- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Ligand-Target Interaction in vitro by Cellular Thermal Shift Assay and Isothermal Dose-response Fingerprint Assay
Published: Vol 14, Iss 15, Aug 5, 2024 DOI: 10.21769/BioProtoc.5047 Views: 4904
Reviewed by: Dipak Kumar PoriaSrajan KapoorGundeep KaurAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Proximity Labelling to Quantify Kv7.4 and Dynein Protein Interaction in Freshly Isolated Rat Vascular Smooth Muscle Cells
Jennifer van der Horst and Thomas A. Jepps
Mar 20, 2024 1562 Views
Purification of Native Acetyl CoA Carboxylase From Mammalian Cells
Yaxue Sun [...] Hongtao Zhu
Feb 20, 2025 2190 Views
Ub-POD: A Ubiquitin-Specific Proximity-Dependent Labeling Technique to Identify E3 Ubiquitin Ligase Substrates in Human Cells
Urbi Mukhopadhyay [...] Sagar Bhogaraju
Jun 20, 2025 2425 Views
Abstract
The cellular thermal shift assay (CETSA) and isothermal dose-response fingerprint assay (ITDRF CETSA) have been introduced as powerful tools for investigating target engagement by measuring ligand-triggered thermodynamic stabilization of cellular target proteins. Yet, these techniques have rarely been used to evaluate the thermal stability of RNA-binding proteins (RBPs) when exposed to ligands. Here, we present an adjusted approach using CETSA and ITDRFCETSA to determine the interaction between enasidenib and RBM45. Our assay is sensitive and time-efficient and can potentially be adapted for studying the interactions of RBM45 protein with other potential candidates.
Key features
• This protocol builds upon the method developed by Molina et al. and extends its application to new protein classes, such as RBPs.
Keywords: Ligand–target interactionGraphical overview
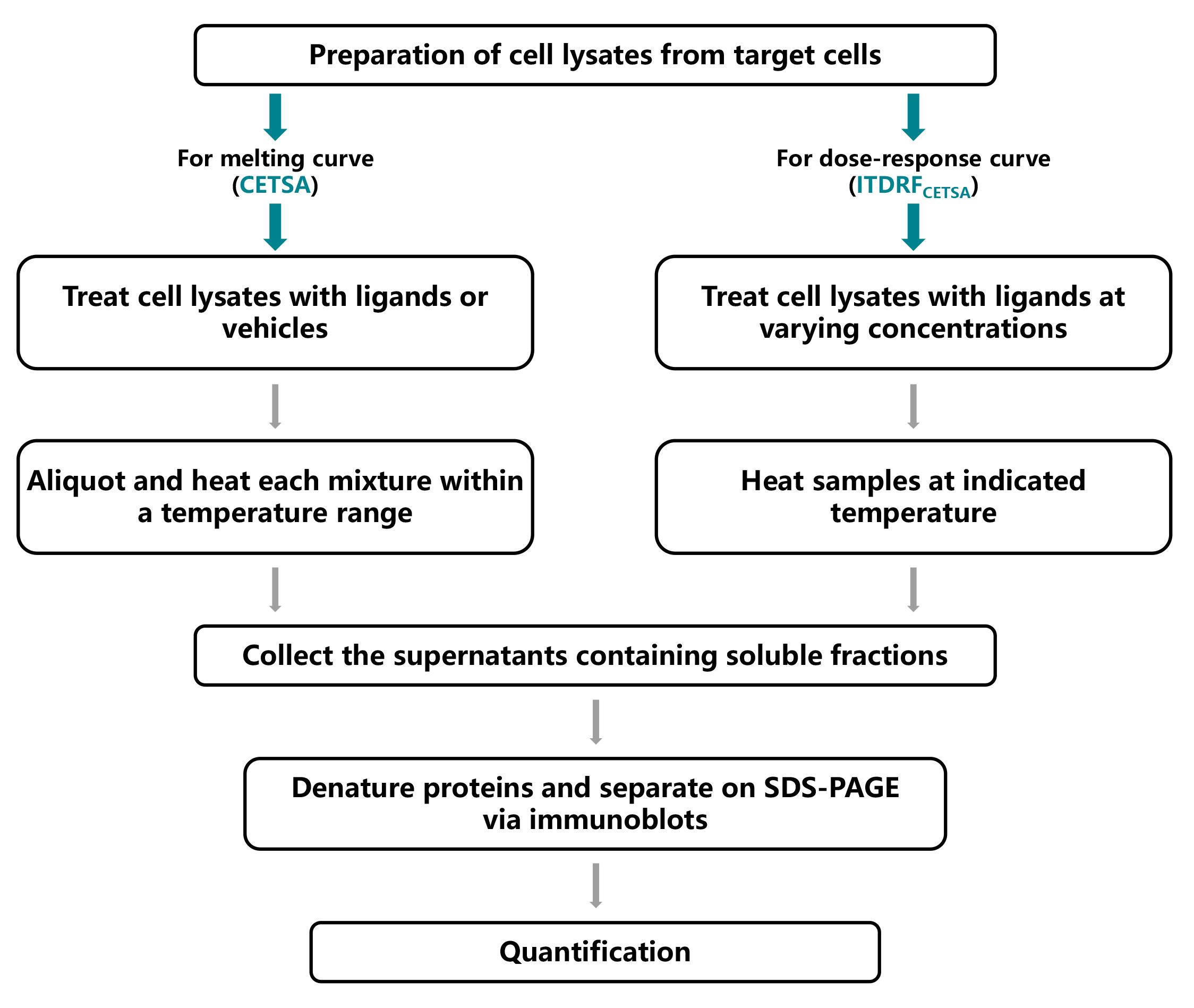
Background
RNA-binding proteins (RBPs) contribute considerably to post-transcriptional regulation; thus, their dysregulation has been linked to a variety of illnesses, including malignancies [1]. Thereafter, targeting RBPs with specific ligands to perturb cancer development has attracted much attention [2]. Recent studies, including ours, have unraveled the role of RNA-binding motif protein 45 (RBM45) in promoting hepatocellular carcinoma (HCC) progression [3,4], highlighting its importance in cancer development. More crucially, our previous work, which combines a molecular docking approach as well as molecular and cellular techniques, identified enasidenib, a previously considered mutant isocitrate dehydrogenase 2 (mIDH2) inhibitor that binds to RBM45. Therefore, a better clarification of the binding affinity between enasidenib and RBM45 would be beneficial for the design of RBM45-targeted therapeutics.
