- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Monitoring Protein Stability In Vivo Using an Intein-Based Biosensor
(*contributed equally to this work) Published: Vol 15, Iss 8, Apr 20, 2025 DOI: 10.21769/BioProtoc.5271 Views: 1571
Reviewed by: Philipp A.M. SchmidpeterSneha RayAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Assay for Protealysin-like Protease Inhibitor Activity
Igor M. Berdyshev [...] Ilya V. Demidyuk
Oct 5, 2022 2160 Views
H2 Production from Methyl Viologen–Dependent Hydrogenase Activity Monitored by Gas Chromatography
Nuttavut Kosem
Dec 5, 2023 1754 Views
Endo-1,4-β-D-xylanase Assay Using Azo-Xylan and Variants Thereof
Luca Bombardi [...] Salvatore Fusco
Apr 20, 2025 1914 Views
Abstract
Inteins are elements translated within host proteins and removed via a unique protein splicing reaction. In this process, the two peptide bonds flanking the intein are rearranged, releasing the intein and leaving a standard peptide bond in its place. Due to their ability to shuffle peptide bonds in a specific and controlled manner, inteins have proven valuable in protein engineering, leading to the development of numerous impactful technologies. In one application, intein-based biosensors link the activity of a host protein to intein excision. Recently, we developed a biosensor to measure protein stability in vivo, in which the removal of an intein-protein fusion is required for antibiotic resistance. In our protocol, cells expressing our biosensor are logarithmically diluted and spotted on agar plates containing increasing levels of antibiotics. Following incubation, quantitative survival curves can be generated. We also developed a dual protein stability sensor where both antibiotic resistance and fluorescence can be used as readouts and demonstrated that co-expression of the chaperonin GroEL can promote survival and fluorescence. Taken together, our novel intein-based biosensor adds to the available tools to measure protein stability within the cellular environment.
Key features
• Biosensor demonstrates a 100,000-fold difference in survival between stable and unstable test proteins.
• Dual biosensor quantitatively links protein stability to antibiotic resistance and fluorescence simultaneously.
Keywords: InteinBackground
Inteins (intervening proteins) are polypeptide segments translated within host proteins that are removed through a protein splicing reaction [1,2]. In the intein-mediated splicing reaction, the intein is released by the formation of a peptide bond between the flanking sequences, termed N- and C-exteins, which form the mature host protein. This reaction can proceed without any external factors, though the rate at which splicing occurs is highly dependent on the conditions present. The unique ability of inteins to rearrange peptide bonds has led to the invention of numerous applications in protein engineering [2,3]. Abundant and widespread within the microbial world [4], prior work has demonstrated that some inteins have adapted to serve as environmental sensors, displaying their capacity to regulate protein function in response to conditions such as metals, heat, salt, oxidative stress, and DNA damage [2,5,6].
To monitor intein activity in response to different conditions, protein splicing reporters are often used. These reporters are flexible in design, as their only requirement is the presence of a cysteine, serine, or threonine as the first residue following the intein [1]. Previously, we developed a kanamycin intein splicing reporter (KISR), with it providing an easy method to detect selective small molecule inhibitors of protein splicing within the cellular environment [7–10]. As inteins are absent in humans but present within human pathogens including Mycobacterium tuberculosis and Cryptococcus neoformans, splicing inhibitors may serve as novel antimicrobials, making them increasingly important targets to study [11,12]. Kanamycin A is a commonly used aminoglycoside antibiotic that inhibits translation. Bacteria with a mature aminoglycoside phosphotransferase (KanR), however, can survive in the presence of the antibiotic. The KISR reporter makes use of KanR as the exteins flanking an intein, making it so intein splicing activity confers antibiotic resistance to the experimental cells transformed with the reporter [7–10]. In the presence of small molecule inhibitors, the KISR will be unable to splice, leaving the cells vulnerable to the antibiotic. Therefore, this reporter allows for easy observation of what molecules can act as inhibitors and to what degree they inhibit, as stronger inhibitors will lead to reduced growth.
We recently developed an intein-based biosensor that adapts the KISR framework into a tripartite folding biosensor, where a protein of interest (POI) is inserted within the intein between conserved splicing blocks (Figure 1). Tripartite protein folding biosensors serve to measure the stability of a POI by linking it to reporter activity within the cellular environment [13]. Our intein-based tripartite biosensor design, while serving a similar purpose, distinguishes itself from previous biosensors through the insertion of the POI into an intein, which is then flanked with reporter extein sequences [14]. Through splicing activity, the POI is removed as the extein sequences are joined into a functional reporter molecule. This allows the biosensor to avoid the challenges associated with previous designs that kept a POI fused to the reporter, such as limitations on the size of POI insertion or restrictions on reporter function. Initial work with our biosensor was carried out using the E. coli maltose binding protein (MBP) as a POI, with it being inserted into the homing endonuclease (HEN) loop of the Pyrococcus horikoshii RadA intein (RadA-I) [14]. The HEN loop of RadA-I has been noted to accommodate POI insertion, as previous work has shown that the intein remains splicing active upon insertion of ZsGreen into the loop [15,16]. The RadA-I intein was also selected due to its rapid and accurate splicing across a variety of extein contexts [17–19], which indicated that it could accommodate reporter exteins. The RadA-I-MBP fusion was inserted within KanR (KanR-RadA-I-MBP), with kanamycin resistance serving as the reporter in a manner similar to KISR. This biosensor was then tested in a variety of conditions, where it was observed that a decrease in MBP stability (due to mutations) or inhibition of splicing activity resulted in reduced survival of cells, as they were not provided kanamycin resistance [14]. This supported the viability of our intein-based biosensor approach, as the stability of the POI was directly linked to reporter activity. Further work, which made use of the fluorescent protein TagRFP675 as the POI, showed the potential of our biosensor design as a dual-folding sensor, with both antibiotic resistance and fluorescence linked to POI stability [14]. Potential applications of our intein-based biosensor are broad due to the relaxed requirements of intein splicing, making this approach viable for linking protein stability to any desired in vivo readout [14].
