- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Developing a Ministroke Model in Mouse Barrel Cortex
Published: Vol 15, Iss 6, Mar 20, 2025 DOI: 10.21769/BioProtoc.5244 Views: 2306
Reviewed by: Samantha HallerRaniki KumariAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Transient Middle Cerebral Artery Occlusion with an Intraluminal Suture Enables Reproducible Induction of Ischemic Stroke in Mice
Luke R. Lemmerman [...] Daniel Gallego-Perez
Feb 5, 2022 7556 Views
Operant Self-medication for Assessment of Spontaneous Pain Relief and Drug Abuse Liability in Mouse Models of Chronic Pain
David Cabañero [...] Rafael Maldonado
Mar 5, 2022 3046 Views
Utilizing EdU to Track Leukocyte Recruitment to the Brain
Zoie K. Lipfert [...] David P. Sullivan
Dec 5, 2025 1555 Views
Abstract
Stroke is a worldwide leading cause of death and long-term disability, with ischemic strokes making up approximately 85% of all cases. There is a significant need for an ideal animal model that accurately replicates the disease’s pathology to study the molecular mechanisms of brain injury. Various experimental models have been created to induce middle cerebral artery occlusion (MCAO), including intraluminal MCAO, photothrombotic models, endothelin-1 injections, and electrocoagulation. However, these often result in large infarct or lesion volumes accompanied by considerable variability. In this study, we present a ministroke model that specifically targets the mouse barrel cortex, making it suitable for investigating the mechanisms of minor strokes and stroke recurrence. In our model, the distal branch of the right middle cerebral artery (MCA), which supplies the sensorimotor cortex, is permanently ligated using 10-0 sutures. This is followed by a 7-min occlusion of the bilateral common carotid arteries (CCAs) and subsequent reperfusion. This approach produces a mild stroke characterized by small and consistent lesion volumes and very low mortality rates. A well-trained experimenter can achieve nearly zero mortality with this technique. Furthermore, this model of localized ischemia induces lesions in the functionally defined barrel cortex, allowing the use of the vibrissae-evoked forelimb placing test to assess functional outcomes.
Key features
• Introduces a novel ministroke model targeting the mouse barrel cortex, specifically designed for studying minor strokes.
• Achieves consistently small infarct sizes and low mortality rates, allowing for long-term assessments of functional outcomes.
• Requires specialized surgical skills for the permanent ligation of the right middle cerebral artery and temporary occlusion of common carotid arteries.
• The adhesive removal test and the vibrissae-evoked forelimb placing test exhibit remarkable sensitivity in this model.
Keywords: Ischemic strokeGraphical overview
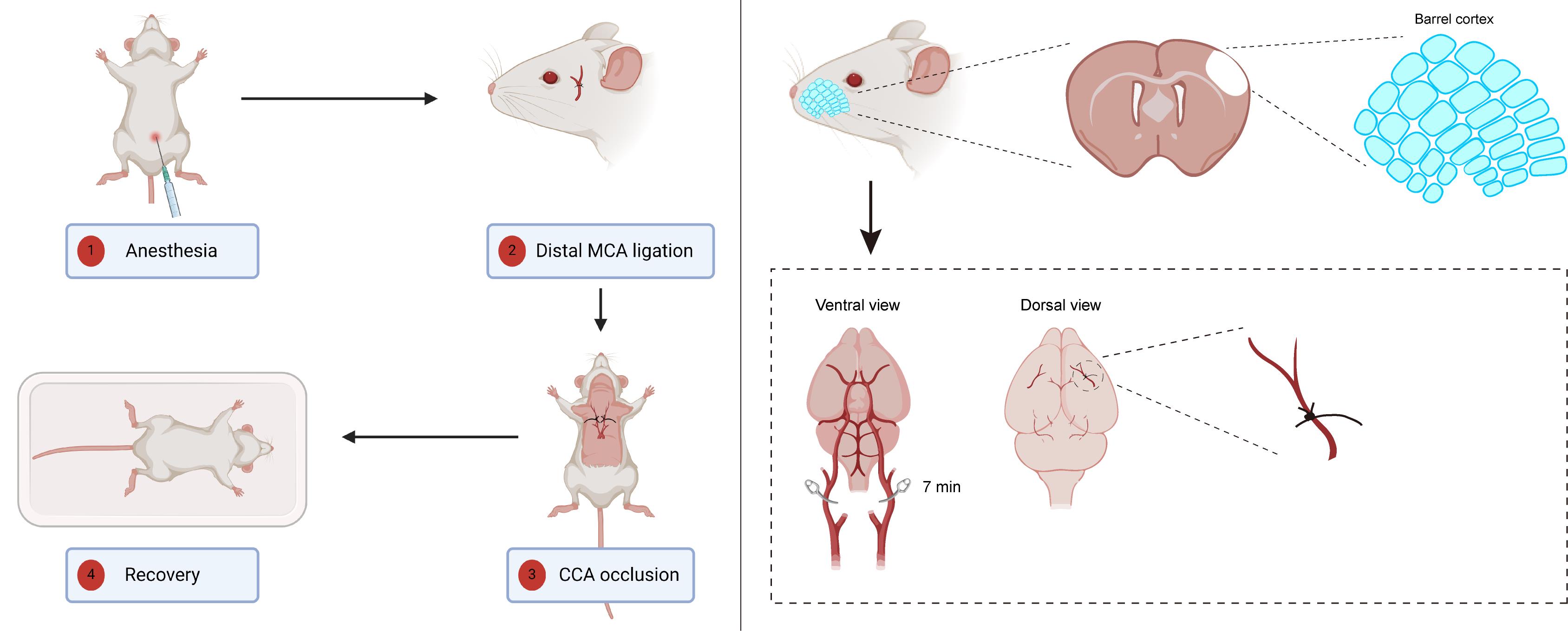
Ministroke model in mouse barrel cortex. Left panel: An overview of the surgical procedure. Right panel: An illustration depicting the two primary steps of this model; the upper section illustrates the location of the barrel cortex, while the lower section highlights the blood vessels that require ligation or occlusion. The left panel of the graphical overview was created in BioRender.
