- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Rapid Plasmid-Free Generation of Recombinant Positive-Strand RNA Viruses That Use IRES-Mediated Translation Using an Expansion of the Circular Polymerase Extension Reaction (CPER)
Published: Vol 15, Iss 8, Apr 20, 2025 DOI: 10.21769/BioProtoc.5275 Views: 2063
Reviewed by: Emilie BesnardAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Protocol to Retrieve Unknown Flanking DNA Using Fork PCR for Genome Walking
Hongjing Wu [...] Haixing Li
Jan 20, 2025 2393 Views
Protocol to Identify Unknown Flanking DNA Using Partially Overlapping Primer-based PCR for Genome Walking
Mengya Jia [...] Haixing Li
Feb 5, 2025 1363 Views
Protocol to Mine Unknown Flanking DNA Using PER-PCR for Genome Walking
Zhou Yu [...] Haixing Li
Feb 20, 2025 1495 Views
Abstract
Reverse genetics systems in virology are technologies used to generate recombinant viruses, enabling the manipulation of viral genes. Recombinant viruses facilitate the investigation of pathogenesis and the development of antivirals. In studies of positive-sense single-stranded RNA (ssRNA) viruses, a reverse genetics approach typically uses infectious viral cDNA clones derived from bacterial artificial chromosomes and plasmids or from the in vitro ligation of viral cDNA fragments. However, these methods are time-consuming, involve complex procedures, and do not always successfully generate recombinant viruses. Possible reasons for unsuccessful outcomes include i) viral sequences exhibiting toxicity in bacterial systems, ii) the duplication of viral genes observed in some strains, complicating the acquisition of correct cDNA clones, and iii) certain cell lines being highly susceptible to infection but difficult to transfect with nucleotides. For these reasons, a simple and rapid reverse genetics system is needed to accelerate research on ssRNA viruses. The circular polymerase extension reaction (CPER) method offers a solution by eliminating the need for molecular cloning in bacteria, enabling the generation of recombinant viruses over a shorter timeframe. This method has been widely adopted for the study of ssRNA viruses, including SARS-CoV-2 and flaviviruses. Recently, we expanded the CPER method for ssRNA viruses using internal ribosome entry site (IRES)-mediated translation. This protocol details the experimental procedures, using bovine viral diarrhea virus as an example—one of the most challenging viruses for generating viral cDNA clones because of the factors listed above.
Key features
• Rapid generation of recombinant positive-strand RNA viruses.
• The CPER method eliminates the need for molecular cloning in bacteria, enabling the rapid generation of recombinant viruses.
• The CPER method for ssRNA viruses enables efficient translation of viruses using IRES by incorporating the gene cassette of RNA Pol-I promoters and terminators.
Keywords: Reverse geneticsGraphical overview
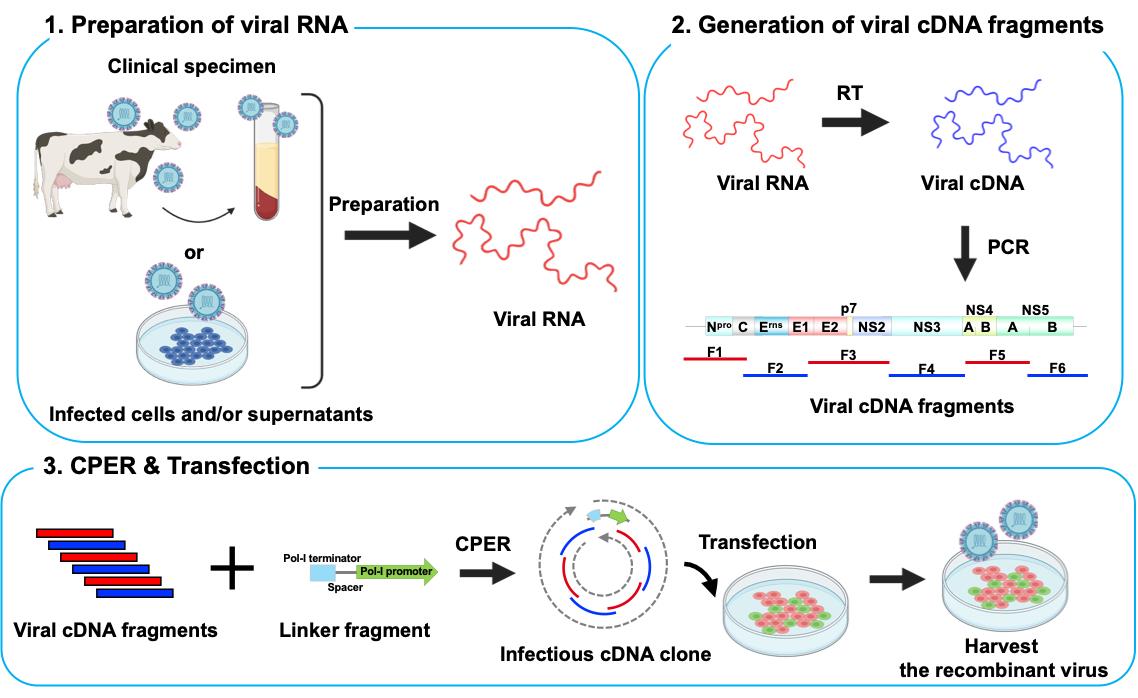
Background