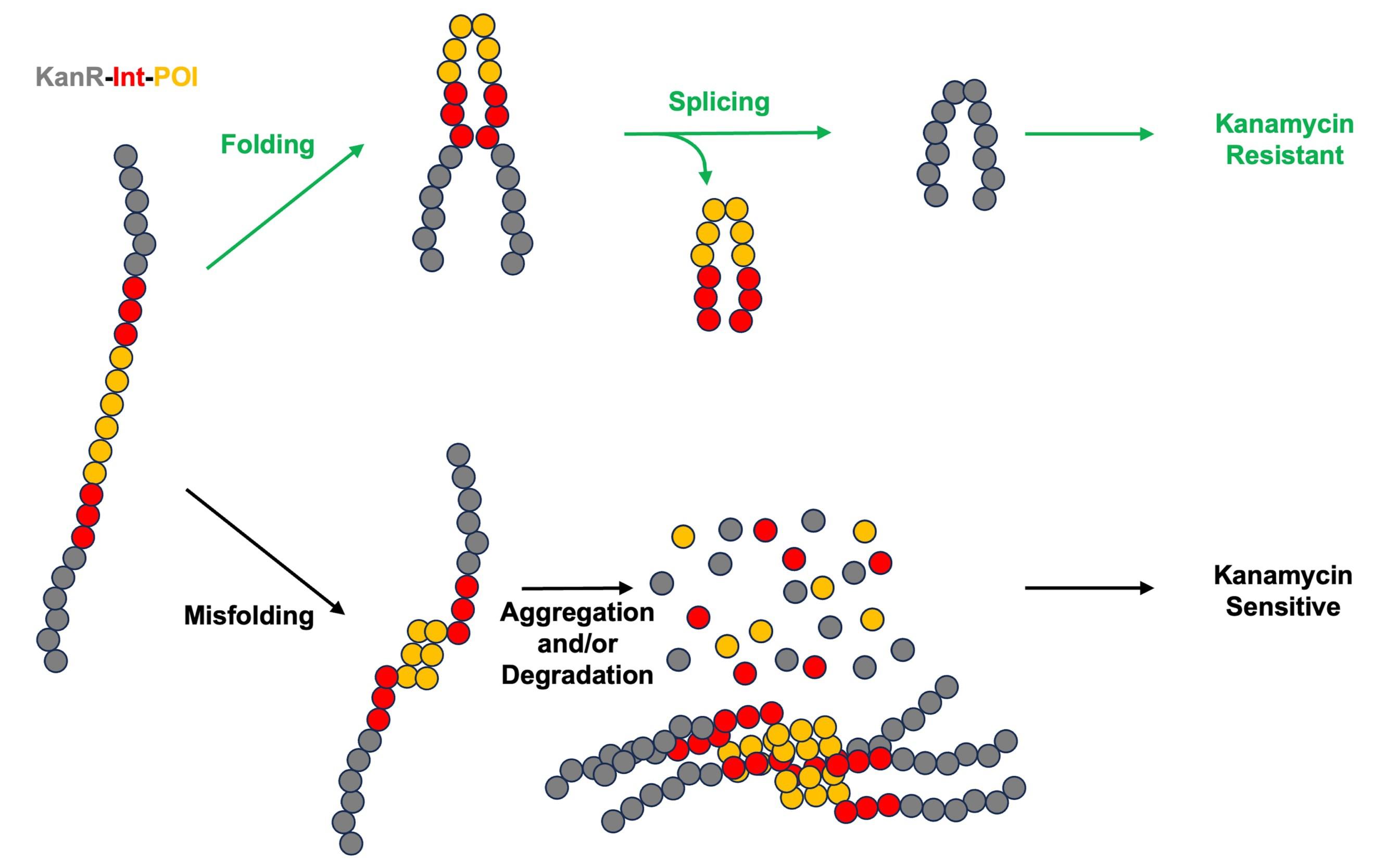
Figure 1. Intein-based protein stability sensor. KanR is colored in gray, intein is colored in red, and the protein of interest (POI) is colored in orange.
Materials and reagents
Biological materials
1. NEB5α E. coli (New England Biolabs, C2987H) (Table 1)
2. MC4100(DE3) [20]
Table 1. E. coli strains used in this study.
| Strain number | Plasmid 1 | Plasmid 2 | Strain | ||
|---|---|---|---|---|---|
| CWL599 | KanR-IntWT-MBPMUT | ApR | NEB5a | ||
| CWL600 | KanR-IntWT-MBPWT | ApR | NEB5a | ||
| CWL640 | KanR-IntMUT-MBPWT | ApR | NEB5a | ||
| AS917 | KanR-IntWT-MBPMUT | ApR | pBAD33mut-Empty | CmR | MC4100(DE3) |
| AS923 | KanR-IntWT-MBPMUT | ApR | pBAD33-GroEL | CmR | MC4100(DE3) |
| AS925 | KanR-IntWT-MBPWT | ApR | pBAD33mut-Empty | CmR | MC4100(DE3) |
| AS931 | KanR-IntWT-MBPWT | ApR | pBAD33-GroEL | CmR | MC4100(DE3) |
| AS933 | KanR-IntWT-TagRFP675 | ApR | pBAD33mut-Empty | CmR | MC4100(DE3) |
| AS939 | KanR-IntWT-TagRFP675 | ApR | pBAD33-GroEL | CmR | MC4100(DE3) |
Reagents
1. Difco LB broth, Miller (BD, catalog number: 244610)
2. Difco LB agar, Miller (BD, catalog number: 244510)
3. Ampicillin sodium salt (Fisher, catalog number: BP906)
4. Kanamycin sulfate (Fisher, catalog number: BP1760)
5. Chloramphenicol (Fisher, catalog number: BP904100)
6. Ethanol (Sigma-Aldrich, catalog number: E7023)
7. L-Arabinose (Chem-IMPEX, catalog number: 29750)
8. Phosphate-buffered saline (PBS) (10×) pH 7.4, RNase-free (Fisher, catalog number: AM9625)
Solutions
1. LB medium (see Recipes)
2. LB agar (see Recipes)
3. 100 mg/mL ampicillin stock solution (1,000×) (see Recipes)
4. 50 mg/mL kanamycin stock solution (1,000×) (see Recipes)
5. 35 mg/mL chloramphenicol stock solution (1,000×) (see Recipes)
6. 20% L-arabinose stock solution (100×) (see Recipes)
Recipes
1. LB medium
25 g of LB broth
Bring to 1 L with ddH2O
Autoclave to sterilize
2. LB agar
40 g of LB agar
Bring to 1 L with ddH2O
Autoclave to sterilize
3. 100 mg/mL ampicillin stock solution (1,000×)
1 g of ampicillin sodium salt
Bring to 10 mL with ultrapure H2O
Place in 10 mL syringe and pass through 0.22 mm filter to sterilize
Aliquot in 1.5 mL tubes and store at -20 °C
4. 50 mg/mL kanamycin stock solution (1,000×)
500 mg of kanamycin sulfate
Bring to 10 mL with ultrapure H2O
Place in 10 mL syringe and pass through 0.22 mm filter to sterilize
Aliquot in 1.5 mL tubes and store at -20 °C
5. 35 mg/mL chloramphenicol stock solution (1,000×)
350 mg of chloramphenicol
Bring to 10 mL with ethanol
Place in 10 mL syringe and pass through 0.22 mm filter to sterilize
Aliquot in 1.5 mL tubes and store at -20 °C
6. 20% L-arabinose stock solution (100×)
2 g of L-arabinose
Bring to 10 mL with ultrapure H2O
Place in 10 mL syringe and pass through 0.22 mm filter to sterilize
Aliquot in 1.5 mL tubes and store at -20 °C
Laboratory supplies
1. 1, 10, 20, 200, and 1,000 μL pipette tips (USA Scientific, Inc. TipOne, catalog numbers: 1111-3800, 1120-1810, 1111-0706, and 1111-2820)
2. 15 mL conical tubes (Greiner Bio-One, catalog number: 188 271)
3. Tissue culture plate 96 wells (Fisher Scientific, catalog number: FB012931)
4. Nonbinding 96-well plates (Greiner Bio-One, catalog number: 655904)
5. Black 96-well plates, black (Corning, catalog number: NBS3991)
6. Petri dishes (Fisher Scientific, catalog number: FB0875712)
7. 1.5 mL cuvette (Fisher Scientific, catalog number: 14955127)
8. 1.5 mL microcentrifuge tubes (USA Scientific, catalog number: 1615-5500)
9. 10 mL syringe (BD, catalog number: 302995)
10. 0.22 mm syringe filters (Fisher Scientific, catalog number: 09-719A)
11. Roll & GrowTM spherical glass plating beads (MP Biomedicals, catalog number: 115000550)
12. Cell scraper (CELLTREAT, catalog number: 229310)
Equipment
1. Benchtop refrigerated shaking incubator (Thermo Scientific, catalog number: SHKE4000)
2. Spectrophotometer (Eppendorf, catalog number: 6131 02779)
3. Amersham Imager 600 (GE, catalog number: 29-0834-61)
4. Microplate reader (Tecan, model: Infinite M200 Pro)
5. P20 Pipetman (Gilson, catalog number: FA10003M)
6. P200 Pipetman (Gilson, catalog number: F144058M)
7. P1000 Pipetman (Gilson, catalog number: FA10006M)
8. Multichannel pipette (Thermo Scientific, catalog number: 46300100)
9. Incubator (Fisher Scientific, model: 11-690-637D)
10. Infrared thermometer (Etekcity, model: 817915020807)
11. Bunsen burner
12. 1 cm grid paper
Procedure
Procedure 1: Single expression of an intein-based biosensor
A. Prepare overnight cultures
1. In sterile conditions, prepare an overnight culture for each of the three NEB5α strains (CWL599, CWL600, and CWL640) by inoculating a culture tube that contains 5 mL of LB media and 5 μL of 1,000× ampicillin stock solution.
