- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Social Stimulation Paradigm to Ameliorate Memory Deficit in Alzheimer's Disease
Published: Vol 14, Iss 15, Aug 5, 2024 DOI: 10.21769/BioProtoc.5046 Views: 1768
Reviewed by: Salim GasmiRachael E. HokensonAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1290 Views
The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1804 Views
Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1465 Views
Abstract
Alzheimer's disease (AD) poses a global health threat, progressively robbing patients of their memory and cognitive abilities. While it is recognized that meaningful social contact can alleviate the symptoms of dementia in AD patients, the precise mechanisms by which social stimulation mitigates AD symptoms remain poorly understood. We found that social interaction with novel mice, also known as novel social, simulated meaningful socializing. Therefore, we developed the multiple novel social (MNS) stimulation paradigm to train AD model mice and found that MNS effectively alleviated cognitive deficits in AD mice. This discovery not only opens up a new avenue for investigating the relationship between social stimulation and Alzheimer's disease but also lays the groundwork for delving into the underlying mechanisms, thereby providing crucial theoretical support for developing novel strategies to treat Alzheimer's disease.
Key features
• Designing a new social stimulation method to simulate meaningful social interactions in daily life.
• The MNS stimulation protocol spans 14 days, with one novel mouse introduced to the subject mice each day.
• The subjects were 2.5-month-old FAD4T mice, simulating patients with mild cognitive impairment (MCI).
• Results of behavioral tests confirm the efficacy of MNS in reducing cognitive deficits in the AD model.
Keywords: Alzheimer's diseaseGraphical overview
Background
Alzheimer’s disease (AD) is one of the most influential and common neurodegenerative diseases in the world. It also ranks as the fifth leading cause of mortality among elderly individuals worldwide [1,2]. Memory and cognitive decline are recognized as the most incapacitating aspects of Alzheimer's disease [3]. In recent years, there have been emerging drugs in clinical practice that show promise in alleviating the dementia symptoms associated with Alzheimer’s disease [4–6]. However, the precise effects and potential side effects of these medications on cognitive function and disease progression are still being closely monitored. Non-pharmacological therapies have also been increasingly recognized and valued. Non-pharmacological treatments, such as engaging in physical activities, cognitive exercises, social interactions, and managing vascular and metabolic risks, are integrated alongside drug therapies for Alzheimer's disease [2].
Despite the recognition of non-pharmacological interventions, there remains a research gap regarding the mechanisms underlying the benefits of social activities. Studies have shown that an enriched social network and frequent social contact can prevent dementia and increase patients' cognitive reserve [7–11]. Additionally, social isolation exacerbates anxiety and asymmetrical brain atrophy in Alzheimer's patients [12]. Conversely, complex social interactions can improve episodic memory, enhance brain reserve, and reduce the risk of dementia [13]. Although the benefits of social stimulation for Alzheimer's patients have been well established, the specific biological mechanisms remain unclear.
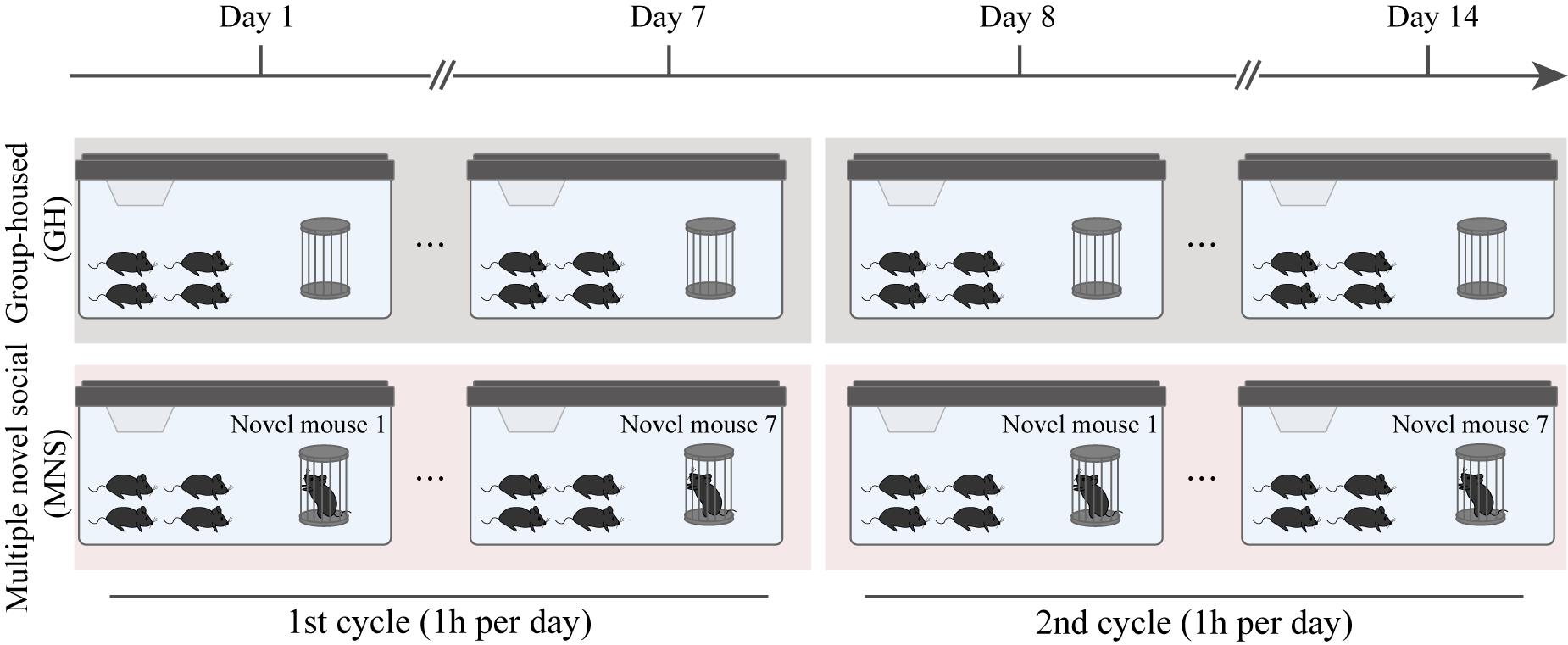