In virology, the reverse genetics system is a technology for generating recombinant viruses that enables the manipulation of viral genes. Using this system, we can define the function of viral genes/proteins and investigate the mechanisms of viral pathogenicity [1]. To date, infectious viral cDNA clones from bacterial artificial chromosomes and plasmids or from in vitro ligation of viral cDNA fragments have been used for reverse genetics in studies on positive-sense single-stranded RNA (ssRNA) viruses [2–5]. Although these methods allow for the generation of recombinant viruses, they are time-consuming and involve complicated procedures. Thus, a simple and efficient reverse genetics system is required, particularly in the context of pandemics caused by emerging/re-emerging ssRNA viruses.
A method for the generation of recombinant ssRNA viruses using circular polymerase extension reaction (CPER) has been developed [6,7]. In this method, full-length viral cDNA fragments with overlapping ends and an RNA Pol-II promoter-encoding linker are assembled into a circular genome by DNA polymerase and are directly transfected into cells to recover infectious viruses. This technology was applied to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which possesses one of the largest genomes (~30 kb) among the ssRNA viruses, enabling the rapid generation of recombinant viruses. It also enabled the rapid characterization of SARS-CoV-2 variants of concern and variants of interest after their emergence [8–12].
Flaviviruses and SARS-CoV-2 perform translation in a cap-dependent manner, whereas certain ssRNA viruses, such as hepatitis C virus (HCV) and bovine viral diarrhea virus (BVDV), perform cap-independent internal ribosome entry site (IRES)-mediated translation. The CPER method, optimized for efficient capping after viral RNA transcription, may not be directly applicable to ssRNA viruses that use IRES-mediated translation. Therefore, we adapted the CPER method using a gene cassette containing an RNA Pol-I promoter and terminator sequence that is needed for the efficient translation of RNA viruses [13].
BVDV, now renamed as Pestivirus bovis, belongs to the Pestivirus genus of the Flaviviridae family and possesses ssRNA that undergoes IRES-mediated translation. BVDV is the causative agent of bovine viral diarrhea, which leads to economic losses in agriculture. Generating recombinant BVDV using conventional reverse genetics has proven challenging for three reasons: i) the viral sequence exhibits toxicity in bacteria [14]; ii) gene duplication in some BVDV strains makes it difficult to obtain the correct cDNA clones [15]; and iii) MDBK cells, the most common cell line for BVDV propagation, show high susceptibility but are difficult to transfect with nucleotides [16].
In this protocol, we describe an optimized CPER method for ssRNA viruses using IRES-mediated translation, with BVDV serving as an example. Our method overcomes commonly encountered challenges and successfully produces recombinant viruses over a short timeframe. In addition, we demonstrate the generation of recombinant viruses without the need for isolation from permissive cell cultures, which is useful for the rapid characterization of viral strains, particularly during a pandemic.
