- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
Published: Vol 14, Iss 16, Aug 20, 2024 DOI: 10.21769/BioProtoc.5054 Views: 2310
Reviewed by: Andrea GramaticaRaju MondalAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2749 Views
Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2630 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1562 Views
Abstract
Most terrestrial plants are associated with symbiotic Glomeromycotina fungi, commonly known as arbuscular mycorrhizal (AM) fungi. AM fungi increase plant biomass in phosphate-depleted conditions by allocating mineral nutrients to the host; therefore, host roots actively exude various specialized metabolites and orchestrate symbiotic partners. The hyphal branching activity induced by strigolactones (SLs), a category of plant hormones, was previously discovered using an in vitro assay system. For this bioassay, AM fungi of the Gigaspora genus (Gigasporaeae) are commonly used due to their linear hyphal elongation and because the simple branching pattern is convenient for microscopic observation. However, many researchers have also used Glomeraceae fungi, such as Rhizophagus species, as the symbiotic partner of host plants, although they often exhibit a complex hyphal branching pattern. Here, we describe a method to produce and quantify the hyphal branches of the popular model AM fungus Rhizophagus irregularis. In this system, R. irregularis spores are sandwiched between gels, and chemicals of interest are diffused from the surface of the gel to the germinating spores. This method enables the positive effect of a synthetic SL on R. irregularis hyphal branching to be reproduced. This method could thus be useful to quantify the physiological effects of synthesized chemicals or plant-derived specialized metabolites on R. irregularis.
Key features
• Development of an in vitro hyphal branching assay using germinating spores of Rhizophagus irregularis.
• This in vitro assay system builds upon a method developed by Kameoka et al. [1] but modified to make it more applicable to hydrophilic compounds.
• Optimized for R. irregularis to count the hyphal branches.
• This bioassay requires at least 12 days to be done.
Keywords: AM symbiosisGraphical overview
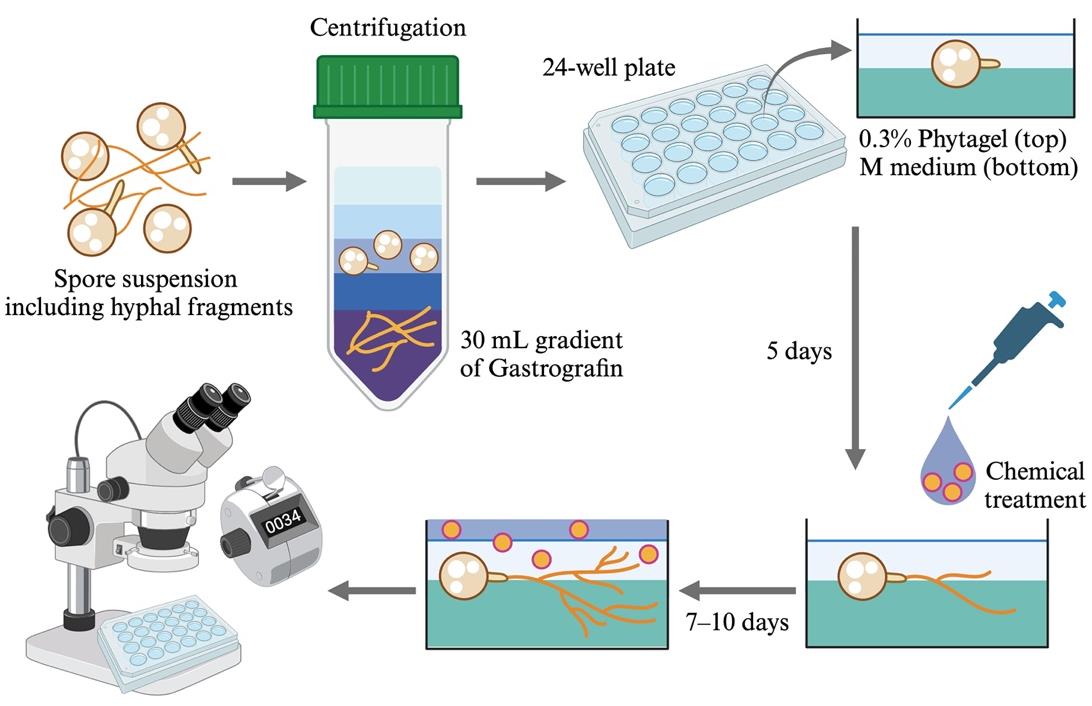
Simplified overview of the hyphal branching assay using Rhizophagus irregularis spores
Background
In nature, plants are surrounded by diverse and numerous microbes in their leaves (phyllosphere) and roots (rhizosphere). Since these microbes beneficially or detrimentally modulate the host’s growth, host plants utilize their own specialized metabolites to manipulate the assembly of microbes. In particular, Glomeromycotina fungi, termed arbuscular mycorrhizal (AM) fungi, are representative beneficial microbes that have been found to be associated with more than 70% of terrestrial plant species [2]. AM fungi allocate inorganic phosphate from the soil to the host plant and, in return, the host plant shares its photosynthates with the symbiotic fungi. This mutual interaction begins with a category of root-secreted plant hormones, the strigolactones (SLs), which possess hyphal branching activity in AM fungi [3]. SLs were initially identified from cotton root exudates and described as germination stimulants of root parasitic plants [4]. Forty years later, their hyphal branching activity was reported using an AM fungus of the Gigaspora genus, Gigaspora margarita [3,5]. Prior to that discovery, Gigaspora fungi had been used in bioassays to identify signal compounds in host root exudates [6–9]. Thus, in vitro assay systems using AM fungi are essential to clarify the chemical communication between AM fungi and host plants.