Background
Our protocol focuses on the study of ischemic strokes, specifically on the long-term effects and mechanisms of brain injury following mild ischemic events. Ischemic stroke, particularly acute ischemic stroke (AIS), poses significant challenges for treatment and rehabilitation due to its high prevalence and complexities surrounding timely medical intervention. As traditional treatments, such as intravenous tissue plasminogen activator (tPA), are limited by strict time windows, there is an urgent need for alternative therapeutic strategies that can address the chronic phase of the stroke [1].
Several methodologies have been developed to model ischemic strokes in laboratory settings, with the middle cerebral artery occlusion (MCAO) model being the most widely adopted [2]. MCAO can be executed via the filament method or embolic occlusion, providing a reproducible model of focal ischemia that closely mirrors human stroke patterns. However, MCAO often produces large infarct volumes and can lead to atypical damage, such as hypothalamic injury, which does not commonly occur in human cases. Conversely, the endothelin-1 (ET-1) model facilitates targeted ischemia through localized vasoconstriction, allowing for precise examination of specific regions [3]. Yet, its inability to fully replicate the complex pathophysiological processes of natural strokes presents a limitation.
The photothrombotic model, utilizing a photosensitive dye to induce localized thrombosis via light activation, offers spatial and temporal control over ischemic areas but falls short of mimicking the inflammatory responses associated with strokes [4]. In contrast, our ministroke model involves a combination of permanent distal middle cerebral artery (MCA) ligation and transient bilateral common carotid artery occlusion, resulting in a mild stroke phenotype. This method yields small, consistent lesion volumes and maintains low mortality rates, allowing for the exploration of long-term neuroimmune interactions and other chronic effects of ischemia.
This model presents limitations, such as the requirement for a cranial window and fewer behavioral tests applicable. Despite these limitations, the advantages of our ministroke protocol lie in its ability to produce mild strokes that facilitate the study of chronic pathophysiological processes without the confounding effects of larger infarcts. Unlike models that induce severe injury with high mortality rates, our approach enables researchers to investigate neuroinflammatory responses over extended periods, making it particularly valuable for studying recovery mechanisms and potential therapeutic interventions.
In addition to its primary application in the mechanistic research of ischemic stroke, our protocol can be elegantly adapted for studies focused on therapeutic testing and validation. The ministroke model serves as an invaluable platform for the evaluation of novel neuroprotective agents and rehabilitation strategies. Moreover, the model’s capacity to examine long-term neuroimmune interactions renders it particularly suitable for investigating the role of inflammation in post-stroke recovery. This protocol may also be employed to establish stroke recurrence models. The insights gained from this model could inform strategies to mitigate the long-term consequences of brain injury and improve therapeutic outcomes for stroke patients. Overall, our ministroke model represents a promising tool for advancing the understanding of ischemic stroke and its chronic implications, thereby contributing to the development of effective treatments.