We found that a single novel social (SNS) stimulation with an unfamiliar mouse effectively activated the α-secretase activity in the ventral hippocampus of both wildtype (WT) and AD model mice and inhibited the amyloidogenic-cleavage pathway but was not enough to reduce the accumulation of Aβ in AD model mice [14]. Therefore, we implemented a 14-day multiple novel social (MNS) protocol to extend the intervention effect of this paradigm by increasing the number of social stimuli, allowing the subject mice to experience 14 novel social interactions. Given that the social memory of mice is transient, typically lasting only a few days [15], we cycled through seven different novel mice (designated N1–N7) for the first and second seven-day periods of the protocol. It should be noted that we also recommend using 14 different unfamiliar mice for the 14-day MNS protocol. As a control, group-housed (GH) subject mice were only able to interact with their familiar littermates.
After the MNS treatment, through the novel object recognition test and 3-chamber test, we found that the MNS stimulation paradigm significantly improved the cognitive ability and social memory of AD mice. Therefore, the MNS stimulation paradigm represents a promising method to alleviate dementia symptoms in Alzheimer’s disease.
Materials and reagents
Mice [all animals were housed in standard laboratory conditions with ad libitum access to food and water, as well as a 12/12 h light/dark cycle (light: 7:00 AM–7:00 PM), a temperature range of 22–26 °C, and a humidity level of 55%–60%. The mice used in this study were cared for in accordance with the authorized protocols of Southeast University, Nanjing, China)].
FAD4T mice [C57BL/6 genetic background, 10–12 weeks old. FAD4T mice co-expressed human APP with the Swedish (KM670/671NL) and India (V717F) variants, together with human mutant PS1 (M146L, L286V), driven by the mouse Thy1 promoter (Gempharmatech Co., Ltd, catalog number: T053302)]
Mice used for novel stimulation (N1–N7) and social test (S1–S2) (C57BL/6J genetic background, 10–12 weeks old, 22–25 g) (Gempharmatech Co., Ltd, catalog number: N000013, Jiangsu, China)
Nitrile laboratory gloves (Guangming, China)
Paper towels (Breeze, China)
75% Ethanol (Alladin, catalog number: A171299)
Equipment
Mouse breeding room with constant temperature system, ventilation system, and automatic light timing system
Home cage (380 mm × 180 mm × 170 mm, with a height under the partition bar inside the cage of 13 cm, which meets the requirements of the GB14925 national standard. It can accommodate 5–8 experimental mice weighing 20–30 g) (SHINVA, China)
Small electronic scale (127 mm × 106 mm × 19 mm) (Kubei, China)
Metal pen holder (golden, diameter 8 cm, height 10.3 cm, weight 140 g) (Wuling, China)
Timer (82 mm × 62 mm × 24 mm) (Anytime, model: XL-009B)
Spray bottle (500 mL) (LDPE, Sangon Biotech, catalog number: F505008)
Objects for novel object recognition (NOR) test (two bright-red metal cubes, 83 mm × 83 mm × 83 mm, were used as similar objects; one light-purple metal cylinder, 85 mm in diameter and 84 mm in height, was used as the novel object) (Aiziling, China)
Mouse behavioral testing room with constant temperature system, ventilation system, and automatic light timing system
Behavioral testing apparatus (Xinruan, China)
NOR apparatus
A square Plexiglas box (50 cm × 50 cm × 50 cm) with four 50 cm-high sidewalls and an open roof. In addition, two metal boxes with heavy objects inside were used as similar objects, which maintained consistency in size, color, and shape. Another metal box with a heavy object, similar in size but different in color and shape, was used as the novel object
3-chamber apparatus
A rectangular box made of Plexiglas (50 cm × 25 cm) with a detachable base, creating three distinct compartments divided by two partitions. Each partition contained a small door (10 cm × 5 cm) to facilitate mouse’s movement between the different chambers. In addition, there were two metal pen holders that were identical in size, shape, and color, used for restraining stranger mice.