G. margarita usually forms large spores (approximately 200 μm in diameter), as suggested by the genus name [10]. In addition to a uniquely large spore size, the fungus exhibits a simple hyphal branching pattern, forming thick and linear hyphae [3,5]. Therefore, G. margarita has often been used for in vitro bioassays. However, recent studies aimed at revealing the molecular mechanisms regulating AM symbiosis have mainly applied Rhizophagus irregularis because fungal genomic information, including of several intraspecies lines, is available [11–13]. Another reason is that Rhizophagus fungi have more beneficial traits (i.e., plant growth promotion) than Gigaspora fungi [14,15]. On the other hand, Rhizophagus fungi have small spores (approximately 50 μm in diameter), thin and winding hyphae, and a complex branching pattern [16–19]. Taken together, the hyphal branching assay using R. irregularis is technically difficult [20]. This is the reason why bioassay using R. irregularis is performed by measuring the total length of hyphae [20–22].
In hyphal branching assays using Gigaspora fungi, chemicals of interest have been loaded onto paper discs [3,5]. On the other hand, diffusion assays are limited to the local treatment of reagents and require specialized techniques. In our previous study, we modified an in vitro bioassay system that was originally applied for asymbiotic sporulation of R. irregularis [1]. Using our protocol, which is technically easy, chemicals can be evenly applied to all parts of R. irregularis. Compared to the protocols for Gigaspora fungi, this system is useful for assessing the hyphal branching activity induced by various chemicals in this model AM fungus.
Materials and reagents
Biological materials
Rhizophagus irregularis DAOM197198 (4,000 spores/mL) (PremierTech)
Reagents
Acetone (FUJIFILM WAKO Pure Chemical, CAS number: 67-64-1)
Ethanol (99.5%) (FUJIFILM WAKO Pure Chemical, CAS number: 64-17-5)
Gastrografin for oral/enema use (Bayer, 597.3 g/L amidotrizoic acid, CAS number: 117-96-4)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 10034-99-8)
Potassium nitrate (KNO3) (FUJIFILM WAKO Pure Chemical, CAS number: 7757-79-1)
Potassium chloride (KCl) (FUJIFILM WAKO Pure Chemical, CAS number: 7447-40-7)
Potassium dihydrogen phosphate (KH2PO4) (FUJIFILM WAKO Pure Chemical, CAS number: 7778-77-0)
Calcium nitrate tetrahydrate [Ca(NO3)2·4H2O] (FUJIFILM WAKO Pure Chemical, CAS number: 13477-34-4)
Sucrose (FUJIFILM, CAS number: 57-50-1)
Fe(III)-EDTA (NaFeEDTA·3H2O) (DOJINDO, CAS number: 15708-41-5)
Potassium iodide (KI) (FUJIFILM WAKO Pure Chemical, CAS number: 7681-11-0)
Manganese chloride tetrahydrate (MnCl2·4H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 13446-34-9)
Zinc sulfate heptahydrate (ZnSO4·7H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 7446-20-0)
Boric acid (H3BO3) (FUJIFILM WAKO Pure Chemical, CAS number: 10043-35-3)
Copper sulfate pentahydrate (CuSO4·5H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 7758-98-7)
Disodium molybdate(VI) dihydrate (Na2MoO4·2H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 10102-40-6)
Potassium hydroxide (KOH) (FUJIFILM WAKO Pure Chemical, CAS number: 1310-58-3)
Glycine (FUJIFILM WAKO Pure Chemical, CAS number: 56-40-6)
Thiamine hydrochloride (FUJIFILM WAKO Pure Chemical, CAS number: 67-03-8)
Pyridoxine hydrochloride (FUJIFILM WAKO Pure Chemical, CAS number: 58-56-0)
Nicotinic acid (FUJIFILM WAKO Pure Chemical, CAS number: 59-67-6)
Myo-inositol (FUJIFILM WAKO Pure Chemical, CAS number: 87-89-8)
Phytagel (Sigma-Aldrich, CAS number: 71010-52-1)
rac-GR24 (StrigoLab, CAS number: 76974-79-3)
Solutions
M medium (see Recipes) [16]
Calcium stock of M medium (see Recipes)
1,000× vitamin stock of M medium (see Recipes)
0.3% phytagel containing 3 mM MgSO4·7H2O (see Recipes)
Recipes
M medium
Reagent Final concentration Quantity or Volume MgSO4·7H2O 2.97 mM 731 mg KNO3 0.79 mM 80 mg KCl 0.87 mM 65 mg KH2PO4 0.035 mM 4.8 mg Sucrose 10% (w/v) 10,000 mg NaFeEDTA·3H2O 0.022 mM 9.18 mg KI 0.0045 mM 0.75 mg MnCl2·4H2O 0.030 mM 6 mg ZnSO4·7H2O 0.0092 mM 2.65 mg H3BO3 0.024 mM 1.5 mg CuSO4·5H2O 0.00052 mM 0.13 mg Na2MoO4·2H2O 9.92 nM 0.0024 mg Phytagel 4% (w/v) 4,000 mg H2O n/a 1,000 mL Total (optional) n/a 1,000 mL Note: Adjust pH at 5.5 using KOH solution and autoclave at 121 °C for 20 min. n/a, not applicable.