Materials and reagents
Biological materials
1. 8–10-week-old C57BL/6N male mice (Beijing Vital River Laboratory Animal Technology Co., Ltd., catalog number: 213)
Reagents
1. 1.25% ready-to-use avertin solution (Nanjing Aibei Biotechnology Co., Ltd., catalog number: M2920)
2. 0.9% sodium chloride solution (saline) (Sigma-Aldrich, catalog number: S8776-100ML)
3. Buprenorphine hydrochloride solution (Sigma-Aldrich, catalog number: B7536)
Laboratory supplies
1. Cotton tip applicator (Winner Medical, catalog number: 601-015216)
2. Sterile cotton ball (Winner Medical, catalog number: 601-015215)
3. Prolene non-absorbable suture, blue 10-0 3.8 mm 3/8 circle taper point needle 13 cm (Ethicon, catalog number: W2790)
4. Non-absorbable surgical silk suture, 4-0 (Ethicon, catalog number: 736G)
5. Non-absorbable surgical silk suture, 6-0 (Ethicon, catalog number: 706G)
6. Nair hair removal lotion with soothing aloe & lanolin (Church & Dwight, catalog number: UPC022600223290)
7. Vetbond tissue adhesive (3M, catalog number: 1469SB)
8. 75% ethanol wipes (Beautyblend, catalog number: BLD.R-8070)
9. Eye ointment (Puralube Vet Ointment, Dechra, catalog number: NDC 17033-211-38)
Equipment
1. Dissecting microscope (Ningbo Novel Optics Technology Co., Ltd, catalog number: JSZ8)
2. Ophthalmic surgery microscissors (Jinzhong surgical instrument, catalog number: Y3F020)
3. Ophthalmic forceps (Jinzhong surgical instrument, catalog number: JD1050)
4. Microforceps (Jinzhong surgical instrument, catalog number: WA3080)
5. Ophthalmic scissors (Jinzhong surgical instrument, catalog number: Y00030)
6. Scalpel blades (Jinzhong surgical instrument, catalog number: J0B020)
7. Electric drill (Slite, catalog number: P-500-2)
8. Warming pad (REPTIZOO, catalog number: AHM11)
Procedure
A. Surgery preparation
1. Sterilize all surgical instruments and materials before surgery.
Note: Autoclave all surgical instruments and soak non-autoclavable materials in 75% ethanol and rinse them with sterile water before the next surgery.
2. Rinse 4-0 sutures in a sterile 50 mL centrifuge tube.
3. Put a heating pad on the base of the dissecting microscope and set and maintain it at 37 °C.
4. Put a heating pad on a new sterile cage and set and maintain it at 37 °C.
Note: The cage will function as a habitat, facilitating the recovery of consciousness following the completion of the surgery.
B. Ligation of the distal branch of the right MCA
1. Weigh and record the weight of the mouse and calculate the dose of the anesthetic.
Note: The suggested dose of 1.25% avertin is 0.2 mL per 10 g of body weight.
2. Perform intraperitoneal injection of the desired volume of 1.25% avertin to deeply anesthetize the mouse.
Note: Mice can be anesthetized with isoflurane utilizing a gas anesthesia apparatus instead.
3. Wait for 5 min until the mouse is fully unconscious.
Note: Gently pinch the mouse’s hind limb to ascertain the depth of anesthesia.
4. Gently apply eye ointment to the eyes to avoid corneal drying and adhesion after Vetbond tissue adhesive treatment.
Note: Alternatively, rinse sterile cotton in saline and apply it to the eyes.
5. Gently apply the Nair hair removal lotion to the area between the right eye and right ear and wait for 2 min. Then, wipe the detached hair and the Nair hair removal lotion with 75% ethanol wipes (Figure 1).
Note: Alternatively, hair can be removed using an electric razor.
Caution: The application of Nair hair removal lotion should not exceed 2 min; otherwise, it may result in skin damage.
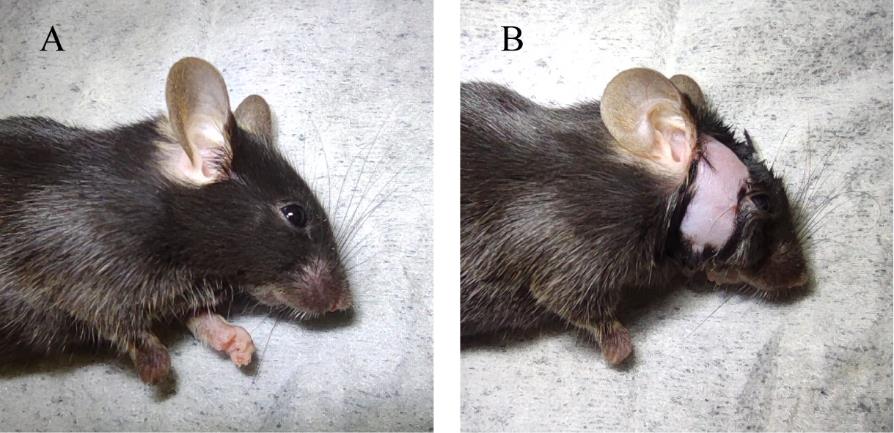
Figure 1. Hair removal prior to surgery. A. Before the application of Nair hair removal lotion. B. Following the complete removal of hair.