Computer (Lenovo, model number: Tianyi 510pro-14IMB)
Camera (MOKOSE, model number: HDC10)
Software and datasets
Video tracking and analyzing software (Noldus, EthoVision XT 13 software)
Statistical analysis software (Prism 8.0.1, GraphPad, https://www.graphpad.com/)
Procedure
Pre-experimental preparation
Choose FAD4T mice that are 2.5 months old, ensuring that they exhibit standard body size and good health. Randomly allocate them to control and experimental groups, with each group maintaining 3–4 mice per cage.
Prepare seven cages for the novel mice that will be used for novel social stimulation. Each cage should contain about 3–5 mice. These novel mice should be from different parents, similar in age and body size to the FAD4T mice, and of the same sex. Place the cages at various locations in the breeding room to avoid olfactory similarity between the mice in each cage (these cages should be spaced at least three cage positions apart from each other).
Prepare 75% ethanol, paper towels, and metal pen holders. First, thoroughly clean the metal pen holder with 75% ethanol; then, carefully wipe it dry with a paper towel to ensure that no odor or impurities remain inside the pen holder.
To acclimate the FAD4T mice to the pen holders, place clean pen holders in their cages at 10:00 AM each day for 1 h, starting three days before the social stimulation is scheduled to begin.
Multiple novel social stimulation protocol
On the first day of social stimulation (Day 1), at 1:00 PM, place a clean pen holder in the cages of both the control and experimental FAD4T mice groups. Meanwhile, introduce the first wild-type novel mouse into the pen holder in the experimental FAD4T mice's cage, serving as the novel social stimulation “novel mouse” (N1). After 1 h, remove the N1 and pen holder, wash it with 75% ethanol, and then dry it with a paper towel.
On Day 2, repeat the procedure at 1:00 PM, introducing another wild-type novel mouse as the second social stimulation mouse (N2). After 1 h, remove the N2 and clean the pen holder with 75% ethanol, followed by drying it with a paper towel.
Continue this sequence and stimulate the subject mice with novel mice (N3–N7) daily until Day 7.
On Day 8, at 1:00 PM, start a new cycle of novel social stimulation starting from N1, which was used for novel stimulation on Day 1.
Repeat the novel social stimuli with N2–N7 from Day 9 to Day 14.
Post-stimulation assessment of cognitive ability and memory in FAD4T mice
Cognitive ability assessment (novel object recognition, NOR) (Figure 1)
Training phase
Individually place each mouse into the NOR apparatus, which contains two identical objects (A and A’). Give the mouse 10 min to explore these objects and utilize EthoVision XT 13 software to record and analyze the behavior of each mouse with a heat map. Then, return it to the home cage.
Testing phase.
Twenty-four hours later, replace object A’ with a new, distinct object (B), which differs in both color and shape. Individually place each mouse into the arena and provide a 5 min period for it to freely explore. Utilize EthoVision XT 13 software to record and analyze the behavior of each mouse with a heat map and return it to home cage.