Calcium stock of M medium
Reagent Final concentration Quantity or Volume Ca(NO3)2·4H2O 1.22 M 2,880 mg H2O n/a 10 mL Total (optional) n/a 10 mL Note: Sterilize the stock using a 0.45 μm filter and store at room temperature (23–25 °C) until use. n/a, not applicable.
1,000× vitamin stock of M medium
Reagent Quantity or Volume Glycine 30 mg Thiamine hydrochloride 1 mg Pyridoxine hydrochloride 1 mg Nicotinic acid 5 mg Myo-inositol 500 mg H2O 10 mL Total (optional) 10 mL Note: Sterilize the mixture using a 0.45 μm filter and store at -30 °C until use.
0.3% phytagel containing 3 mM MgSO4·7H2O
Reagent Final concentration Quantity or Volume MgSO4·7H2O 3 mM 739.4 mg Phytagel 0.3% (w/v) 3,000 mg H2O n/a 1,000 mL Total (optional) n/a 1,000 mL Note: No need to adjust pH. Autoclave at 121 °C for 20 min. n/a, not applicable.
Laboratory supplies
24-well plates (TrueLine, catalog number: TR5002)
1.5 mL microcentrifuge tube (BIO-BIK, catalog number: CF-0150)
15 mL centrifuge tube (Labcon, catalog number: 3132-345)
50 mL centrifuge tube (Labcon, catalog number: 3181-345)
10 mL disposable serological pipette (ASONE, catalog number: 2-4131-14)
Cell strainer (40 μm mesh) (Falcon, catalog number: 352340)
1 mL needleless plastic syringe (TERUMO, catalog number: SS-01T)
0.45 μm PTFE filter (Shimadzu, catalog number: GLCTD-HPTFE1345)
Inspection film tape (aglis, catalog number: LP0002K)
Equipment
Electronic pipette (Labnet, model: FASTPETTE Pro)
Laminar flow hood (SANYO, model: MCV-B131S)
Centrifuge (KUBOTA, model: 3740)
Swing rotor for 50 mL centrifuge tubes (KUBOTA, model: SD-242)
Autoclave (TOMY SEIKO, model: LSX-300)
Vacuum concentrator (Thermo Fisher Scientific, model: Savant SpeedVac DNA130)
Plant growth chamber (NIPPON MEDICAL & CHEMICAL INSTRUMENTS, model: LH-411S)
Stereomicroscope (Olympus, model: SZX16) equipped with a digital camera (Olympus, model: DP-26)
Imaging software (Olympus, model: CellSens standard v1.18)
Software and datasets
Microsoft Office Excel 10
R v4.2.0 (https://www.r-project.org/)
Procedure
Spore separation from inoculum
To observe and count hyphal branches, fragments of fungal hyphae should be removed from the R. irregularis spore suspension in advance. This step builds upon the protocol developed previously [23]. Maintain the suspension and collected spores axenic by working on a laminar flow food.
Prepare 8%, 16%, 32%, and 50% (v/v) Gastrografin solutions in sterile 50 mL centrifuge tubes. Invert the tubes to mix the dense and thick reagents with sterile distilled water.
In a new 50 mL centrifuge tube, pour each Gastrografin solution very gently using an electronic pipette equipped with a 10 mL disposable pipette as follows:
First, pour 10 mL of 50% Gastrografin, followed by 5 mL of 32%, 16%, and 8% Gastrografin. Do not disturb the layers.
Second, load 5 mL of spore suspension onto the surface of 8% Gastrografin softly. The spore concentration of the final solution can be changed.
Centrifuge the tubes at 500× g for 10 min at room temperature in a swing rotor for 50 mL centrifuge tubes.
Note: Decelerate the rotor slowly if possible.