Materials and reagents
Biological materials
1. Bovine MDBK cells (ATCC, catalog number: CCL-22)
2. Lenti-X 293T cells (TAKARA BIO, catalog number: 632180)
3. Serum from cattle persistently infected with BVDV (BVDV-1b strain Shihoro/B_6, Hirose et al. [17])
Reagents
1. BVDV antibody-free FBS (Japan Bio Serum, catalog number: 621-00535)
2. DMEM (4.5 g/L glucose) with L-glutamine, without sodium pyruvate, liquid (Nacalai Tesque, catalog number: 08459-64)
3. Penicillin-streptomycin mixed solution (Nacalai Tesque, catalog number: 26253-84)
4. PrimeSTAR® GXL DNA polymerase (TAKARA BIO, catalog number: 12292-04)
5. KOD One® PCR master mix (Toyobo, catalog number: 18538-01)
6. 1 kb DNA ladder (TAKARA BIO, catalog number: 3412A)
7. SuperScriptTM IV VILOTM master mix (Thermo Fisher Scientific, catalog number: 11756050)
8. Opti-MEM (Thermo Fisher Scientific, catalog number: 07088-54)
9. TransIT®-LT1 transfection reagent (Mirus, catalog number: MIR2304)
10. Acetone (Nacalai Tesque, catalog number: 00309-35)
11. D-PBS (-) without Ca and Mg, liquid (Nacalai Tesque, catalog number: 14249-24)
12. Anti-pestiviral NS3 antibody (prepared in-house, see Kameyama et al. [18])
13. Goat anti-mouse IgG Alexa Fluor 488-conjugated secondary antibody (Thermo Fisher Scientific, catalog number: A32723)
14. PureLinkTM RNA Mini kit (Thermo Fisher Scientific, catalog number: 12183018A)
15. FastGene Gel/PCR Extraction kit (NIPPON Genetics, catalog number: FG-91202)
16. Primer sets (Fasmac; Table 1)
Table 1. Primer sets used for CPER of BVDV-1b
| Fragment | Genome size (bp) | Orientation | Nucleotide sequence (5'–3') |
|---|---|---|---|
| BVDV-1b Fragment 1 | 1303 | Forward | GTATACGAGGTTAGGCAAGTTC |
| Reverse | GATTTTCTCTGGCCAGATC | ||
| BVDV-1b Fragment 2 | 2401 | Forward | GATCTGGCCAGAGAAAATC |
| Reverse | CTCTCTTAGTAGTAGGTATAGTAG | ||
| BVDV-1b Fragment 3 | 2316 | Forward | CTACTATACCTACTACTAAGAGAG |
| Reverse | CATGTATTGATAGACTGACTCAGCTGC | ||
| BVDV-1b Fragment 4 | 2501 | Forward | GCAGCTGAGTCAGTCTATCAATACATG |
| Reverse | CTTATCTTCCCTTCAGAGTCCATC | ||
| BVDV-1b Fragment 5 | 2236 | Forward | GATGGACTCTGAAGGGAAGATAAG |
| Reverse | GTGCTTCTCTGAGTCCAGTAC | ||
| BVDV-1b Fragment 6 | 1652 | Forward | GTACTGGACTCAGAGAAGCAC |
| Reverse | GGGGCTGTTAAGGGTTTTCCCTAGTC | ||
| BVDV-1b Linker fragment | 738 | Forward | ACCCTTAACAGCCCCCCCCCCCAACTTCGGAGGTCGAC |
| Reverse | GAACTTGCCTAACCTCGTATACAATAACCCGGCGGCCCAAAATGC |
Solutions
1. Maintenance medium (see Recipes)
2. 2% FBS medium (see Recipes)
Recipes
1. Maintenance medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM | Not applicable | 500 mL |
| BVDV antibody-free FBS | 9.1% | 50 mL |
| Penicillin-streptomycin | 1% | 5 mL |
2. 2% FBS medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM | Not applicable | 500 mL |
| BVDV antibody-free FBS | 2% | 10 mL |
| Penicillin-streptomycin | 1% | 5 mL |
Laboratory supplies
1. Collagen-coated microplate 6-well with lid, collagen type I (IWAKI, catalog number: 4810-010N)
2. Collagen-coated microplate 24-well with lid, collagen type I (IWAKI, catalog number: 4820-010)
3. Flat bottom micro tube 1.5 mL (BIO-BIK, catalog number: CF-0150)
4. 0.2 mL 8-strip PCR tube caps with dome top, natural (WATSON BIO LAB, catalog number:137-432C)
Equipment
1. CO2 incubator (PHCbi, model: MCO-170AICUVD)
2. NanoDrop Lite UV-Vis spectrophotometer ND-LITE (Thermo Fisher Scientific, catalog number: 32-1001)
3. Fluorescence microscope (KEYENCE, model: BZ-X810)
Software and datasets
1. Prism v. 10.2.2 (GraphPad, 7/31/2024)
Procedure
A. Preparation of viral RNA
1. Follow the manufacturer’s protocol to purify total RNA from the serum of cattle persistently infected with BVDV using the PureLinkTM RNA Mini kit (see General note 1). Use 100 μL of serum stored at -80 °C.