Recently, several label-free techniques have been developed to determine ligand-target engagement using intact cells or cell lysates. These techniques include drug affinity responsive target stability (DARTS) [5], stability of proteins from rates of oxidation (SPROX) [6], and cellular thermal shift assay (CETSA) [7]. Among these, CETSA is more widely applicable since it can determine the specific ligand-induced target thermodynamic stabilization in biological settings such as whole cell lysates, intact cells, or even tissues [7]. Typically, the shift in thermal stability is assessed by quantifying the density of the remaining soluble target proteins using available antibodies via immunoblots analysis. As a result, thermal melt curves are generated, in which the target protein of interest, with or without ligands, is exposed to a panel of temperatures in the CETSA experiment. Alternatively, an isothermal dose-response curve is also generated, in which the target protein is subjected to increasing concentrations of compounds under a constant temperature (a checkpoint at which the unliganded target protein is degraded as determined by the CETSA experiment), referred to as the isothermal dose-response fingerprint assay (ITDRFCETSA) [8]. Undoubtedly, CETSA and ITDRFCETSA techniques offer the opportunity to investigate whether ligands engage their intended targets in complicated environments and determine at what concentration they exert their effects.
Moreover, the CETSA approach has been demonstrated to be effective in determining the thermal stability of enzymes such as dihydrofolate reductase (DHFR) and thymidylate synthase (TS) [7], kinases including cyclin-dependent kinases CDK4 and CDK6 [7], mitogen‐activated protein kinases p38α and ERK1/2 [8]), as well as membrane proteins like solute carriers (SLCs) [9], in the absence and presence of potential ligands. However, the CETSA technique has rarely been applied to assess the thermodynamic stabilization of RBPs when subjected to compounds. Furthermore, a cell-based CETSA decreases the sensitivity to the effects of low-affinity ligands due to drug dissociation from the target after cell lysis, making lysate-based CETSA a preferred choice [9]. Here, we report the modified cell lysate CETSA and ITDRFCETSA techniques for determining the interaction between enasidenib and RBM45. Our assay is sensitive and time saving and can potentially be adapted to investigate RBM45 protein with other candidate ligands.