Collect the supernatant, including the upper three layers, using the electronic pipette (Figure 1).
Note: R. irregularis spores must be floating in the 16% Gastrografin layer, and hyphal fragments should be precipitated to the bottom of the tube. Spore density differs among AM fungal species. In the case of R. clarus, spores float in the 32% Gastrografin layer.
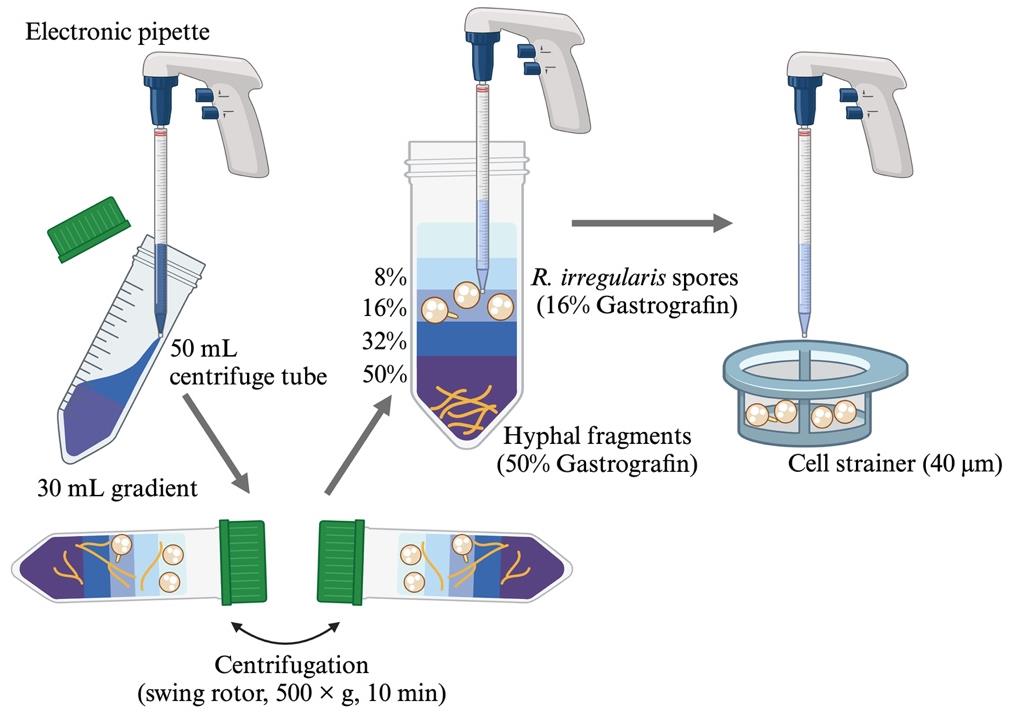
Figure 1. Isolation of Rhizophagus irregularis spores from the inoculumPour the supernatant onto a sterile cell strainer and rinse the collected spores with 10 mL of sterile distilled water three times.
Put the cell strainer on a Petri dish filled with sterile distilled water and move the spores to a new 15 mL centrifuge tube.
Note: Ideally, adjust spore concentration to approximately 2,000 spores/mL.
Keep the spore suspension at 4 °C in the dark.
Preparation of germinating R. irregularis spores and chemical treatment
Add 1 mL of sterile calcium stock of M medium and 1 mL of 1,000× vitamin stock of M medium (see Recipes) to 1 L of autoclaved M medium.
Pour 350 μL of M medium into each well of a sterile 24-well plate and keep the plate still until the gel solidifies.
Dilute the purified spore suspension to approximately 1,000 spores/mL with sterile distilled water.
Drop an 8 μL aliquot of the purified spore suspension onto the center of each M medium.
After placing spores on the media, check if approximately 8 spores are placed on the medium center using a stereomicroscope. Adjust the number under a laminar flow condition if one well contains more or fewer spores. In case of more spores, remove extra spores by sucking them with a 200 μL micropipette.
Open the 24-well plate in the laminar flow hood for approximately 5 min to evaporate excessive water (Figure 2).
Note: The spores move around on the medium surface if too much water remains.
Pour 150 μL of 0.3% phytagel containing 3 mM MgSO4·7H2O (see Recipes). The gel should be cooled sufficiently in advance to allow it to be touched (approximately 40 °C). The 0.3% phytagel should be poured gently into the perimeter of the M medium using a micropipette (see Figure 2).