B. Generation of viral cDNA fragments
1. Reverse transcribe total RNA into cDNA using the SuperScriptTM IV VILOTM master mix. Mix 16 μL of total RNA sample with 4 μL of SuperScriptTM IV VILOTM master mix and heat at 25 °C for 10 min, 50 °C for 10 min, and 85 °C for 5 min.
2. Amplify a total of six viral gene fragments covering the entire viral genome and a linker fragment encoding a Pol-I terminator, spacer, and a Pol-I promoter by KOD One® PCR master mix with gene-specific primer sets (primer sets in Table 1; the recipe of this reaction mixture is in Table 2; thermocycling conditions for the PCR reaction are in Table 3) [19].
Table 2. Reaction mixture
| Reagent | Volume |
|---|---|
| cDNA | 1 μL |
| Forward primer (10 μM) | 1.5 μL |
| Reverse primer (10 μM) | 1.5 μL |
| KOD One® PCR master mix | 25 μL |
| H2O | 21 μL |
Table 3. Thermocycling conditions for PCR
| Step | Temp. (°C) | Duration | No. of cycles |
| Denaturation | 98 | 10 s | 30 |
| Annealing | 50 | 5 s | |
| Extension | 68 | 40 s | |
| Final extension | 68 | 4 min | 1 |
| Hold | 4 | ∞ | - |
3. Assess the size of the fragments by gel electrophoresis using a commercial 1 kb DNA ladder as a reference.
4. Follow the manufacturer’s protocol to purify the PCR fragments using the FastGene Gel/PCR Extraction kit.
5. Measure DNA concentration using NanoDrop.
6. Because CPER is based on assembly PCR, the correct joining of fragments via the overlapping regions is critical for making the complete circular cDNA assembly. Thus, use PCR assembly to connect neighboring fragments by PrimeSTAR® GXL DNA polymerase (see Troubleshooting 1).
C. Circular polymerase extension reaction (CPER)
1. Mix each gene fragment of BVDV cDNA and the linker fragment at 0.1 pmol per fragment.
2. Assemble all fragments of equal molar amounts using PrimeSTAR® GXL DNA polymerase (the recipe of this reaction mixture is in Table 4; thermocycling conditions for the CPER are detailed in Table 5).
Table 4. CPER mixture
| Reagent | Volume |
|---|---|
| Each gene fragment of BVDV cDNA and the linker fragment | 0.1 pmol per fragment |
| dNTP mixture | 4 μL |
| 5× PrimeSTAR GXL buffer | 10 μL |
| PrimeSTAR GXL DNA polymerase | 1 μL |
| H2O | Up to 50 μL |
Table 5. Thermocycling conditions for the CPER
| Step | Temp. (°C) | Duration | No. of cycles |
| Initial denaturation | 98 | 2 min | 1 |
| Denaturation | 98 | 10 s | 20 |
| Annealing | 55 | 15 s | |
| Extension | 68 | 12 min | |
| Final extension | 68 | 12 min | 1 |
| Hold | 4 | ∞ | - |
D. Transfection
1. Prepare cells.
a. One day before transfection, co-culture MDBK and 293T cells in a 2:1 ratio (see General note 2) in maintenance medium (see Recipes) in a collagen-coated 6-well microplate. Seed cells by plating 7 × 105 cells per well.
b. Upon 80% confluence, replace maintenance medium with 2% FBS medium (see Recipes).
2. Immediately after the CPER, add 25 μL of CPER product to 200 μL of Opti-MEM warmed to 37 °C and mix by pipetting.
3. Add 12 μL of TransIT-LT1® transfection reagent and mix by slow pipetting.
4. Centrifuge at 5,000× g for several seconds and incubate for 10 min.
5. Add the whole mixture to the cells and culture in a CO2 incubator at 37 °C in a humidified atmosphere with 5% CO2.
6. After 4 days of transfection, collect 100 μL of culture supernatant in a flat bottom microtube and store at -80 °C.
E. Detection of viruses by immunofluorescence staining
1. Inoculate newly seeded MDBK cells at 80% confluence in a collagen-coated 24-well microplate with 100 μL of culture supernatant.
2. After 5 days of infection, wash with D-PBS and fix cells with acetone for 10 min at 4 °C. After washing with D-PBS three times, the plate can be stored at 4 °C.
3. Incubate with the indicated anti-pestiviral NS3 antibody (1:1,000 dilution) in D-PBS for 1 h at 37 °C in a humidified atmosphere.
4. After washing with D-PBS three times, incubate cells in the dark with goat anti-mouse IgG Alexa Fluor 488-conjugated (1:1,000 dilution) secondary antibody in D-PBS for 1 h at room temperature.