6. Position the mouse laterally to reveal the right side of its face. Then, create a vertical incision in the designated area adjacent to the eyes with a scalpel blade (Figure 2A).
Caution: Avoid cutting the large blood vessel adjacent to the eye, as indicated by the arrowhead in Figure 2A.
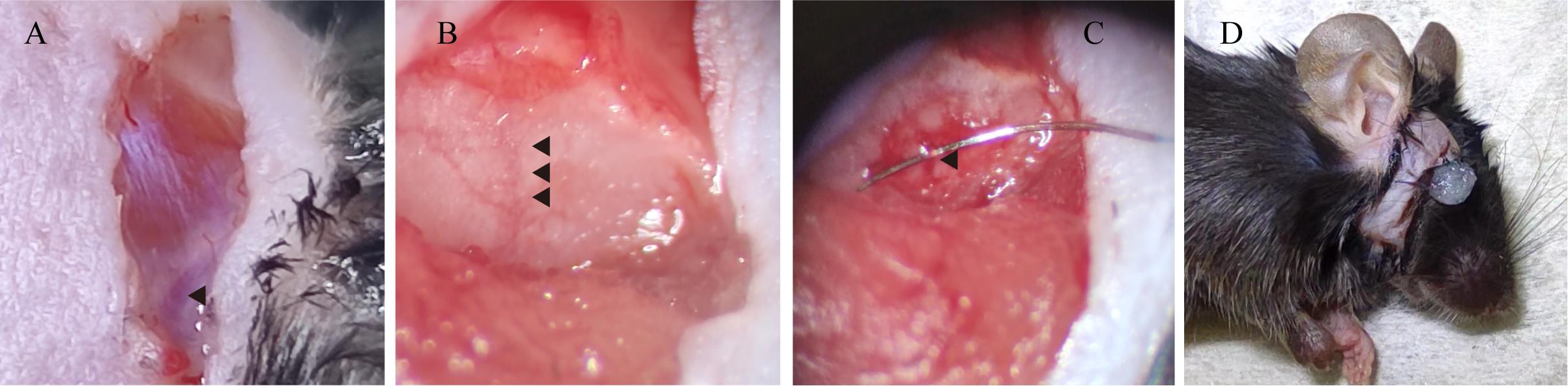
Figure 2. Create a vertical incision to reveal the distal branch of the right middle cerebral artery (MCA). A. View of the facial incision, with an arrow indicating a prominent blood vessel. B. View of the distal branch of the right MCA. C. Microscopic visualization of the 10-0 nylon suture thread positioned at the distal segment of the MCA. D. View of the facial incision following the sealing of the wound with Vetbond tissue adhesive.
7. Carefully detach the muscle from the skull using ophthalmic surgery microscissors to expose the skull.
Caution: Minimize the cutting of any blood vessels whenever possible; otherwise, it may result in bleeding.
Note: In the event of bleeding, promptly apply gentle pressure to the affected area with sterile cotton until the bleeding ceases.
8. Identify the Y-shaped blood vessel situated beneath the skull using the dissecting microscope and proceed to penetrate the skull with an electric drill (diameter 1.3 mm) (Figure 2B).
Caution: Regulate the intensity of the drilling to prevent any harm to the blood vessel, thereby averting the risk of hemorrhage.
Note: If the Y-shaped blood vessel is indistinct, gently rinse the area with saline and subsequently absorb any residual liquid with sterile cotton.
9. Carefully excise the remnants of the skull and the meninges using microforceps to reveal the distal branch of the MCA.
Caution: Exercise extraordinary caution to avoid inflicting damage to the blood vessel while detaching the meninges.
10. Carefully thread a 10-0 suture through the blood vessel, initially securing it with a double knot followed by a reverse knot (Figure 2C).
Caution: Exercise exceptional care to avoid causing any harm to the blood vessel during the suturing process. Regulate the tension of the knots to avert the risk of vessel rupture.
Note: The 10-0 sutures are imperceptible to the naked eye and must be examined under a dissecting microscope. Adjust the pressure applied to the needle to prevent the sutures from being ejected.
11. Trim the excess 10-0 suture, restore the muscle, and seal the incision with Vetbond tissue adhesive (Figure 2D).
Note: The incision may be initially sutured with a 6-0 suture before being secured with Vetbond tissue adhesive.
C. Temporary occlusion of the bilateral CCAs
1. Position the mouse in a supine orientation and delicately apply the Nair hair removal lotion to the cervical region, allowing it to act for 2 min. Subsequently, clean the area by wiping away the detached hair and the Nair hair removal lotion with 75% ethanol wipes (Figure 3A).
Note: Alternatively, hair removal may be achieved using an electric razor.