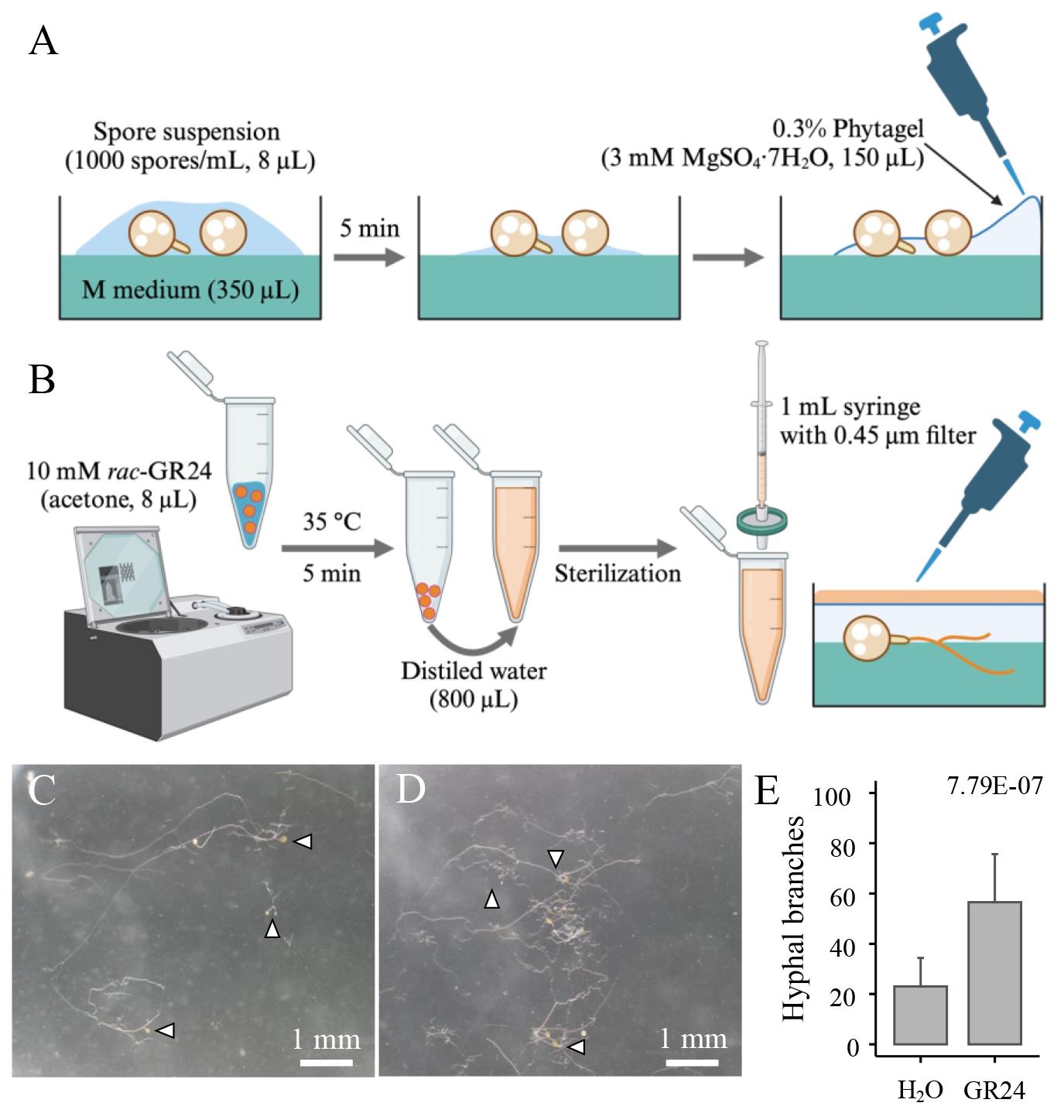
Figure 2. In vitro culture of Rhizophagus irregularis spores and chemical treatment. A. R. irregularis spores (approximately 8 spores) are placed on the center of each well filled with M medium. After drying off excess water, cool 0.3% phytagel with 3 mM MgSO4·7H2O is poured gently into the perimeter of the M medium to cover the spores. B. To exchange the solvent of rac-GR24 (positive control) from acetone to distilled water, the acetone stock is centrifuged in vacuo. The residues are redissolved in distilled water and sterilized using the 0.45 μm PTFE filter. Images show germinating R. irregularis spores (arrowheads) treated with H2O (C) and 100 nM GR24 (D) for 5 days. E. The number of hyphal branches in R. irregularis treated with H2O and 100 nM GR24. Dots and error bars represent individual values and the standard deviation, respectively (n > 15). The E-value was lower than 0.05 in the Wilcoxon rank-sum test.Close the 24-well plate lid and seal it with two layers of inspection film to prevent the dehydration of the gels.
Place the plate horizontally in a growth chamber at 25 °C and incubate it for 5 days in the dark.
Note: In our condition, R. irregularis spores usually germinate in this period.
After 5 days, treat 200 μL of chemicals diluted in sterile distilled water onto each gel containing germinating spores. Use the positive control rac-GR24 diluted in acetone as follows:
Add an 8 μL aliquot of 10 mM rac-GR24 in a 1.5 mL tube.
Centrifuge the tube in a vacuum concentrator at 35 °C for 5 min. If acetone is left in the tube, adjust the evaporation time to remove the solvent completely.