2. Incubate at 37 °C with shaking at 250 rpm for approximately 20 h.
B. Prepare agar plates
1. Prepare LB agar.
2. Warm LB agar until liquid, then allow it to cool to approximately 65 °C.
3. Control plates: add 1 μL of 1,000× ampicillin stock solution per 1 mL of LB agar.
4. Experimental plates: add 1 μL of 1,000× ampicillin and 1 μL of 1,000× kanamycin stock solution per 1 mL of LB agar.
5. Pour the agar into Petri dishes and allow it to solidify overnight at room temperature.
C. Cell titer standardization
1. Turn on the spectrophotometer to allow it to warm up (Figure 2).
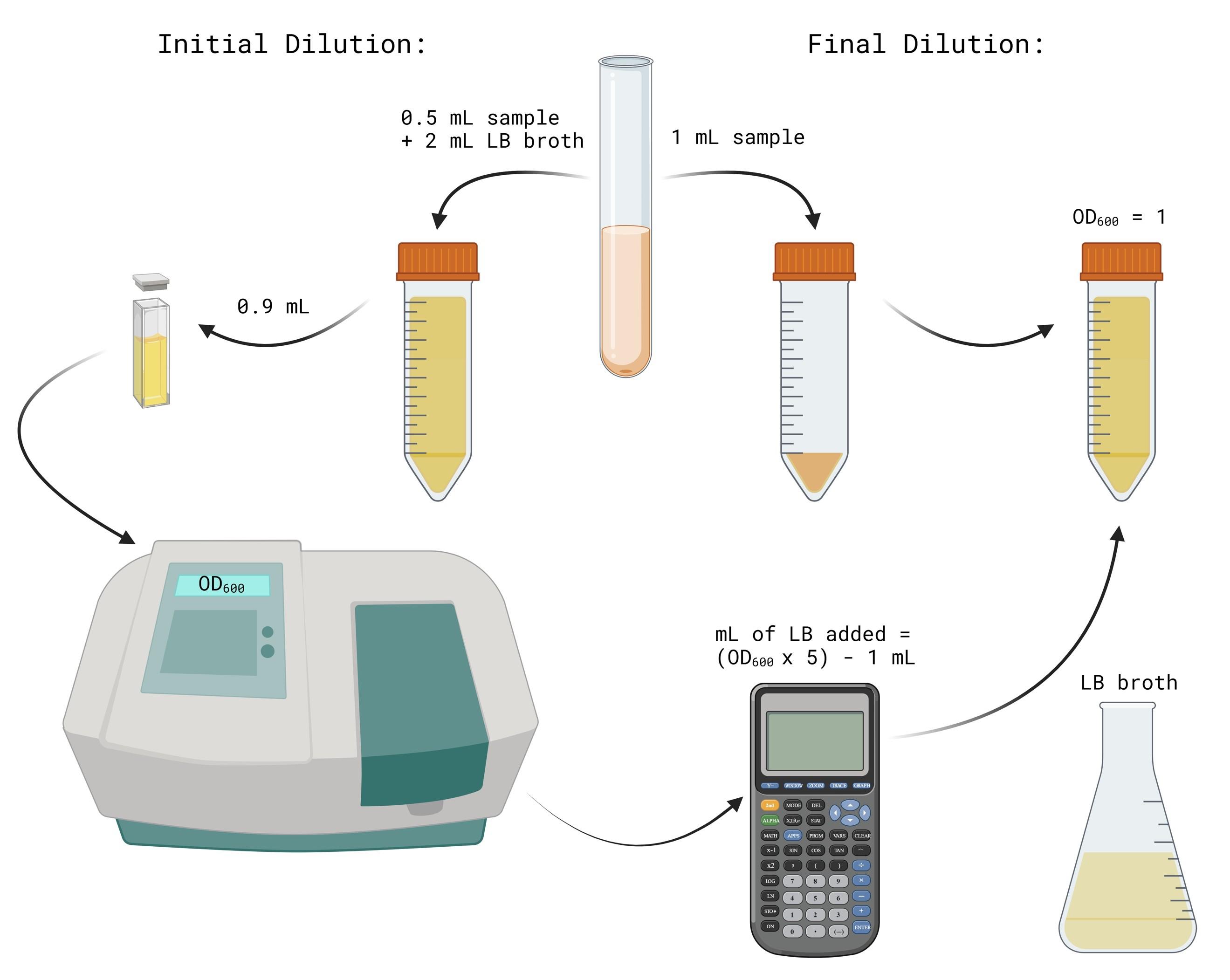
Figure 2. Procedure for normalizing overnight cultures to OD600 = 1. Image generated using BioRender.
2. Label two 15 mL conical tubes for each 5 mL culture as initial dilution and final dilution for each sample.
3. Fill the initial dilution tubes with 2 mL of LB broth.
4. Transfer 0.5 mL of the CWL 599 culture into its respective initial dilution tube. Pipette up and down three times to mix.
5. Repeat step C4 for the remaining samples.
6. Take 1 mL of each initial dilution mixture and transfer into its respective final dilution tube.
7. Transfer 0.9 mL of each initial dilution mixture into 2 mL cuvettes. Measure and record their OD600 using the spectrophotometer.