5. After washing with D-PBS four times, observe under the fluorescence microscope using the BX-Z filter GFP (Figure 1).
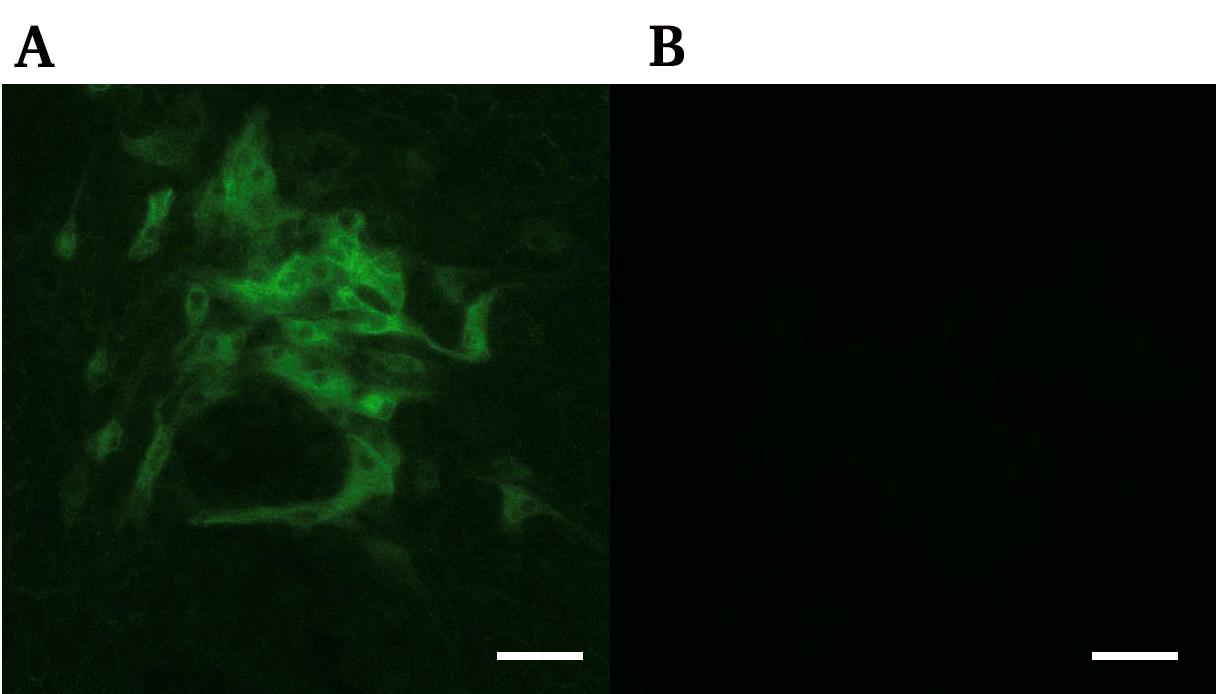
Figure 1. Image of immunofluorescence staining showing bovine viral diarrhea virus (BVDV)-positive cells. Antiviral NS3 immunofluorescence image of infected MDBK cells (A) and non-infectious condition (B). Scale bar, 100 μm.
Data analysis
Significant differences in viral titers between different ratios of co-cultures (Figure 2) were analyzed by student’s t-test using GraphPad Prism software.