Materials and reagents
Cell line
Human liver cancer cell line SK-HEP-1 was purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China).
Materials
1.5 mL tubes (Corning Axygen, catalog number: MCT-150-C)
200 μL tubes (Corning Axygen, catalog number: PCR-02-C)
10 cm dishes (Corning Axygen, catalog number: 430167)
Reagents
MEM medium (Thermo Fisher Scientific, catalog number: 61100061)
Fetal bovine serum (FBS) (Royacel SERUM, catalog number: RYS-KF22-01)
Non-essential amino acids (100×) (Thermo Fisher Scientific, catalog number: 11140050)
Sodium pyruvate 100 mM solution (Thermo Fisher Scientific, catalog number: 11360070)
Penicillin-Streptomycin (100×) (New Cell & Molecular Biotech Co., Ltd., catalog number: C100C5)
1× phosphate buffered saline (PBS), pH 7.4 (Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd, catalog number: ZQ-1300)
0.25% trypsin-EDTA (New Cell & Molecular Biotech Co., Ltd., catalog number: C125C1)
RIPA lysis buffer (Beyotime Biotechnology, catalog number: P0013B)
ProtLytic protease inhibitor cocktail (EDTA-Free, 100× in DMSO) (New Cell & Molecular Biotech Co., Ltd., catalog number: P001)
Liquid nitrogen
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 67-68-5)
Enasidenib (Jiangsu Aikon Biopharmaceutical R&D Co., Ltd., catalog number: 4409639)
Bicinchoninic acid (BCA) Protein Assay kit (enhanced) (Beyotime Biotechnology, catalog number: P0009)
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer, 5× (Beyotime Biotechnology, catalog number: P0015)
Rabbit-polyclonal-anti-RBM45 (ABclonal, catalog number: A13843)
Goat anti-Rabbit IgG (H+L) secondary antibody, HRP (Thermo Fisher Scientific, catalog number: 31460)
Enhanced chemiluminescence (ECL) detection reagent (Vazyme, catalog number: E411-03/04/05)
Equipment
Gene amplification instrument (Bioer Technology, model: G1000 GeneExplorer)
Metal Bath (Labnet)
Electrophoresis systems & transfer equipment (Bio-Rad)
Image analyzer (Tanon, model: 5200)
Refrigerated high-speed benchtop centrifuge (Thermo Fisher Scientific, model: 75005289)
Rotating incubator (Haimen Kylin-Bell Lab Instruments Co., Ltd., model: QB-228)
Software and datasets
ImageJ 1.46r
Microsoft Excel
GraphPad Prism 9.0.0
Procedure
Preparation of cell lysates for CETSA
Note: SK-HEP-1 cells were cultured in 10 cm dishes in MEM medium supplemented with 10% FBS, 1% non-essential amino acids, 1 mM sodium pyruvate, and 1% penicillin-streptomycin at 37 °C with 5% CO2 atmosphere. Cells were characterized by short tandem repeat profiling and tested for mycoplasma contamination upon receipt. Cells were split at a 1:3 ratio when they reached 80%–90% confluence.
Digest cells with 0.25% trypsin-EDTA when reaching the indicated confluence (suitable for two dishes, approximately 4 × 106 cells per dish). Transfer to two 1.5 mL tubes separately and pellet them by centrifugation (1,000× g for 5 min at room temperature).
Remove the supernatant, wash cells with cold PBS once, and collect cell pellets by centrifugation (1,000× g for 5 min at room temperature).
Resuspend cell pellets with 1 mL of RIPA lysis buffer containing protease inhibitor cocktail (1×) to each cell pellet.
Fast freeze the above-mentioned cells using liquid nitrogen and thaw (on ice). Repeat the freeze-thaw cycle three times.