8. If the OD600 value is above 1, add LB to the final dilution tube to bring the solution to an OD600 of 1. The equation to solve for X mL of LB added is: X = ((OD600 Value) × 5) – 1 mL.
D. Serial dilution
1. Turn on the Bunsen burner and work near its base to prevent contamination while the nonbinding 96-well plate lid is open (Figure 3).
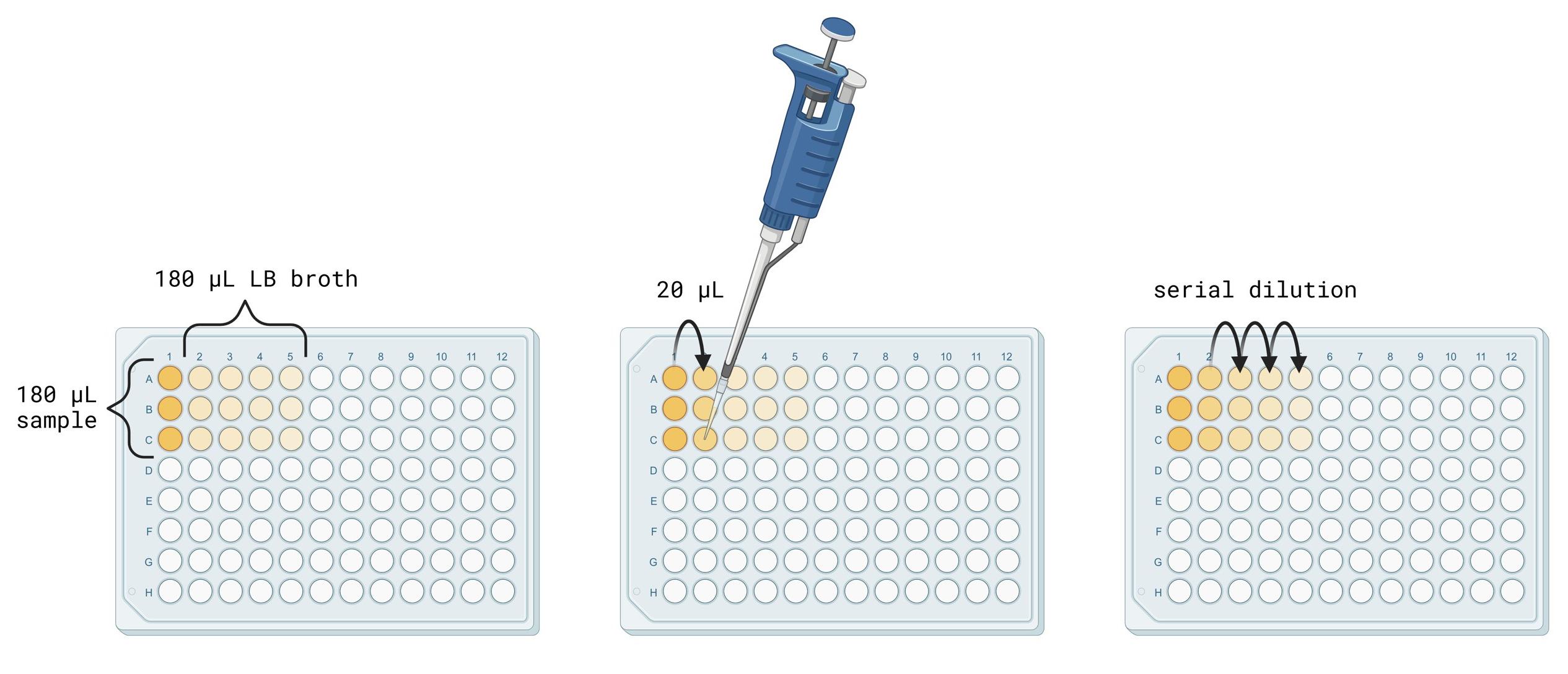
Figure 3. Procedure for making serial dilutions. Image generated using BioRender.
2. Assign row A to CWL 599, row B to CWL 600, and row C to CWL 640 of the nonbinding 96-well plate.
3. Using a P200 Pipetman, pipette 180 μL of LB broth into wells 2–5 in rows A–C.
4. Pipette 180 μL of each cell culture at an OD600 value of 1 into its respective row in the empty wells in column 1. Change pipette tips between samples.
5. Using a P20 Pipetman, transfer 20 μL of sample from well A1 into well A2.
6. After drawing up the 20 μL sample, pump the plunger on the Pipetman three times to mix the 20 μL sample into the 180 μL of LB. Discard the pipette tip after mixing.
7. Transfer 20 μL of sample from well A2 into well A3.
8. After drawing up the 20 μL sample, pump the plunger on the Pipetman three times to mix the 20 μL sample into the 180 μL of LB. Discard the pipette tip after mixing.
9. Transfer 20 μL of sample from well A3 into well A4.
10. After drawing up the 20 μL sample, pump the plunger on the Pipetman three times to mix the 20 μL sample into the 180 μL of LB. Discard the pipette tip after mixing.
11. Transfer 20 μL of sample from well A4 into well A5.
12. After drawing up the 20 μL sample, pump the plunger on the Pipetman three times to mix the 20 μL sample into the 180 μL of LB. Discard the pipette tip after mixing.
13. Repeat step D11 for the remaining samples.
E. Spot titers
1. Print a 1 × 1 cm grid on paper using Excel as a template (this can be reused).
2. If plates were stored at 4 °C, warm the control and experimental agar plates in a 30 °C incubator for 10–15 min before use. If the plates were prepared the previous day, they can remain at room temperature.
3. Label plates with strain, date, and the antibiotic(s) included.
4. Arrange three control plates and three experimental plates on the grid paper.
5. Set a P10 multichannel pipette to six drops of 1.25 μL.
6. Using a P10 multichannel pipette, draw up CWL 599 samples from row A. Spot them onto each agar plate by lightly touching the tip to the surface. Avoid piercing the agar. Open the plate lid only briefly while spotting to prevent contamination. Discard pipette tips after spotting each row (Figure 4).
7. Draw up CWL 600 samples from row B and spot them below the CWL 599 spots on each agar plate. Ensure you just touch the pipette tip to the surface. Discard tips after each plate.
8. Draw up CWL 640 samples from row C and spot them below the CWL 600 spots on each agar plate. Ensure you just touch the pipette tip to the surface. Discard tips after each plate.
9. Allow the sample spots to dry at room temperature. Then, invert the plates upside-down and incubate them at 37 °C overnight (16–20 h) (Figure 5).
Note: Survival may vary depending on incubation temperature.
