjust, then Threshold. Select Default and B&W settings and ensure the Dark background checkbox is checked.
4. Adjust the slide bars to optimize image brightness and eliminate background noise.
Note: Precise adjustments are essential to accurately distinguish fluorescence intensity differences between the infected areas on the left (Mock) and right (Effector) sides of the leaf.
5. Proceed to Analysis, Set Measurement, and check the boxes for Area, Mean grey value, Integrated density, and Limit to threshold.
6. Click on Analyze, then Tools, followed by ROI Manager to open the ROI Manager.
7. Select Oval shape and draw around the infected area on the left side of the leaf to encompass all signals as the region of interest (ROI). Add this ROI by clicking Add in the ROI Manager, then click Rename to label it as Mock, and finally click OK.
8. Repeat step 7 to set the ROI on the right side of the leaf, using identical settings including the size of the circle. Rename this ROI as Effector.
9. In the ROI manager, select both Mock and Effector, then click Measure.
10. Repeat steps 1–9 for the remaining replicates and export the collected data.
11. Normalize the data with Mock set to 1.
12. Analyze the compiled data using GraphPad Prism 8 software for further statistical analysis and interpretation. To compare results individually, perform a Student’s t-test to indicate the statistical significance of the differences (Figure 5). Differences between the GFP intensity of Mock and Effector reflect the influence of the effector on plant immunity.
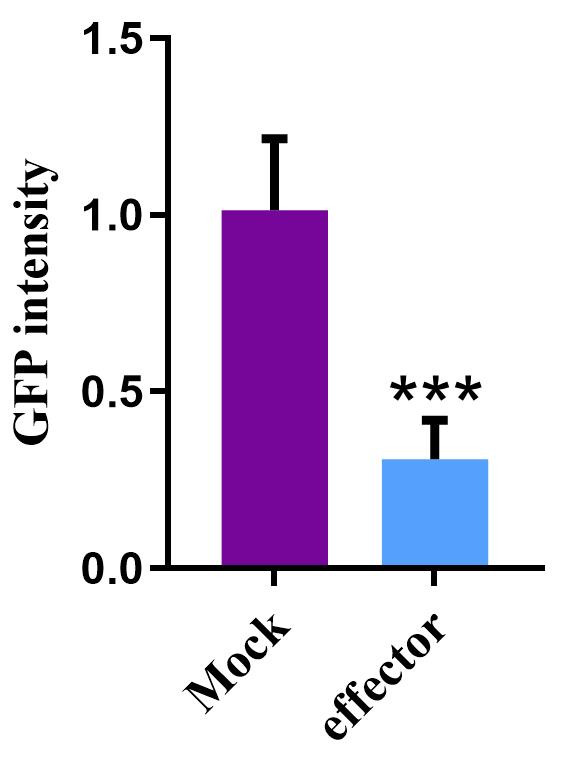
Figure 5. Effector function evaluation using the tobacco mosaic virus–green fluorescent protein (TMV-GFP) system. Values indicate the relative GFP intensity compared to the Mock. Data are represented as the mean ± SEM (n = 9, *** P ≤ 0.001).
Validation of protocol
This protocol or its parts have been used in the following research articles:
• Cao et al. [12]. A robust high-throughput functional screening assay for plant pathogen effectors using the TMV-GFP vector. The Plant Journal. (Figures 2, 3, 4, 5, 6, and 7)
General notes and troubleshooting
1. Caution: To use plant material efficiently, inject 2–3 sets of mock/effectors on a leaf during screening.
2. Pause point: The protocol can be paused after Agrobacterium resuspension.
3. Critical step: Ensure Agrobacterium suspension is at the correct OD600 for efficient infiltration.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2022YFD1602000) and the National Natural Science Foundation of China (32272641; 32372483; 32302296). We acknowledge the previous work described in [12], The Plant Journal.
Competing interests
The authors declare no competing interests.