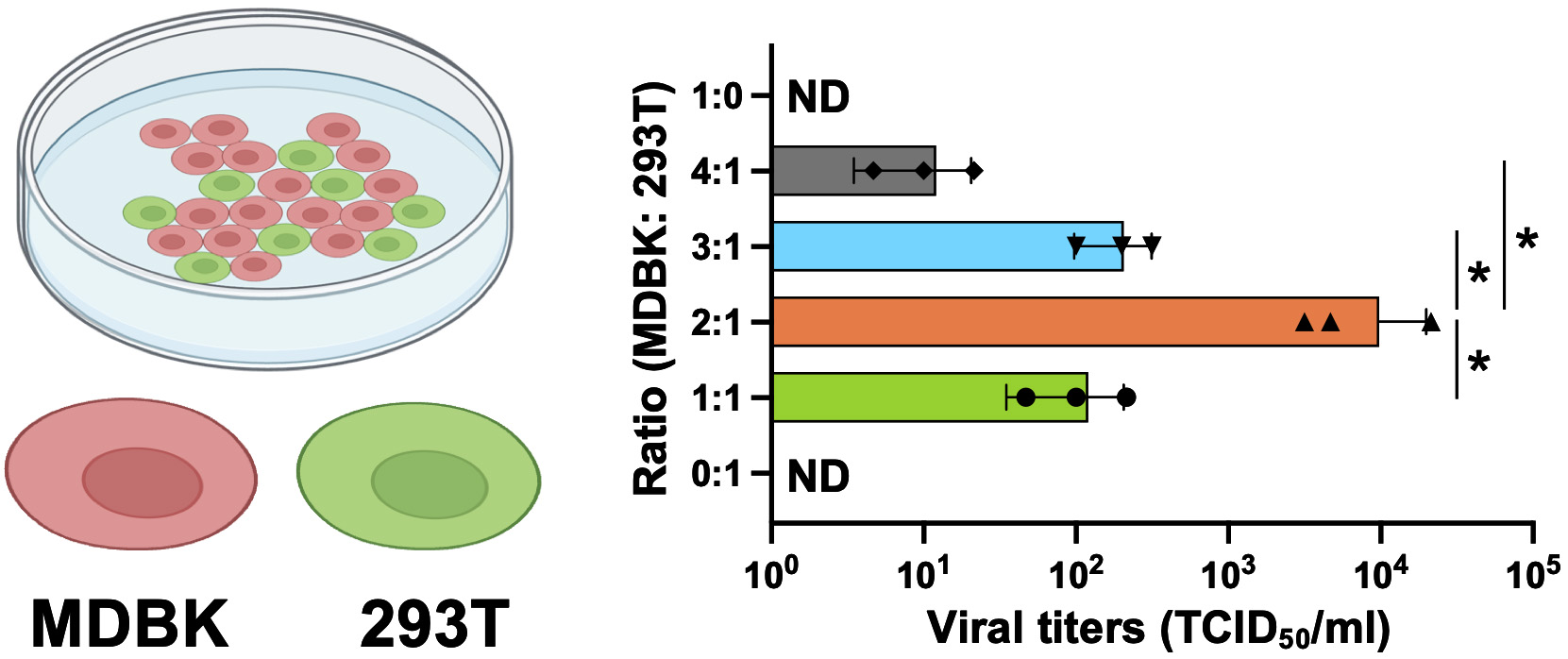
Figure 2. Optimization of the co-culture of two cell lines for the production of recombinant bovine viral diarrhea virus (BVDV). The two cell lines employed in this study were bovine MDBK, which is highly permissive for BVDV infection, and 293T, which shows high transfection efficiency and protein expression. The infectious titers of the prepared working virus were measured by 50% tissue culture infectious dose (TCID50) for BVDV. The value of TCID50/mL was calculated using the Reed–Muench method [20]. The ratios of cell numbers and infectious titers are shown as a bar graph. Asterisks indicate significant differences (*p < 0.05) between a pair.
Validation of protocol
This protocol, or parts of it, have been applied and validated in the following research article:
• Tamura et al. [13]. A rapid and versatile reverse genetics approach for generating recombinant positive-strand RNA viruses that use IRES-mediated translation (see Figure 2B, H, I).
General notes and troubleshooting
General notes
1. Viral RNA purified from infected cells or culture supernatants can also be used.
2. Because MDBK cells, which are difficult to transfect, are the primary choice for BVDV research, we employed a co-culture strategy with 293T cells to deliver sufficient viral cDNA into MDBK cells. The 293T monocultures were unable to produce BVDV, but recombinant BVDV was produced in transfected co-cultures with a ratio of 2:1 (MDBK:293T), which was the ratio demonstrating the greatest efficacy (Figure 2).
3. The protocol can also generate HCV, hepatitis A virus (HAV), and encephalomyocarditis virus (EMCV) using primer sets and cells appropriate for each virus.
Troubleshooting
Problem 1: Transfection is unsuccessful.
Possible cause: Neighboring fragments are not joined.
Solution: Change the overlapping region or length between neighboring fragments, use PCR assembly to connect neighboring fragments, and assess the size of joined fragments by gel electrophoresis.
Problem 2: PCR for amplifying a total of six viral gene fragments is unsuccessful.
Possible cause: The cDNA amount of the viral genome is insufficient for PCR.
Solution: Purify viral RNA from high-viral serum, infected cells, or culture supernatants.