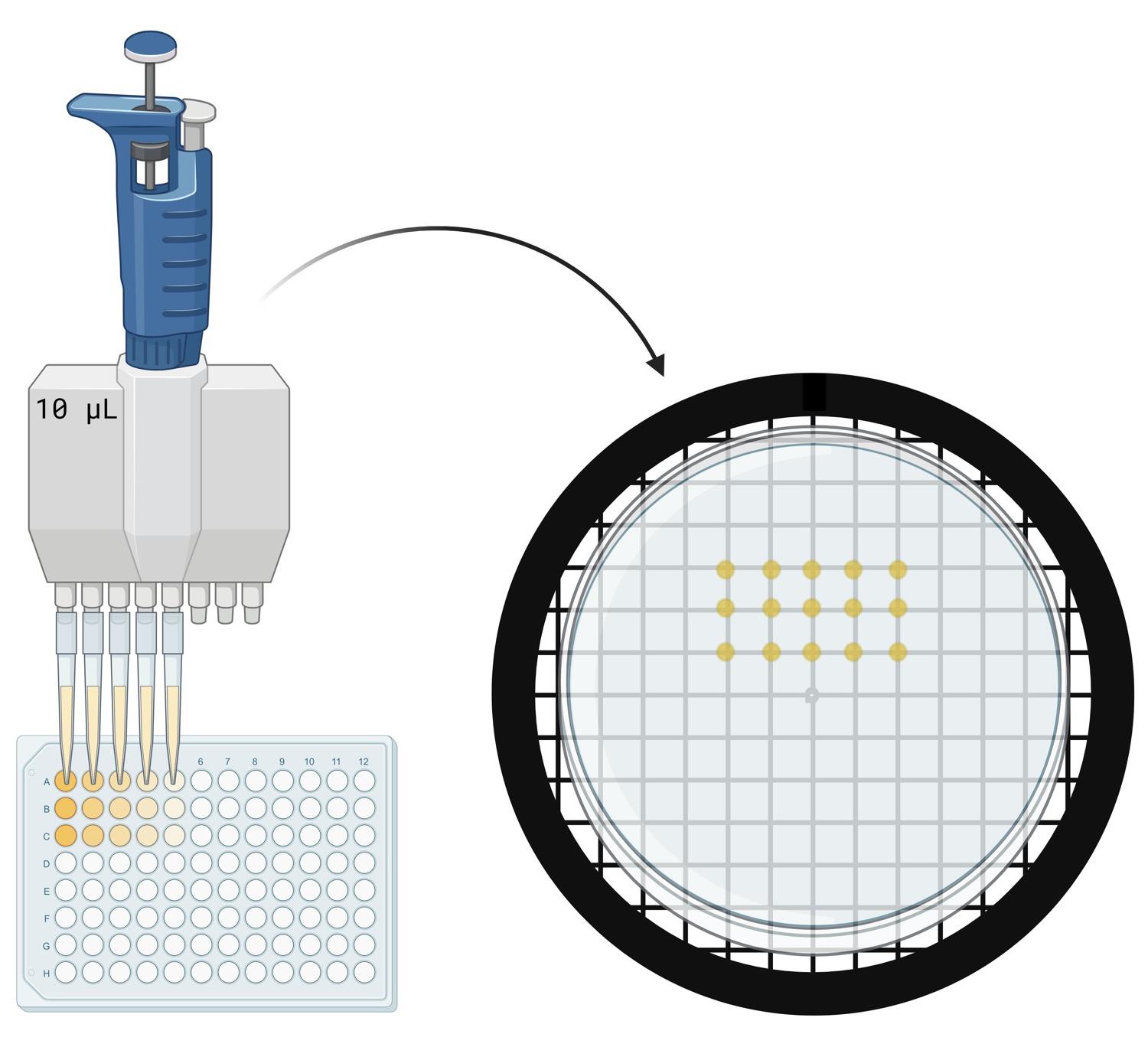
Figure 4. Procedure for spotting cells on agar plates. Image generated using BioRender.
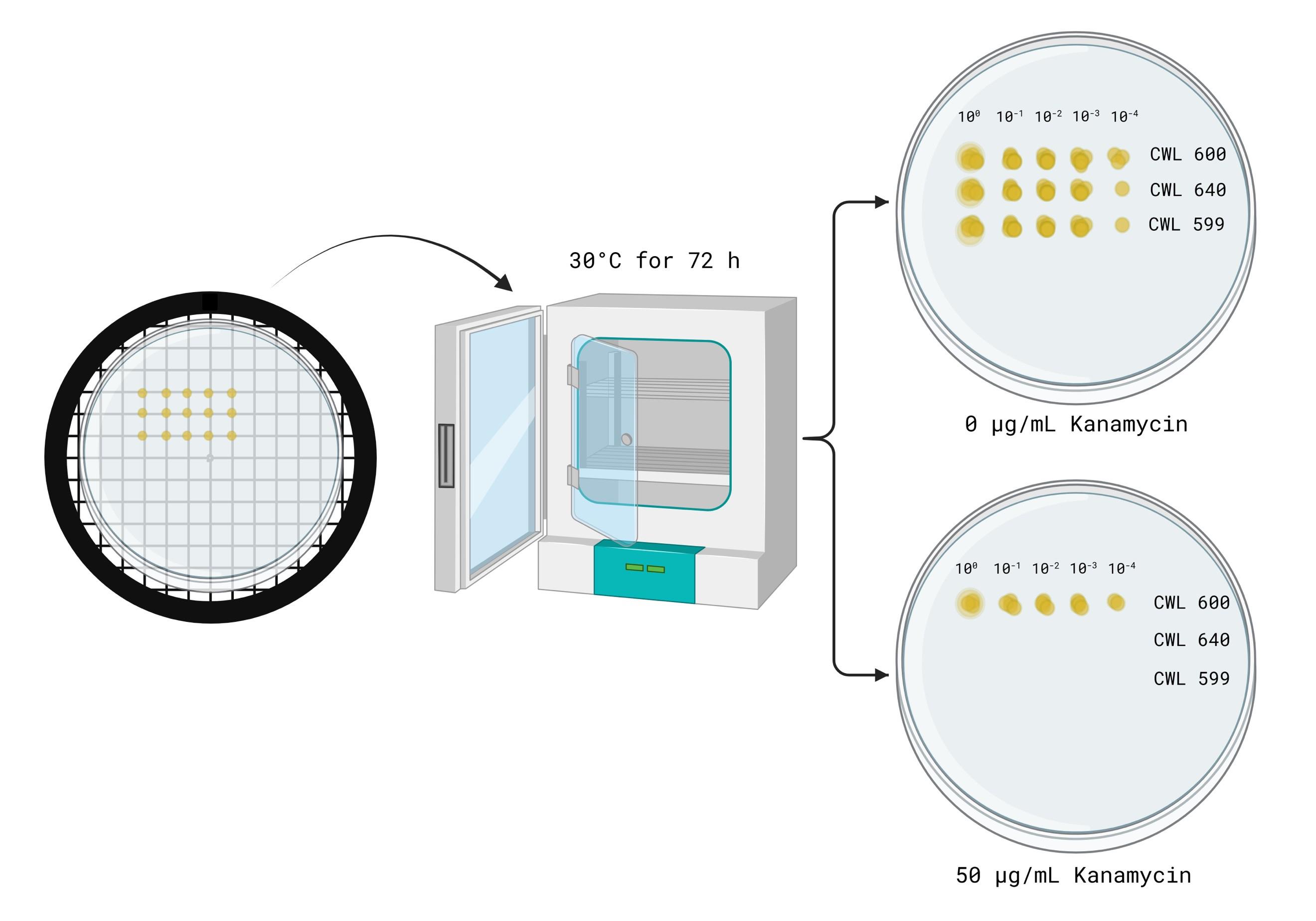
Figure 5. Spot titer plate incubation and expected results. Image generated using BioRender.