Caution: The application of Nair hair removal lotion must not exceed 2 min, as prolonged exposure may lead to skin damage.
2. Using sharp scissors, create a horizontal incision in the neck area, penetrating through the skin.
Note: It is essential to weigh the advantages of a larger incision against those of a smaller one. While larger incisions facilitate enhanced visualization of cervical structures, they also elevate the surgical burden on the experimental animals.
3. Carefully compress the incision site to reveal the submandibular glands; then, use ophthalmic forceps to fully uncover them (Figure 3B and 3C).
4. Using microforceps, delicately separate the muscle to expose the carotid sheath.
Note: Following the trachea will facilitate the identification of the carotid sheath.
5. Carefully dissect the carotid sheath, isolating the common carotid artery with precision while separating it from the vagus nerve, which is situated laterally (Figure 4A and 4B).
Note: The vagus nerve is easily recognizable by its distinct white coloration, contrasting with that of the common carotid artery.
6. Delicately pierce the surrounding fascia and thread a length of 4-0 suture through the common carotid artery (Figure 4C).
Caution: Refrain from elevating the microforceps to prevent rupture of the common carotid artery. Avoid causing damage to the blood vessels. Do not thread the 4-0 suture through the vagus nerve, as this may result in pain.
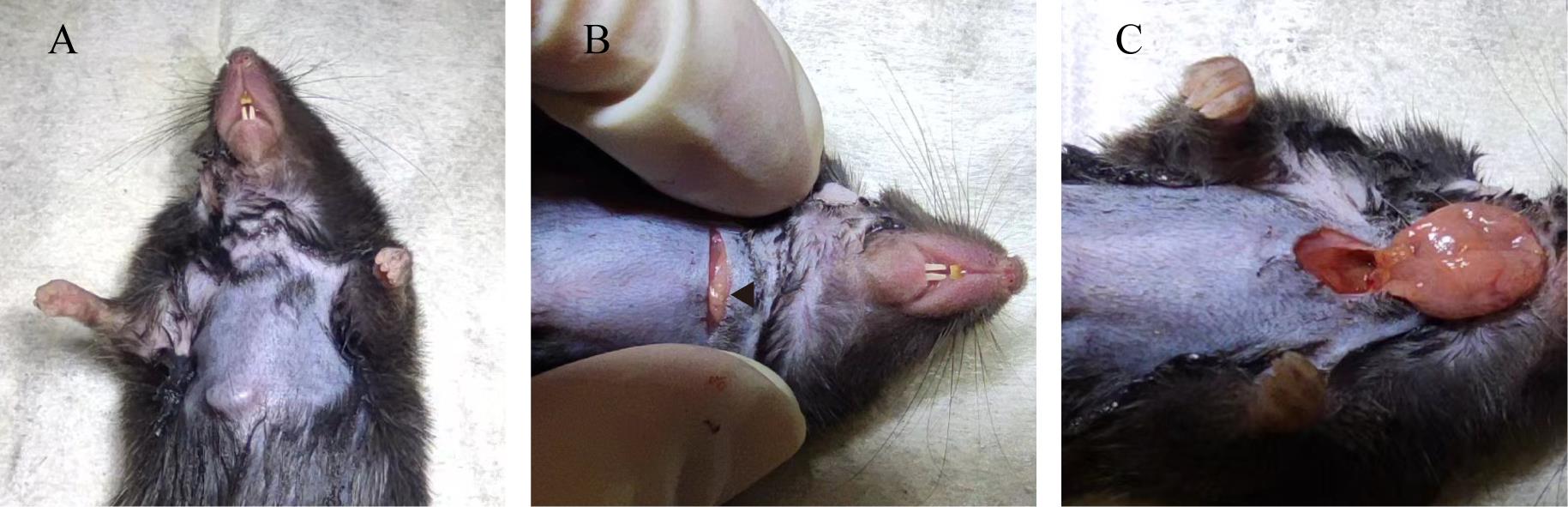
Figure 3. Execute a ventral neck incision to unveil the common carotid artery (CCA). A. View of the neck region following the application of Nair hair removal lotion. B. Ventral perspectives of the neck incision, with an arrow highlighting the submandibular glands. C. Ventral perspectives of the neck incision after complete exposure of the submandibular glands.