After evaporating the solvent, add 800 μL of distilled water to the tube.
In the laminar flow hood, sterilize the 100 nM rac-GR24 using a 1 mL needleless syringe with a 0.45 μm PTFE filter.
Pour 200 μL of the 100 nM rac-GR24 solution onto the gels.
Seal plates twice with inspection film tape and place them in the same growth chamber at 25 °C. Incubate plates for 7–10 days in the dark.
Quantification of R. irregularis hyphal branching
Under a stereomicroscope, count the number of hyphal branches except for that of the initially elongating hypha (Figure 3). Remove the 24-well plate lid if it becomes misty during observation.
Notes:
Count hyphal branches from the runner hyphae (see Figure 3A, B).
Ignore the short hyphae formed at the boundary of the subtending and runner hyphae. The hyphae often show vigorous branching without any chemical treatment (Figure 3C, D).
Basically, the hyphal branches of a single spore are considered to be one biological replicate. If multiple spores are tied with a subtending hypha, it is counted as one germinating spore (see Figure 3B).
Check the differences among treatments using a statistical calculation (see Data analysis).
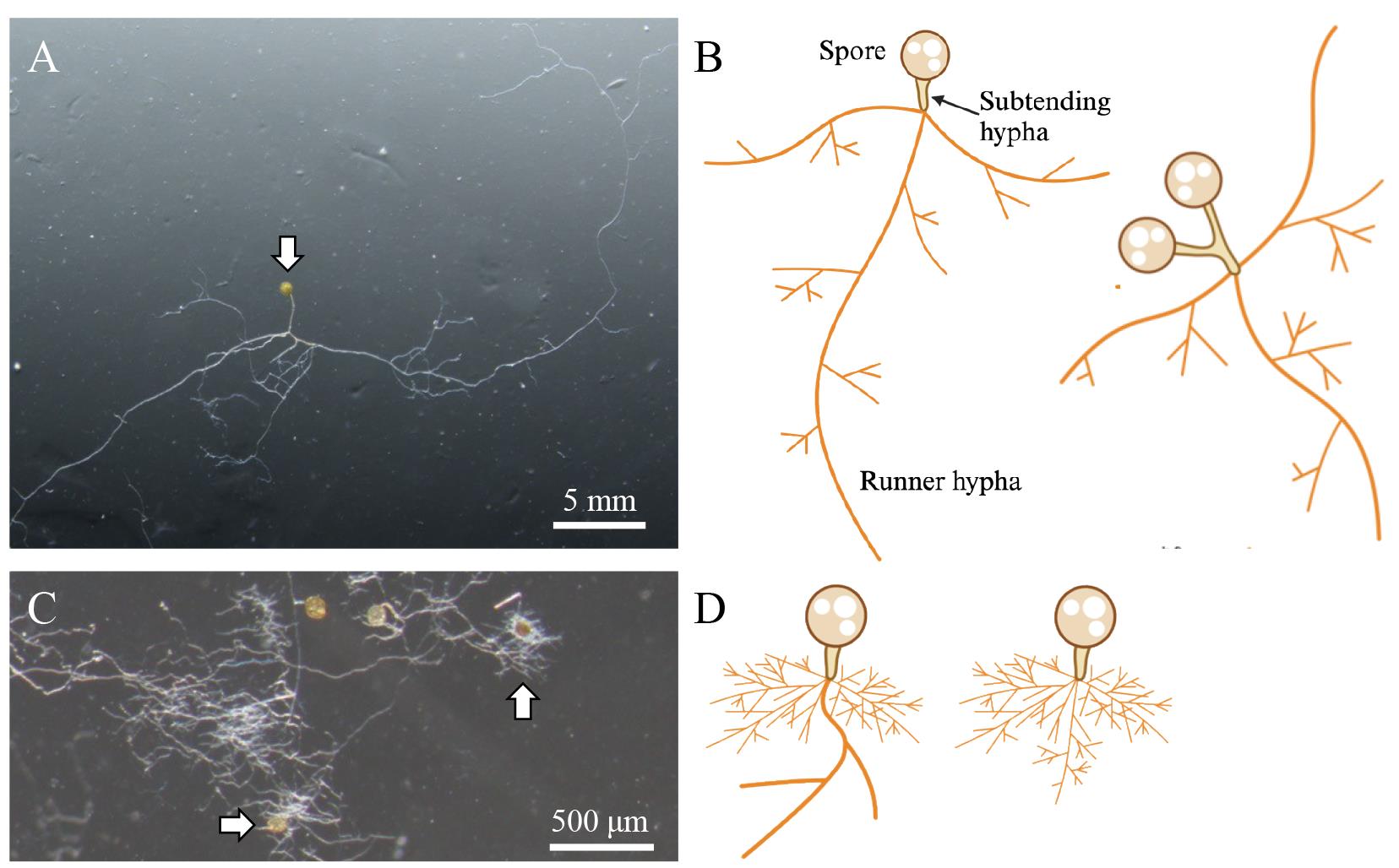
Figure 3. Hyphal structures and growth patterns of Rhizophagus irregularis. A, B. Count the number of hyphal tips emerging from runner hyphae to quantify the hyphal branching. When you find multiple spores tied with a subtending hypha (B, right drawing), count them as one biological replicate. C, D. Germinating spores with highly branching hyphal clusters near the subtending hyphae. Exclude these spores from your quantification. Arrows indicate germinating R. irregularis spores drawn in (B) and (D).