Procedure 2: Dual expression of intein- or fluorescence-based biosensor with GroEL chaperone
A. Prepare overnight cultures
1. Under sterile conditions, prepare an overnight culture for each of the six MC4100 strains (AS917, AS923, AS925, AS931, AS933, and AS939) by inoculating 3 mL of LB medium with 6 μL of 1,000× ampicillin and 3 μL of 1,000× chloramphenicol stock solutions.
2. Incubate at 37 °C shaking at 250 rpm overnight (16–20 h).
B. Prepare agar plates
1. Prepare LB agar.
2. Autoclave the LB agar for 30 min at 121 °C and allow it to cool to approximately 65 °C.
3. Control plates: Per 1 mL of LB agar, add 2 μL of 1,000× ampicillin, 1 μL of 1,000× chloramphenicol, and 10 μL of 100× L-arabinose stock solution.
4. Experimental plates: Per 1 mL of LB agar, add 2 μL of 1,000× ampicillin, 1 μL of 1,000× chloramphenicol, 10 μL of 100× L-arabinose, and various concentrations of kanamycin from the 1,000× kanamycin stock solution: 0.1 μL (5 μg/mL), 0.2 μL (10 μg/mL), and 0.4 μL (20 μg/mL).
5. Non-inducing plates for GroEL chaperone: Per 1 mL of LB agar, add 2 μL of 1,000× ampicillin, 1 μL of 1,000× chloramphenicol, and various concentrations of kanamycin from the 1,000× kanamycin stock solution: 0.1 μL (5 μg/mL), 0.2 μL (10 μg/mL), and 0.4 μL (20 μg/mL).
6. Pour agar into Petri dishes and allow it to solidify overnight at room temperature.
C. Cell titer standardization
Follow the same protocol as described in Procedure 1 (nonbinding 96-well plates can be used if necessary).
D. Serial dilution
Follow the same protocol as described in Procedure 1 (nonbinding 96-well plates can be used if necessary).
E. Spot titers
1. Print a 1×1 cm grid on paper using Excel as a template (this can be reused).
2. If plates were stored at 4 °C, warm the control and experimental agar plates in a 37 °C incubator for 10–15 min before use. If the plates were prepared the previous day, they can remain at room temperature.
3. Label each plate with the strain, date, and the antibiotic(s) included.
4. Arrange three control plates and three experimental plates on the grid paper.
5. Set a P10 multichannel pipette to 5 μL.
6. Using a P10 multichannel pipette, draw up each sample (AS917, AS923, AS925, AS931, AS933, and AS939) from rows A to F.
7. Spot 5 μL of each sample onto each agar plate by lightly touching the tip to the surface. Avoid piercing the agar. Open the lid only briefly while spotting to prevent contamination. Discard pipette tips after spotting each row.
8. Allow the sample spots to dry at room temperature. Then, invert the plates upside-down and incubate them at 37 °C overnight (16–20 h) (Figure 6). Note: Survival may vary depending on incubation temperature and kanamycin concentration.
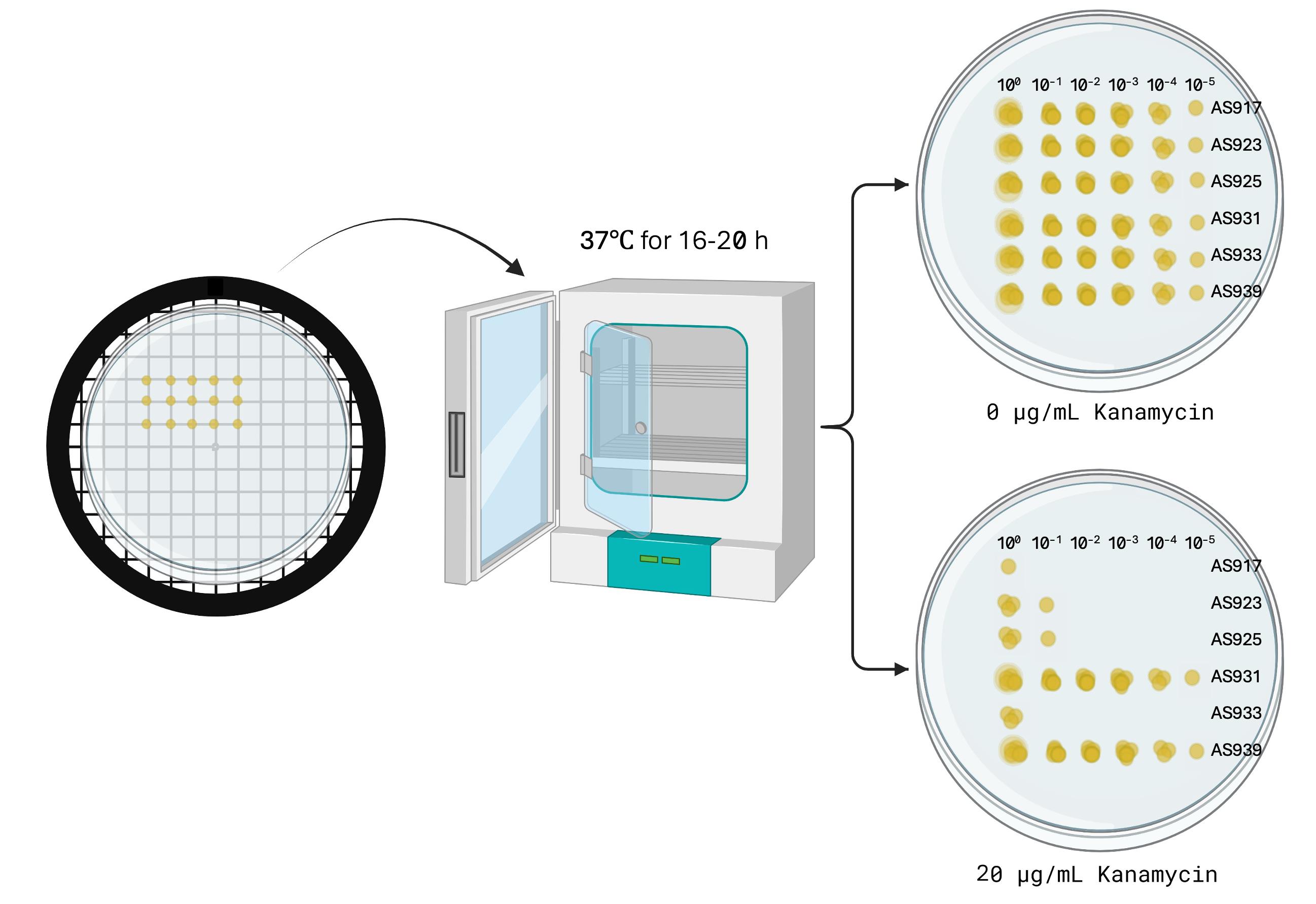
Figure 6. Spot titer plate incubation and expected results. Image generated using BioRender.