Acknowledgments
We thank H. Kubo, M. Tetsuka, and S. Shimamura for their secretarial work and H. Maruyama, M. Hanazaki, T. Matsuoka, R. Kudo, M. Hommura, A. Imajoh, and K. Oyama for their technical assistance. We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
This work was supported in part by AMED Research Program on Emerging and Re-emerging Infectious Diseases (JP22fk0108617 to Takasuke Fukuhara); AMED SCARDA Hokkaido University Institute for Vaccine Research and Development (HU-IVReD) (JP223fa627005 to Takasuke Fukuhara); AMED CREST (JP22gm1610008 to Takasuke Fukuhara); JSPS KAKENHI Grant-in-Aid for Scientific Research B (21H02736 to Takasuke Fukuhara); JSPS KAKENHI Fund for the Promotion of Joint International Research (International Leading Research) (JP23K20041 to Takasuke Fukuhara); JST SPRING (JPMJSP2119 to Hirotaka Yamamoto); Takeda Science Foundation (to Takasuke Fukuhara); Hokkaido University Support Program for Frontier Research (to Takasuke Fukuhara); Hokuto Foundation for Bioscience (to Tomokazu Tamura); Grants-in-Aid for R&D of Young Scientist from Northern Advancement Center for Science & Technology (NOASTEC) of Hokkaido (T-1-26 to Tomokazu Tamura); and Kuribayashi Scholarship Academic Foundation (to Tomokazu Tamura).
This protocol was developed and used in [13].
Competing interests
The authors declare no competing interests.
References
- Venter, J. C., Glass, J. I., Hutchison, C. A. and Vashee, S. (2022). Synthetic chromosomes, genomes, viruses, and cells. Cell. 185(15): 2708–2724. https://doi.org/10.1016/j.cell.2022.06.046
- Almazán, F., DeDiego, M. L., Galán, C., Escors, D., Álvarez, E., Ortego, J., Sola, I., Zuñiga, S., Alonso, S., Moreno, J. L., et al. (2006). Construction of a Severe Acute Respiratory Syndrome Coronavirus Infectious cDNA Clone and a Replicon To Study Coronavirus RNA Synthesis. J Virol. 80(21): 10900–10906. https://doi.org/10.1128/jvi.00385-06
- Yount, B., Curtis, K. M., Fritz, E. A., Hensley, L. E., Jahrling, P. B., Prentice, E., Denison, M. R., Geisbert, T. W. and Baric, R. S. (2003). Reverse genetics with a full-length infectious cDNA of severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 100(22): 12995–13000. https://doi.org/10.1073/pnas.1735582100
- Scobey, T., Yount, B. L., Sims, A. C., Donaldson, E. F., Agnihothram, S. S., Menachery, V. D., Graham, R. L., Swanstrom, J., Bove, P. F., Kim, J. D., et al. (2013). Reverse genetics with a full-length infectious cDNA of the Middle East respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 110(40): 16157–16162. https://doi.org/10.1073/pnas.1311542110
- Terada, Y., Kuroda, Y., Morikawa, S., Matsuura, Y., Maeda, K. and Kamitani, W. (2019). Establishment of a Virulent Full-Length cDNA Clone for Type I Feline Coronavirus Strain C3663. J Virol. 93(21): e01208–19. https://doi.org/10.1128/jvi.01208-19
- Edmonds, J., van Grinsven, E., Prow, N., Bosco-Lauth, A., Brault, A. C., Bowen, R. A., Hall, R. A. and Khromykh, A. A. (2013). A Novel Bacterium-Free Method for Generation of Flavivirus Infectious DNA by Circular Polymerase Extension Reaction Allows Accurate Recapitulation of Viral Heterogeneity. J Virol. 87(4): 2367–2372. https://doi.org/10.1128/jvi.03162-12
- Tamura, T., Fukuhara, T., Uchida, T., Ono, C., Mori, H., Sato, A., Fauzyah, Y., Okamoto, T., Kurosu, T., Setoh, Y. X., et al. (2018). Characterization of Recombinant Flaviviridae Viruses Possessing a Small Reporter Tag. J Virol. 92(2): e01582–17. https://doi.org/10.1128/jvi.01582-17
- Motozono, C., Toyoda, M., Zahradnik, J., Saito, A., Nasser, H., Tan, T. S., Ngare, I., Kimura, I., Uriu, K., Kosugi, Y., et al. (2021). SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 29(7): 1124–1136.e11. https://doi.org/10.1016/j.chom.2021.06.006