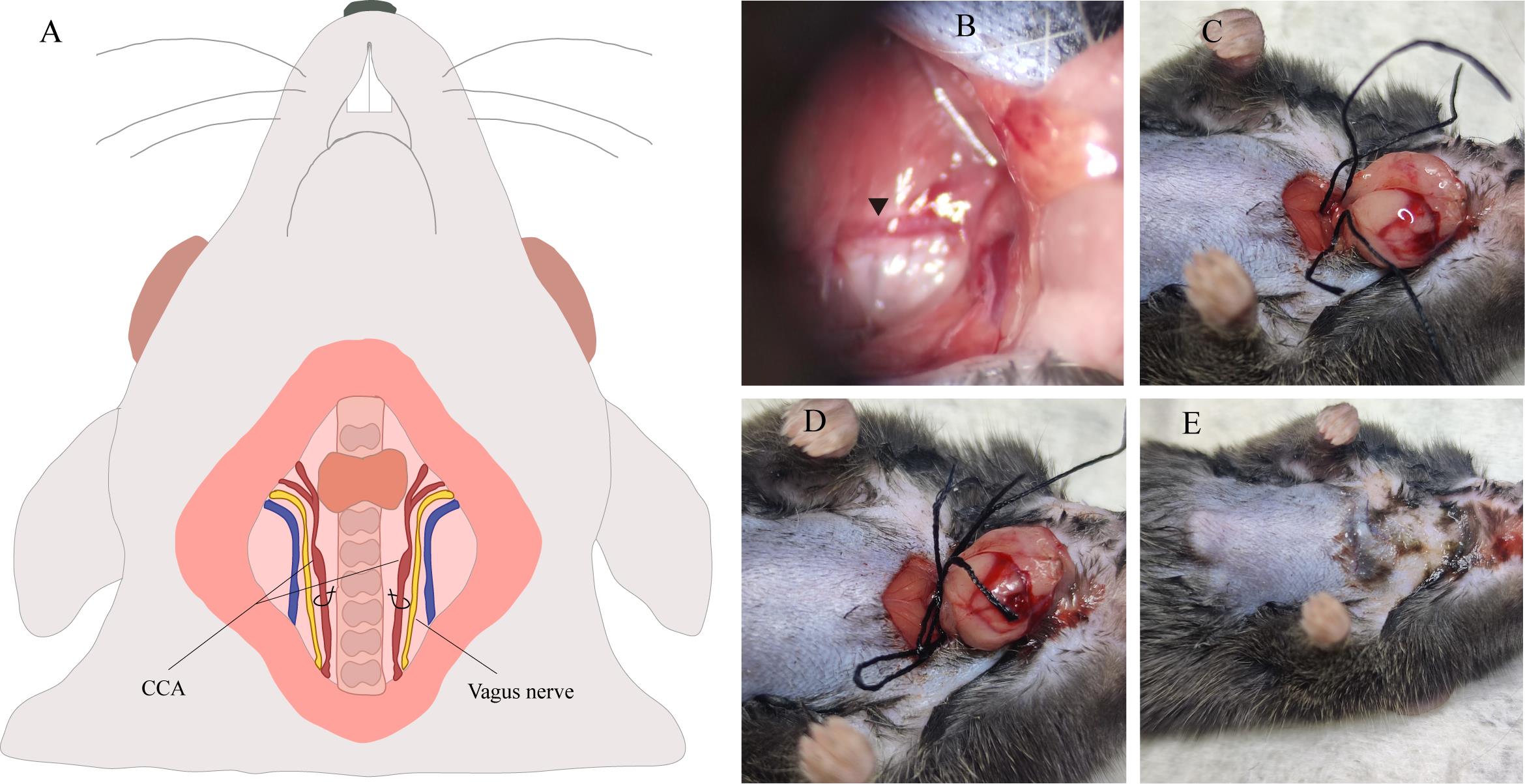
Figure 4. Ligation procedures of the common carotid artery (CCA). A. Illustration of the CCA’s anatomical structure. B. Microscopic examination of the common carotid artery. C. Position two 4-0 silk sutures beneath the CCA. D. Form a slipknot on both sides of the CCA. E. View of the neck incision following the sealing of the wound with Vetbond tissue adhesive.
7. Repeat step C6 on the contralateral common carotid artery.
Caution: Refrain from elevating the microforceps to prevent rupture of the common carotid artery. Avoid causing damage to the blood vessels. Do not thread the 4-0 suture through the vagus nerve, as this may result in pain.
8. Form a slipknot on both sides and maintain it for 7 min (Figure 4D).
9. Upon completion of the allotted time, promptly untie both slipknots and restore the submandibular glands.
Note: The 4-0 suture may adhere to the tissue; if so, rinse with saline and gently detach it.
10. Seal the incision using Vetbond tissue adhesive (Figure 4E).
Note: The incision may initially be sutured with a 4-0 suture before being secured with Vetbond tissue adhesive.
D. Postoperative care
1. Vigilantly observe the mouse during and after the surgical procedure, including, but not limited to, its respiration and body temperature.
2. Ensure the mouse remains on a warming pad until it has fully regained consciousness.
Note: This step is of paramount importance and typically requires a minimum of 4 h. Following surgery, mice are unable to thermoregulate, and immediate reintroduction to their environment may lead to severe hypothermia [5].