F. Fluorescence assay of TagRFP675
1. Spread 50 μL of 1.0 OD600 AS933 and AS939 (prepared in Procedure 2, Section C) onto LB agar plates (control, experimental, and non-inducing plates) using glass plating beads (Figure 7).
2. Invert the plates upside-down and incubate them at 37 °C overnight (16–20 h).
3. Turn on the spectrophotometer and microplate reader to allow them to warm up.
4. Using a P1000 Pipetman, add 1 mL of 1× PBS (pH 7.4) to each plate and scrape the entire cell layer.
5. Transfer the cell suspension to a 1.5 mL microcentrifuge tube.
6. Centrifuge cells at 10,000× g for 1 min and discard supernatant.
7. Resuspend the cell pellet in 1 mL of 1× PBS (pH 7.4) by pipetting several times to ensure even resuspension.
8. Label two 1.5 mL microcentrifuge tubes as initial dilution and final dilution for each sample.
9. Add 900 μL of 1× PBS (pH 7.4) to each initial dilution tube.
10. Using a P200 Pipetman, transfer 100 μL of each resuspended sample into its respective initial dilution tube. Pipette up and down three times to mix.
11. Repeat step F10 for the remaining samples.
12. Transfer 1 mL of each initial dilution mixture into 2 mL cuvettes. Measure and record their OD600 value using a spectrophotometer. If necessary, calculate the final OD600 by multiplying by 10 (the dilution factor).
13. Take 100 μL of each initial dilution mixture and transfer it into its respective final dilution tube.
14. If OD600 exceeds 1, adjust the sample to an OD600 of 1 by adding 1× PBS. Use the formula: X = 0.1× (OD600 ×10(dilution factor) −1), where X is the volume of 1× PBS (in mL) to add.
15. Using a P200 Pipetman, transfer 100 μL of each 1.0 OD600 sample from step F14 into a black 96-well plate. For accuracy, use triplicates (i.e., three wells per sample, filling rows A–C or D–F, if needed). Change pipette tips between samples.
16. Measure and record fluorescence using a microplate reader, setting the excitation wavelength to 598 nm and the emission wavelength to 675 nm.
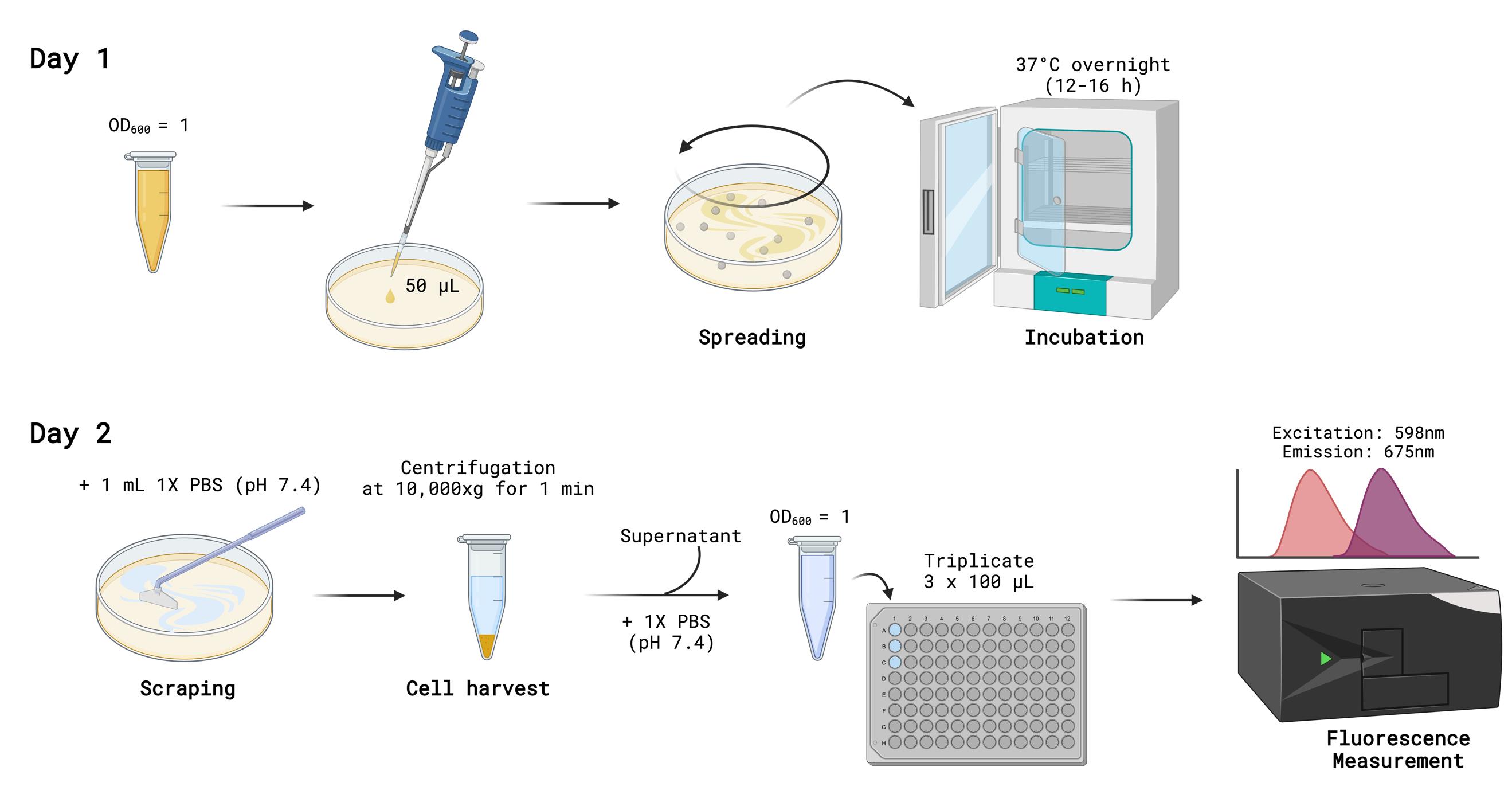
Figure 7. Fluorescence assay of TagRFP675. Image generated using BioRender.
Validation of protocol
This protocol has been used and validated in the following research article:
Son et al [14]. An intein‐based biosensor to measure protein stability in vivo. Protein Sci.
General notes and troubleshooting
1. It is critical that a second antibiotic other than kanamycin be used to maintain the intein-based folding plasmid. The backbone, pUC4K, has resistance to ampicillin, while the pBAD33 backbone has resistance to chloramphenicol, both independently of kanamycin.
2. For the intein-based folding sensor, expression of the KanR-Intein-POI fusion protein is constitutive.
3. Survival in the presence of kanamycin for cells containing the intein-based folding sensor is highly dependent on temperature, incubation time, and antibiotic concentrations. Growth also depends on whether the cells, prior to their exposure to kanamycin, were grown in liquid culture or collected from plates, as well as the growth phase of the cells.