- Saito, A., Irie, T., Suzuki, R., Maemura, T., Nasser, H., Uriu, K., Kosugi, Y., Shirakawa, K., Sadamasu, K., Kimura, I., et al. (2022). Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature. 602(7896): 300–306. https://doi.org/10.1038/s41586-021-04266-9
- Tamura, T., Ito, J., Uriu, K., Zahradnik, J., Kida, I., Anraku, Y., Nasser, H., Shofa, M., Oda, Y., Lytras, S., et al. (2023). Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat Commun. 14(1): 2800. https://doi.org/10.1038/s41467-023-38435-3
- Tamura, T., Irie, T., Deguchi, S., Yajima, H., Tsuda, M., Nasser, H., Mizuma, K., Plianchaisuk, A., Suzuki, S., Uriu, K., et al. (2024). Virological characteristics of the SARS-CoV-2 Omicron XBB.1.5 variant. Nat Commun. 15(1): 1176. https://doi.org/10.1038/s41467-024-45274-3
- Tamura, T., Mizuma, K., Nasser, H., Deguchi, S., Padilla-Blanco, M., Oda, Y., Uriu, K., Tolentino, J. E., Tsujino, S., Suzuki, R., et al. (2024). Virological characteristics of the SARS-CoV-2 BA.2.86 variant. Cell Host Microbe. 32(2): 170–180.e12. https://doi.org/10.1016/j.chom.2024.01.001
- Tamura, T., Yamamoto, H., Ogino, S., Morioka, Y., Tsujino, S., Suzuki, R., Hiono, T., Suzuki, S., Isoda, N., Sakoda, Y., et al. (2024). A rapid and versatile reverse genetics approach for generating recombinant positive-strand RNA viruses that use IRES-mediated translation. J Virol. 98(3): e01638–23. https://doi.org/10.1128/jvi.01638-23
- Ruggli, N. and Rice, C. M. (1999). Functional cDNA Clones of The Flaviviridae: Strategies and Applications. Adv Virus Res. 53: 183–207. https://doi.org/10.1016/s0065-3527(08)60348-6
- Nagai, M., Sakoda, Y., Mori, M., Hayashi, M., Kida, H. and Akashi, H. (2003). Insertion of cellular sequence and RNA recombination in the structural protein coding region of cytopathogenic bovine viral diarrhoea virus. J Gen Virol. 84(2): 447–452. https://doi.org/10.1099/vir.0.18773-0
- Osorio, J. S. and Bionaz, M. (2017). Plasmid transfection in bovine cells: Optimization using a realtime monitoring of green fluorescent protein and effect on gene reporter assay. Gene. 626: 200–208. https://doi.org/10.1016/j.gene.2017.05.025
- Hirose, S., Notsu, K., Ito, S., Sakoda, Y. and Isoda, N. (2021). Transmission Dynamics of Bovine Viral Diarrhea Virus in Hokkaido, Japan by Phylogenetic and Epidemiological Network Approaches. Pathogens. 10(8): 922. https://doi.org/10.3390/pathogens10080922
- Kameyama, K., Sakoda, Y., Tamai, K., Igarashi, H., Tajima, M., Mochizuki, T., Namba, Y. and Kida, H. (2006). Development of an immunochromatographic test kit for rapid detection of bovine viral diarrhea virus antigen. J Virol Methods. 138: 140–146. https://doi.org/10.1016/j.jviromet.2006.08.005
- Masaki, T., Suzuki, R., Saeed, M., Mori, K. I., Matsuda, M., Aizaki, H., Ishii, K., Maki, N., Miyamura, T., Matsuura, Y., et al. (2010). Production of Infectious Hepatitis C Virus by Using RNA Polymerase I-Mediated Transcription. J Virol. 84(11): 5824–5835. https://doi.org/10.1128/jvi.02397-09
- Reed, L. and Muench, H. (1938). A Simple Method Of Estimating Fifty Per Cent Endpoints12. Am J Epidemiol. 27(3): 93–497. https://doi.org/10.1093/oxfordjournals.aje.a118408
Article Information
Publication history
Received: Jan 8, 2025
Accepted: Mar 11, 2025
Available online: Mar 26, 2025
Published: Apr 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
How to cite
Yamamoto, H., Tamura, T. and Fukuhara, T. (2025). Rapid Plasmid-Free Generation of Recombinant Positive-Strand RNA Viruses That Use IRES-Mediated Translation Using an Expansion of the Circular Polymerase Extension Reaction (CPER). Bio-protocol 15(8): e5275. DOI: 10.21769/BioProtoc.5275.
Category
Microbiology > Microbial genetics > DNA > PCR
Molecular Biology > DNA > PCR
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link