Data analysis
Data can be analyzed using Microsoft Excel. We usually prepare three or four wells containing germinating spores for each treatment. For statistical analysis, R is used to perform Wilcoxon’s rank-sum test with a Bonferroni correction in the case of multiple comparisons. Approximately 20 spores per treatment at least should be assessed. The p-value cutoff is 0.05.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article:
Tominaga et al. [24]. Monoterpene glucosides in Eustoma grandiflorum roots promote hyphal branching in arbuscular mycorrhizal fungi. Plant Physiology (Figure 3).
General notes and troubleshooting
General notes
Hyphal fragments should be removed as much as possible because some hyphal branches will emerge from them and this will mask the germinating spores.
Many spores, i.e., exceeding 8, and a longer (more than 10 days) incubation period can make hyphal branches indistinguishable. In addition, R. irregularis likely develops more hyphal branches when the spores are crowded in a medium, although the reasons remain unclear.
Exposing spores and hyphae to air influences R. irregularis growth and branching. The AM fungal spores exhibit straight hyphal elongation and moderate hyphal branching, although we are unsure why.
Troubleshooting
Problem 1: Spores do not germinate well.
Possible cause: The spore suspension includes many dead spores. Alternatively, phytagel is insufficiently cooled.
Solution: Increase the number of spores or prepare a new spore suspension. Make sure to pour sufficiently cooled phytagel onto the spores.
Problem 2: Cannot count the hyphal branches due to a complex branching pattern.
Possible cause: Each well may contain too many germinating spores, or the incubation period might be unnecessarily long.
Solution: Use fewer spores and shorten the period of chemical treatment. Instead, prepare more wells to assess sufficient spores (>10). Importantly, a germinating spore showing massive branches around subtending hypha without a long runner hypha can be ignored from the quantification. We observe germinating spores with traceable hyphal elongation and branching.
Acknowledgments
This protocol was developed by modifying a previous method [1]. The authors thank Dr. Hiromu Kameoka (China Academy of Science, China) for useful discussions on the in vitro culture of R. irregularis. This work was supported by the Japan Society for the Promotion of Science (JSPS) (KAKENHI grant numbers 20J21994 and 23KJ1578). The graphical abstract and figures were created using Biorender.com.
Competing interests
The authors declare that they have no competing financial or non-financial interests.
References
- Kameoka, H., Tsutsui, I., Saito, K., Kikuchi, Y., Handa, Y., Ezawa, T., Hayashi, H., Kawaguchi, M. and Akiyama, K. (2019). Stimulation of asymbiotic sporulation in arbuscular mycorrhizal fungi by fatty acids. Nat Microbiol. 4(10): 1654–1660.
- Brundrett, M. C. and Tedersoo, L. (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220(4): 1108–1115.
- Akiyama, K., Matsuzaki, K. and Hayashi, H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 435(7043): 824–827.
- Cook, C. E., Whichard, L. P., Turner, B., Wall, M. E. and Egley, G. H. (1966). Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science. 154(3753): 1189–1190.
- Akiyama, K., Ogasawara, S., Ito, S. and Hayashi, H. (2010). Structural Requirements of Strigolactones for Hyphal Branching in AM Fungi. Plant Cell Physiol. 51(7): 1104–1117.
- Besserer, A., Puech-Pagès, V., Kiefer, P., Gomez-Roldan, V., Jauneau, A., Roy, S., Portais, J. C., Roux, C., Bécard, G., Séjalon-Delmas, N., et al. (2006). Strigolactones Stimulate Arbuscular Mycorrhizal Fungi by Activating Mitochondria. PLoS Biol. 4(7): e226.
- Nagahashi, G. and Douds, D. (2007). Separated components of root exudate and cytosol stimulate different morphologically identifiable types of branching responses by arbuscular mycorrhizal fungi. Mycol Res. 111(4): 487–492.
- Besserer, A., Bécard, G., Jauneau, A., Roux, C. and Séjalon-Delmas, N. (2008). GR24, a Synthetic Analog of Strigolactones, Stimulates the Mitosis and Growth of the Arbuscular Mycorrhizal Fungus Gigaspora rosea by Boosting Its Energy Metabolism. Plant Physiol. 148(1): 402–413.