3. To alleviate pain, administer 0.05 mg/kg buprenorphine during the initial 48 h following surgery.
Note: Buprenorphine has been demonstrated to function as an effective analgesic in C57BL/6 male mice subjected to ischemic stroke without detrimental effects on infarct volume [6].
Data analysis
Kindly consult the original article for an in-depth description of the behavioral testing procedures [7]. Brain magnetic resonance imaging (MRI) is frequently employed to confirm the occurrence of stroke within the territory of the middle cerebral artery and to compute the volume of infarction. Alternatively, 2,3,5-triphenyltetrazolium chloride (TTC) brain staining at the time of harvest or immunofluorescence staining are extensively utilized to evaluate the infarct volume (Figure 5).
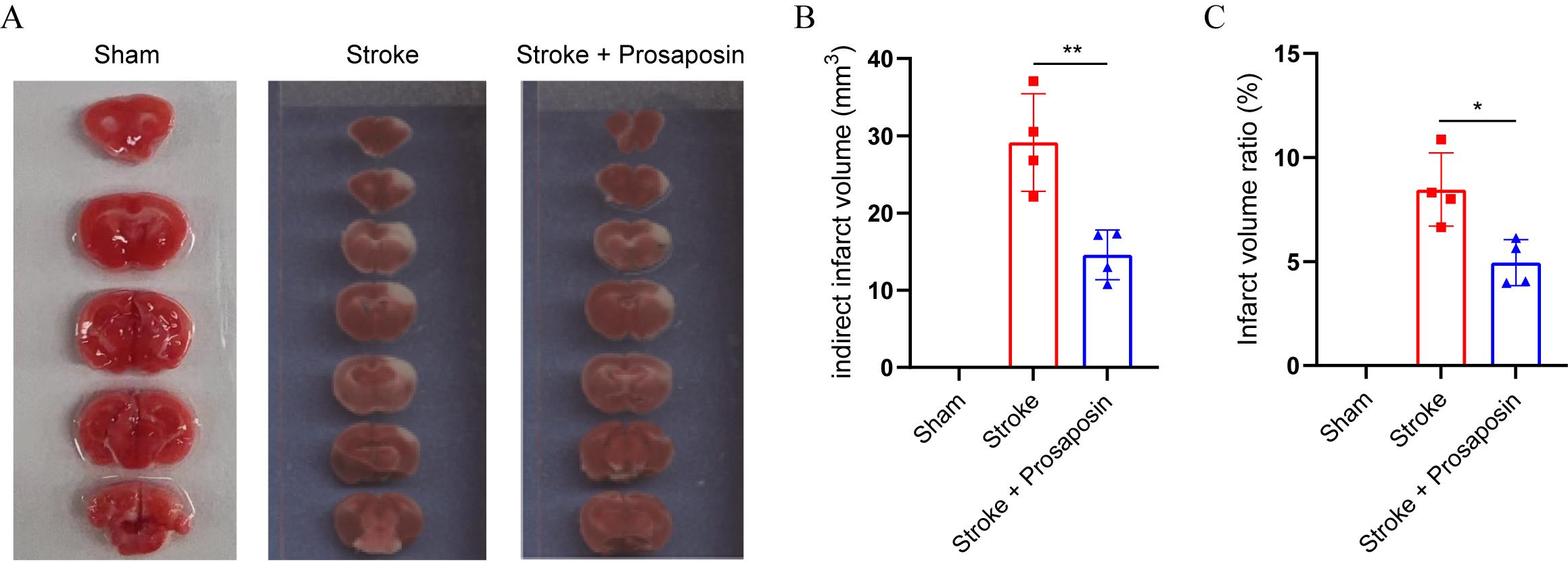
Figure 5. Brain tissue exhibiting 2,3,5-triphenyltetrazolium chloride (TTC) staining 48 h after stroke. A. The TTC cerebral tissue staining reveals the infarcted area in each group. B. Quantitative analysis of the infarct volumes across various groups. C. Quantitative analysis of the infarct volume ratios among various groups. *p < 0.05, **p < 0.01, n = 4 per group, unpaired Student's t-test.
Validation of protocol
This protocol has been used and validated in the following research article:
Wang et al. [7]. Transcriptome analysis reveals dynamic microglial-induced A1 Astrocyte reactivity via C3/C3aR/NF-κB Signaling after ischemic stroke. Mol Neurobiol (Figures 4–8).
General notes and troubleshooting
General notes
1. We usually use male mice only in this model to exclude the influence of estrogen. The role of estrogen in ischemic stroke still needs to be uncovered, as both neurotoxic and neuroprotective effects have been reported [8,9].