4. The initial dilution (Figure 2) may need to be adjusted to ensure that an accurate OD600nm reading is obtained (0.1–1.0). Additionally, based on the measured OD600nm of a strain, the final dilution factor will need to be adjusted to obtain a titer of 100.
Acknowledgments
Conceptualization, J.S.S., A.S., S.H., C.W.L.; Investigation, J.S.S., A.S., S.H., C.WL. Writing—Original Draft, J.S.S., T.M.A., A.S., S.H., C.W.L.; Writing—Review & Editing, J.S.S., T.M.A., A.S., S.H., C.W.L.; Funding acquisition, S.H. and C.W.L.; Supervision, S.H. and C.W.L. This work was supported by National Institutes of Health grants R15GM143662 and P20GM103436 (via KY INBRE) to CWL and NIH R35GM142442 to SH. This protocol is based on our previous work [14].
Competing interests
The authors declare no conflicts of interest.
References
- Mills, K. V., Johnson, M. A. and Perler, F. B. (2014). Protein splicing: how inteins escape from precursor proteins. J Biol Chem. 289(21): 14498–14505. https://doi.org/10.1074/jbc.R113.540310
- Wood, D. W., Belfort, M. and Lennon, C. W. (2023). Inteins—mechanism of protein splicing, emerging regulatory roles, and applications in protein engineering. Front Microbiol. 14: e1305848. https://doi.org/10.3389/fmicb.2023.1305848
- Sarmiento, C. and Camarero, J. A. (2019). Biotechnological Applications of Protein Splicing. Curr Protein Pept Sci. 20(5): 408–424. https://doi.org/10.2174/1389203720666190208110416
- Novikova, O., Jayachandran, P., Kelley, D. S., Morton, Z., Merwin, S., Topilina, N. I. and Belfort, M. (2015). Intein Clustering Suggests Functional Importance in Different Domains of Life. Mol Biol Evol. 33(3): 783–799. https://doi.org/10.1093/molbev/msv271
- Lennon, C. W. and Belfort, M. (2017). Inteins. Curr Biol. 27(6): R204–R206. https://doi.org/10.1016/j.cub.2017.01.016
- Belfort, M. (2017). Mobile self-splicing introns and inteins as environmental sensors. Curr Opin Microbiol. 38: 51–58. https://doi.org/10.1016/j.mib.2017.04.003
- Kelley, D. S., Lennon, C. W., Li, Z., Miller, M. R., Banavali, N. K., Li, H. and Belfort, M. (2018). Mycobacterial DnaB helicase intein as oxidative stress sensor. Nat Commun. 9(1): 4363. https://doi.org/10.1038/s41467-018-06554-x
- Woods, D., Vangaveti, S., Egbanum, I., Sweeney, A. M., Li, Z., Bacot-Davis, V., LeSassier, D. S., Stanger, M., Hardison, G. E., Li, H., et al. (2020). Conditional DnaB Protein Splicing Is Reversibly Inhibited by Zinc in Mycobacteria. mBio. 11(4): e01403–20. https://doi.org/10.1128/mbio.01403-20
- Lennon, C. W., Wahl, D., Goetz, J. R. and Weinberger, J. (2021). Reactive Chlorine Species Reversibly Inhibit DnaB Protein Splicing in Mycobacteria. Microbiol Spectrum. 9(2): e00301–21. https://doi.org/10.1128/spectrum.00301-21
- Wall, D. A., Tarrant, S. P., Wang, C., Mills, K. V. and Lennon, C. W. (2021). Intein Inhibitors as Novel Antimicrobials: Protein Splicing in Human Pathogens, Screening Methods, and Off-Target Considerations. Front Mol Biosci. 8: e752824. https://doi.org/10.3389/fmolb.2021.752824
- Chan, H., Pearson, C. S., Green, C. M., Li, Z., Zhang, J., Belfort, G., Shekhtman, A., Li, H. and Belfort, M. (2016). Exploring Intein Inhibition by Platinum Compounds as an Antimicrobial Strategy. J Biol Chem. 291(43): 22661–22670. https://doi.org/10.1074/jbc.m116.747824
- Li, Z., Tharappel, A. M., Xu, J., Lang, Y., Green, C. M., Zhang, J., Lin, Q., Chaturvedi, S., Zhou, J., Belfort, M., et al. (2021). Small-molecule inhibitors for the Prp8 intein as antifungal agents. Proc Natl Acad Sci USA. 118(2): e2008815118. https://doi.org/10.1073/pnas.2008815118
- Sachsenhauser, V. and Bardwell, J. C. (2018). Directed evolution to improve protein folding in vivo. Curr Opin Struct Biol. 48: 117–123. https://doi.org/10.1016/j.sbi.2017.12.003
- Son, A., Smetana, J. S., Horowitz, S. and Lennon, C. W. (2024). An intein‐based biosensor to measure protein stability in vivo. Protein Sci. 33(3): e4925. https://doi.org/10.1002/pro.4925
- Heiden, B., Mühlberger, E., Lennon, C. W. and Hume, A. J. (2022). Labeling Ebola Virus with a Self-Splicing Fluorescent Reporter. Microorganisms. 10(11): 2110. https://doi.org/10.3390/microorganisms10112110
- Hume, A. J., Deeney, D. J., Smetana, J. S., Turcinovic, J., Connor, J. H., Belfort, M., Mühlberger, E. and Lennon, C. W. (2024). Improved protein splicing through viral passaging. mBio. 15(6): e00984–24. https://doi.org/10.1128/mbio.00984-24
- Ellilä, S., Jurvansuu, J. M. and Iwaï, H. (2011). Evaluation and comparison of protein splicing by exogenous inteins with foreign exteins in Escherichia coli. FEBS Lett. 585(21): 3471–3477. https://doi.org/10.1016/j.febslet.2011.10.005
- Oeemig, J. S., Zhou, D., Kajander, T., Wlodawer, A. and Iwaï, H. (2012). NMR and Crystal Structures of the Pyrococcus horikoshii RadA Intein Guide a Strategy for Engineering a Highly Efficient and Promiscuous Intein. J Mol Biol. 421(1): 85–99. https://doi.org/10.1016/j.jmb.2012.04.029
- Lennon, C. W., Stanger, M. and Belfort, M. (2016). Protein splicing of a recombinase intein induced by ssDNA and DNA damage. Genes Dev. 30(24): 2663–2668. https://doi.org/10.1101/gad.289280.116
- Casadaban, M. J. (1976). Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 104(3): 541–555. https://doi.org/10.1016/0022-2836(76)90119-4
Article Information
Publication history
Received: Feb 5, 2025
Accepted: Mar 11, 2025
Available online: Mar 25, 2025
Published: Apr 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Smetana, J. S., Ariagno, T. M., Son, A., Horowitz, S. and Lennon, C. W. (2025). Monitoring Protein Stability In Vivo Using an Intein-Based Biosensor. Bio-protocol 15(8): e5271. DOI: 10.21769/BioProtoc.5271.
Category
Microbiology > Microbial biochemistry > Protein > Activity
Molecular Biology > Protein > Anti-microbial analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link