Separate the soluble fractions (lysates) from the cell debris by centrifugation (20,000× g for 20 min at 4 °C) and determine the protein concentration using BCA assay kit (Protein concentration in cell lysates usually ranges between 0.1 and 2.0 mg/mL, depending on the abundance of protein expression).
Divide cell lysates evenly into two 1.5 mL tubes, incubate with enasidenib (final concentration: 30 μM) or an equivalent amount of DMSO, and rotate at room temperature for 1 h.
Divide each mixture into 100 μL aliquots in nine 200 μL tubes.
Heat enasidenib or DMSO-treated lysates at the indicated temperatures (40–70 °C) for 4 min using a G1000 GeneExplorer; then, cool the samples at room temperature for 3 min.
Note: The appropriate temperatures need to be adjusted according to the literature or pre-experiments. Here, specific settings were 40, 44, 48, 52, 57, 62, 65, 68, and 70 °C for 4 min, then switch to 25 °C for 3 min.
Collect the supernatants containing soluble fractions by centrifugation (20,000× g for 20 min at 4 °C).
Preparation of cell lysates for ITDRFCETSA
Note: For ITDRFCETSA, it is necessary to determine the temperature at which the unliganded protein starts to degrade, as determined by the CETSA experiment (i.e., the chosen temperature at which the majority of the unliganded protein is degraded and cannot be detected). The steps are nearly identical to those outlined in section A with the following exceptions:
After cell lysates are prepared following liquid nitrogen freeze-thaw and centrifugation, divide cell lysates evenly into four 1.5 mL tubes, incubate with enasidenib (final concentrations: 3, 10, and 30 μM) or DMSO, and rotate at room temperature for 1 h.
Transfer each mixture into 100 μL aliquots in four 200 μL tubes.
Heat various concentrations of enasidenib or DMSO-treated lysates on a G1000 GeneExplorer at the indicated temperature according to CETSA results (here, we chose 62 °C) for 4 min and then cool the samples at room temperature for 3 min.
Immunoblots of cell lysates for CETSA or ITDRFCETSA
Mix one volume of SDS-PAGE sample loading buffer 5× with four volumes of protein sample (here, we added 25 μL of loading buffer into 100 μL of cell lysates).
Heat and denature proteins in a metal bath at 99 °C for 5 min.
Separate the prepared protein samples (10–20 μg) on a 10% SDS-PAGE gel for immunoblots analysis. After transferring proteins from gels to the polyvinylidene fluoride (PVDF) membrane, block the membrane using a 5% milk solution. Incubate the membrane with a primary antibody (anti-RBM45) at a dilution of 1:3,000 overnight at 4 °C, followed by incubation with a secondary antibody (anti-rabbit-HRP) at a dilution of 1:5,000 for 1 h at room temperature. A chemiluminescent signal was generated with ECL detection reagent and captured using an Image analyzer.
Note: To precisely investigate whether enasidenib increases the thermal stabilization of RBM45 protein, the bands of RBM45 originated from enasidenib or DMSO-treated samples were put together upon exposure to maintain the same exposure conditions (with an exposure time ranging from 1 to 5 min).
Data analysis
For CETSA, densitometry analysis of the bands from immunoblots was performed with ImageJ. Specifically, the image background was subtracted (set the rolling ball radius to 50 pixels and choose the Light background option), regions of measurement were selected, and the grey signals of each lane were quantified. Then, the ratio of RBM45 at each indicated temperature was normalized to that at 40 °C (see Figure 1). The relative band intensity at different temperatures was plotted using GraphPad, and the thermal stability of RBM45 was measured by the temperature-dependent cellular thermal shift assay. Notably, the temperature corresponding to the protein that cannot be detected is selected to ITDRFCETSA, and the thermal stability of RBM45 was thus measured by the concentration-dependent cellular thermal shift assay at 62 °C.
Immunoblots and quantification images have been published in Oncogene [4]; for details, please refer to Figure 7e of the mentioned publication.