2. Since this is a ministroke model, it creates lesions only in the barrel cortex region. The adhesive removal test and the vibrissae-evoked forelimb placing test (also known as the paw whisker test) are the most sensitive in this model.
3. This model creates a ministroke with low mortality: a well-trained experimenter could cause nearly zero mortality.
4. Blood vessels are more prone to rupturing and invisible when the tissue is dry. Therefore, we recommend rinsing with saline if this happens.
5. Avertin and isoflurane are chemical compounds that have been used primarily as an anesthetic agent. Their neuroprotective effects are not well established in the literature, and their primary use has been in the context of anesthesia rather than neuroprotection.
Troubleshooting
Problem 1: No infarct was found after sacrifice.
Possible cause: The 10-0 suture is loosened when the mouse scratches the wound.
Solution: Make sure to secure the distal branch of the right MCA with a double knot followed by a reverse knot, or even a triple knot followed by a reverse knot. This rarely occurs if one strictly follows the protocol.
Problem 2: Hemorrhaging during the ligation of the distal branch of the right middle cerebral artery or the occlusion of the bilateral common carotid arteries.
Possible cause: The observed complication may be attributed to an inadvertent transection or avulsion of blood vessels caused by microforceps manipulation during the surgical procedure.
Solution: Maintain a steady hand and delicately regulate the force applied throughout the surgical procedure.
Problem 3: Mortality of the mouse during the recovery phase.
Possible cause: Hypothermia following anesthesia.
Solution: It is essential to maintain the mouse on a temperature-regulated warming pad throughout the whole period until full consciousness is restored, as evidenced by the return of righting reflex and normal locomotor activity.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 82201622) and the Natural Science Foundation of the Inner Mongolia Autonomous Region of China (No. 2020MS03017). The original research paper is found here (DOI: 10.1007/s12035-024-04210-8) [7].
Competing interests
The authors declare no competing interests.
Ethical considerations
All animal procedures were strictly performed within the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Beijing Friendship Hospital (IACUC # 22–2028).
References
- Saini, V., Guada, L. and Yavagal, D. R. (2021). Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology. 97(20 Suppl 2): S6–S16. https://doi.org/10.1212/WNL.0000000000012781.
- Liu, F. and McCullough, L. D. (2011). Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol. 2011: 464701. https://doi.org/10.1155/2011/464701.
- Abeysinghe, H. C. S. and Roulston, C. L. (2018). A Complete Guide to Using the Endothelin-1 Model of Stroke in Conscious Rats for Acute and Long-Term Recovery Studies. Methods Mol Biol. 1717: 115–133. https://doi.org/10.1007/978-1-4939-7526-6_10.
- Uzdensky, A. B. (2018). Photothrombotic Stroke as a Model of Ischemic Stroke. Transl Stroke Res. 9(5): 437–451. https://doi.org/10.1007/s12975-017-0593-8.
- Barber, P. A., Hoyte, L., Colbourne, F. and Buchan, A. M. (2004). Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 35(7): 1720–1725. https://doi.org/10.1161/01.STR.0000129653.22241.d7.
- Jacobsen, K. R., Fauerby, N., Raida, Z., Kalliokoski, O., Hau, J., Johansen, F. F. and Abelson, K. S. (2013). Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57BL/6 male mice. Comp Med. 63(2): 105–113. https://pubmed.ncbi.nlm.nih.gov/23582417/
- Wang, S., Pan, Y., Zhang, C., Zhao, Y., Wang, H., Ma, H., Sun, J., Zhang, S., Yao, J., Xie, D., et al. (2024). Transcriptome Analysis Reveals Dynamic Microglial-Induced A1 Astrocyte Reactivity via C3/C3aR/NF-kappaB Signaling After Ischemic Stroke. Mol Neurobiol. 61(12): 10246–10270. https://doi.org/10.1007/s12035-024-04210-8.
- Selvamani, A. and Sohrabji, F. (2010). The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 30(20): 6852–6861. https://doi.org/10.1523/JNEUROSCI.0761-10.2010.
- Suzuki, S., Brown, C. M. and Wise, P. M. (2009). Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 30(2): 201–211. https://doi.org/10.1016/j.yfrne.2009.04.007.
Article Information
Publication history
Received: Dec 24, 2024
Accepted: Feb 13, 2025
Available online: Mar 4, 2025
Published: Mar 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Wang, S., Wang, S., Sun, Y., Du, Y., Yao, J., Zhang, S., Wu, J., Xie, D. and Wu, Y. (2025). Developing a Ministroke Model in Mouse Barrel Cortex. Bio-protocol 15(6): e5244. DOI: 10.21769/BioProtoc.5244.
Category
Neuroscience > Nervous system disorders > Stroke
Neuroscience > Sensory and motor systems > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link