References
- Hogenhout, S. A., Van der Hoorn, R. A. L., Terauchi, R. and Kamoun, S. (2009). Emerging Concepts in Effector Biology of Plant-Associated Organisms. Mol Plant Microbe Interact. 22(2): 115–122. https://doi.org/10.1094/mpmi-22-2-0115
- Petre, B., Saunders, D. G. O., Sklenar, J., Lorrain, C., Win, J., Duplessis, S. and Kamoun, S. (2015). Candidate Effector Proteins of the Rust Pathogen Melampsora larici-populina Target Diverse Plant Cell Compartments. Mol Plant Microbe Interact. 28(6): 689–700. https://doi.org/10.1094/mpmi-01-15-0003-r
- An, Y. and Zhang, M. (2024). Advances in understanding the plant-Ralstonia solanacearum interactions: Unraveling the dynamics, mechanisms, and implications for crop disease resistance. New Crops. 1: 100014. https://doi.org/10.1016/j.ncrops.2024.100014
- Coll, N. S., Epple, P. and Dangl, J. L. (2011). Programmed cell death in the plant immune system. Cell Death Differ. 18(8): 1247–1256. https://doi.org/10.1038/cdd.2011.37
- Waszczak, C., Carmody, M. and Kangasjärvi, J. (2018). Reactive Oxygen Species in Plant Signaling. Annu Rev Plant Biol. 69(1): 209–236. https://doi.org/10.1146/annurev-arplant-042817-040322
- Ma, W., Xu, X., Cai, L., Cao, Y., Haq, F., Alfano, J. R., Zhu, B., Zou, L. and Chen, G. (2020). A Xanthomonas oryzae type III effector XopL causes cell death through mediating ferredoxin degradation in Nicotiana benthamiana. Phytopathol Res. 2(1): 16. https://doi.org/10.1186/s42483-020-00055-w
- Shi, J., Zhu, Y., Li, M., Ma, Y., Liu, H., Zhang, P., Fang, D., Guo, Y., Xu, P., Qiao, Y., et al. (2020). Establishment of a novel virus‐induced virulence effector assay for the identification of virulence effectors of plant pathogens using a PVX‐based expression vector. Mol Plant Pathol. 21(12): 1654–1661. https://doi.org/10.1111/mpp.13000
- Prautsch, J., Erickson, J. L., Özyürek, S., Gormanns, R., Franke, L., Lu, Y., Marx, J., Niemeyer, F., Parker, J. E., Stuttmann, J., et al. (2023). Effector XopQ-induced stromule formation in Nicotiana benthamiana depends on ETI signaling components ADR1 and NRG1. Plant Physiol. 191(1): 161–176. https://doi.org/10.1093/plphys/kiac481
- Park, M., Baek, E., Yoon, J. Y. and Palukaitis, P. (2017). The Use of a Tobacco mosaic virus-Based Expression Vector System in Chrysanthemum. Plant Pathol J. 33(4): 429–433. https://doi.org/10.5423/ppj.nt.04.2017.0083
- Crameri, A., Whitehorn, E. A., Tate, E. and Stemmer, W. P. (1996). Improved Green Fluorescent Protein by Molecular Evolution Using DNA Shuffling. Nat Biotechnol. 14(3): 315–319. https://doi.org/10.1038/nbt0396-315
- Liu, Y., Schiff, M., Marathe, R. and Dinesh‐Kumar, S. P. (2002). Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N‐mediated resistance to tobacco mosaic virus. Plant J. 30(4): 415–429. https://doi.org/10.1046/j.1365-313x.2002.01297.x
- Cao, P., Shi, H., Zhang, S., Chen, J., Wang, R., Liu, P., Zhu, Y., An, Y. and Zhang, M. (2024). A robust high‐throughput functional screening assay for plant pathogen effectors using the TMV‐GFP vector. Plant J. 119(1): 617–631. https://doi.org/10.1111/tpj.16774
Article Information
Publication history
Received: Dec 30, 2024
Accepted: Mar 18, 2025
Available online: Apr 1, 2025
Published: Apr 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Cao, P., Shi, H., Chen, J., Cui, L., Zhang, M. and An, Y. (2025). Workflow for a Functional Assay of Candidate Effectors From Phytopathogens Using a TMV-GFP-based System. Bio-protocol 15(8): e5287. DOI: 10.21769/BioProtoc.5287.
Category
Plant Science > Plant immunity > Host-microbe interactions
Plant Science > Plant immunity > Disease symptom
Microbiology > Microbe-host interactions
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link