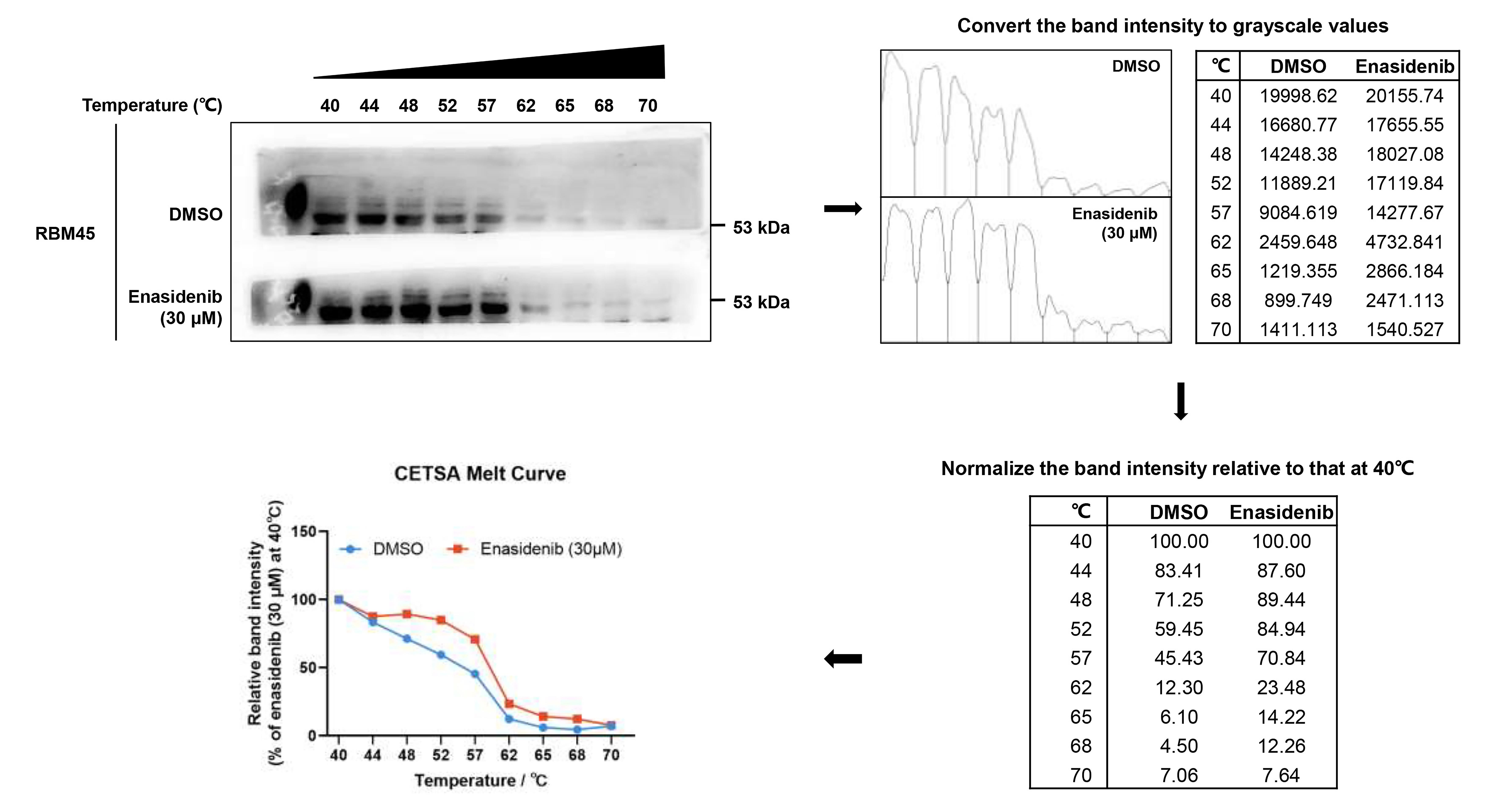
Figure 1. Quantitative analysis workflow for cellular thermal shift assay (CETSA)
Validation of protocol
This protocol or parts of it has been used and validated in the following research article(s):
Du et al. [4]. RNA binding motif protein 45-mediated phosphorylation enhances protein stability of ASCT2 to promote hepatocellular carcinoma progression. Oncogene (Figure 7, panel e).