- Ramos, A. C., Façanha, A. R. and Feijó, J. A. (2008). Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol. 178(1): 177–188.
- Bécard, G. and Piché, Y. (1989). New aspects on the acquisition of biotrophic status by a vesicular—arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytol. 112(1): 77–83.
- Tisserant, E., Malbreil, M., Kuo, A., Kohler, A., Symeonidi, A., Balestrini, R., Charron, P., Duensing, N., Frei dit Frey, N., Gianinazzi-Pearson, V., et al. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc Natl Acad Sci USA. 110(50): 20117–20122.
- Maeda, T., Kobayashi, Y., Kameoka, H., Okuma, N., Takeda, N., Yamaguchi, K., Bino, T., Shigenobu, S. and Kawaguchi, M. (2018). Evidence of non-tandemly repeated rDNAs and their intragenomic heterogeneity in Rhizophagus irregularis. Commun Biol. 1(1): 87.
- Yildirir, G., Sperschneider, J., Malar C, M., Chen, E. C. H., Iwasaki, W., Cornell, C. and Corradi, N. (2021). Long reads and Hi‐C sequencing illuminate the two‐compartment genome of the model arbuscular mycorrhizal symbiont Rhizophagus irregularis. New Phytol. 233(3): 1097–1107.
- Lendenmann, M., Thonar, C., Barnard, R. L., Salmon, Y., Werner, R. A., Frossard, E. and Jansa, J. (2011). Symbiont identity matters: carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza. 21(8): 689–702.
- Kaur, S., Campbell, B. J. and Suseela, V. (2022). Root metabolome of plant–arbuscular mycorrhizal symbiosis mirrors the mutualistic or parasitic mycorrhizal phenotype. New Phytol. 234(2): 672–687.
- Hildebrandt, U., Janetta, K. and Bothe, H. (2002). Towards Growth of Arbuscular Mycorrhizal Fungi Independent of a Plant Host. Appl Environ Microbiol. 68(4): 1919–1924.
- Hildebrandt, U., Ouziad, F., Marner, F. J. and Bothe, H. (2006). The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol Lett. 254(2): 258–267.
- Abdellatif, L., Lokuruge, P. and Hamel, C. (2019). Axenic growth of the arbuscular mycorrhizal fungus Rhizophagus irregularis and growth stimulation by coculture with plant growth-promoting rhizobacteria. Mycorrhiza. 29(6): 591–598.
- Yamato, M., Yamada, H., Maeda, T., Yamamoto, K., Kusakabe, R. and Orihara, T. (2022). Clonal spore populations in sporocarps of arbuscular mycorrhizal fungi. Mycorrhiza. 32: 373–385.
- Tsuzuki, S., Handa, Y., Takeda, N. and Kawaguchi, M. (2016). Strigolactone-Induced Putative Secreted Protein 1 Is Required for the Establishment of Symbiosis by the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis. Mol Plant Microbe Interact. 29(4): 277–286.
- Cosme, M., Fernández, I., Declerck, S., van der Heijden, M. G. A. and Pieterse, C. M. J. (2021). A coumarin exudation pathway mitigates arbuscular mycorrhizal incompatibility in Arabidopsis thaliana. Plant Mol Biol. 106: 319–334.
- Serghi, E. U., Kokkoris, V., Cornell, C., Dettman, J., Stefani, F. and Corradi, N. (2021). Homo- and Dikaryons of the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis Differ in Life History Strategy. Front Plant Sci. 12: e715377.
- Furlan, V., Bartschi, H. and Fortin, J. A. (1980). Media for density gradient extraction of endomycorrhizal spores. Trans Br Mycol Soc. 75(2): 336–338.
- Tominaga, T., Ueno, K., Saito, H., Egusa, M., Yamaguchi, K., Shigenobu, S. and Kaminaka, H. (2023). Monoterpene glucosides in Eustoma grandiflorum roots promote hyphal branching in arbuscular mycorrhizal fungi. Plant Physiol. 193(4): 2677–2690.
Article Information
Publication history
Received: May 20, 2024
Accepted: Jul 14, 2024
Available online: Jul 31, 2024
Published: Aug 20, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tominaga, T. and Kaminaka, H. (2024). In Vitro Hyphal Branching Assay Using Rhizophagus irregularis. Bio-protocol 14(16): e5054. DOI: 10.21769/BioProtoc.5054.
- Tominaga, T., Ueno, K., Saito, H., Egusa, M., Yamaguchi, K., Shigenobu, S. and Kaminaka, H. (2023). Monoterpene glucosides in Eustoma grandiflorum roots promote hyphal branching in arbuscular mycorrhizal fungi. Plant Physiol. 193(4): 2677–2690.
Category
Plant Science > Plant physiology > Endosymbiosis
Biological Sciences > Biological techniques > Microbiology techniques
Microbiology > Microbe-host interactions > Fungus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