General notes and troubleshooting
In the case of CETSA, it is essential to make adjustments to the temperatures employed for a specific protein based on relevant literature or pre-experimental findings. To precisely investigate whether potential ligands enhance the thermal stabilization of the target protein, it is recommended to group together the target protein bands originating from both ligand- and vehicle-treated samples during exposure in order to maintain consistent exposure conditions and enable valid comparisons between different treatment groups.
Regarding ITDRFCETSA, the selected temperature should be modified based on the specific characteristics of the protein and ligand being studied. It is crucial to adapt the temperature based on the results obtained from CETSA experiments.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Nos. 82070883, 82273982, 81872892), the Natural Science Foundation of Jiangsu Province, China (BK20221525) and Scientific Research Foundation for high-level faculty, China Pharmaceutical University. This protocol has been previously described and validated in Oncogene [4].
Competing interests
The authors declare no competing interests.
References
- Hashimoto, S. and Kishimoto, T. (2022). Roles of RNA-binding proteins in immune diseases and cancer. Semin Cancer Biol. 86: 310–324.
- Kathman, S. G., Koo, S. J., Lindsey, G. L., Her, H. L., Blue, S. M., Li, H., Jaensch, S., Remsberg, J. R., Ahn, K., Yeo, G. W., et al. (2023). Remodeling oncogenic transcriptomes by small molecules targeting NONO. Nat Chem Biol. 19(7): 825–836.
- Wang, C., Chen, Z., Yi, Y., Ding, Y., Xu, F., Kang, H., Lin, K., Shu, X., Zhong, Z., Zhang, Z., et al. (2023). RBM45 reprograms lipid metabolism promoting hepatocellular carcinoma via Rictor and ACSL1/ACSL4. Oncogene. 43(5): 328–340.
- Du, D., Qin, M., Shi, L., Liu, C., Jiang, J., Liao, Z., Wang, H., Zhang, Z., Sun, L., Fan, H., et al. (2023). RNA binding motif protein 45-mediated phosphorylation enhances protein stability of ASCT2 to promote hepatocellular carcinoma progression. Oncogene. 42(42): 3127–3141.
- Lomenick, B., Hao, R., Jonai, N., Chin, R. M., Aghajan, M., Warburton, S., Wang, J., Wu, R. P., Gomez, F., Loo, J. A., et al. (2009). Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci USA. 106(51): 21984–21989.
- Strickland, E. C., Geer, M. A., Tran, D. T., Adhikari, J., West, G. M., DeArmond, P. D., Xu, Y. and Fitzgerald, M. C. (2012). Thermodynamic analysis of protein-ligand binding interactions in complex biological mixtures using the stability of proteins from rates of oxidation. Nat Protoc. 8(1): 148–161.
- Molina, D. M., Jafari, R., Ignatushchenko, M., Seki, T., Larsson, E. A., Dan, C., Sreekumar, L., Cao, Y. and Nordlund, P. (2013). Monitoring Drug Target Engagement in Cells and Tissues Using the Cellular Thermal Shift Assay. Science. 341(6141): 84–87.
- Jafari, R., Almqvist, H., Axelsson, H., Ignatushchenko, M., Lundbäck, T., Nordlund, P. and Molina, D. M. (2014). The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 9(9): 2100–2122.
- Hashimoto, M., Girardi, E., Eichner, R. and Superti-Furga, G. (2018). Detection of Chemical Engagement of Solute Carrier Proteins by a Cellular Thermal Shift Assay. ACS Chem Biol. 13(6): 1480–1486.
Article Information
Publication history
Received: Jan 12, 2024
Accepted: Jun 28, 2024
Available online: Jul 19, 2024
Published: Aug 5, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Du, D., Yuan, S. and Xiong, J. (2024). Determination of Ligand-Target Interaction in vitro by Cellular Thermal Shift Assay and Isothermal Dose-response Fingerprint Assay. Bio-protocol 14(15): e5047. DOI: 10.21769/BioProtoc.5047.
Category
Molecular Biology > Protein > Protein-protein interaction
Cancer Biology > Cancer biochemistry > Protein
Drug Discovery > Drug Design
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link