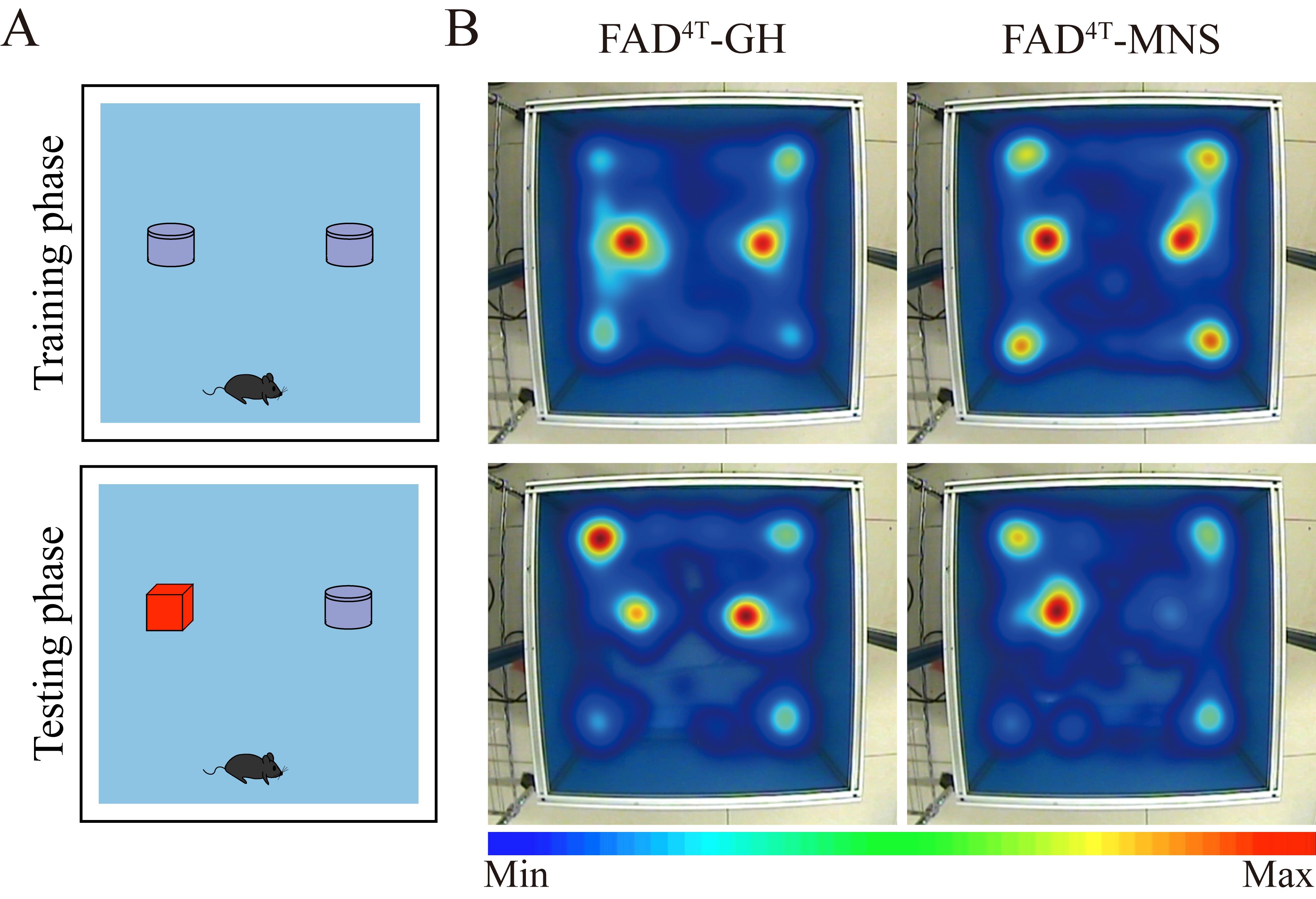
Figure 1. Multiple novel social (MNS) stimulation rescues novel object recognition (NOR) impairment in FAD4T mice. A. Illustration of NOR test. B. Heat map of mouse movement in NOR test, with the training phase on top and the testing phase below. The FAD4T-MNS showed higher exploratory behavior toward the new object compared to the control group.Social memory assessment (3-chamber test) (Figure 2)
In the first stage, place the test mouse in the central chamber, with two wired metal pen holders positioned in the bottom-left and top-right corners of the apparatus. Allow each mouse 10 min to freely explore. Utilize EthoVision XT 13 software to record and analyze the behavior of each mouse with a heat map. Following habituation, gently return the test mouse to the central chamber, with both side doors open.
In the second stage (sociability test), place a stranger mouse (S1) inside one of the metal pen holders. Then, give the subject mouse another 10 min to explore the arena. Utilize EthoVision XT 13 software to record and analyze the behavior of each mouse with a heat map.
In the third stage, return the test mouse to the central chamber for 10 min with the side doors closed. Utilize EthoVision XT 13 software to record and analyze the behavior of each mouse with a heat map.
In the fourth stage (social memory test), introduce the second stranger mouse (S2) into the previously empty metal pen holder, and allow the subject mouse to explore the entire apparatus for 10 min. Utilize EthoVision XT 13 software to record and analyze the behavior of each mouse with a heat map.
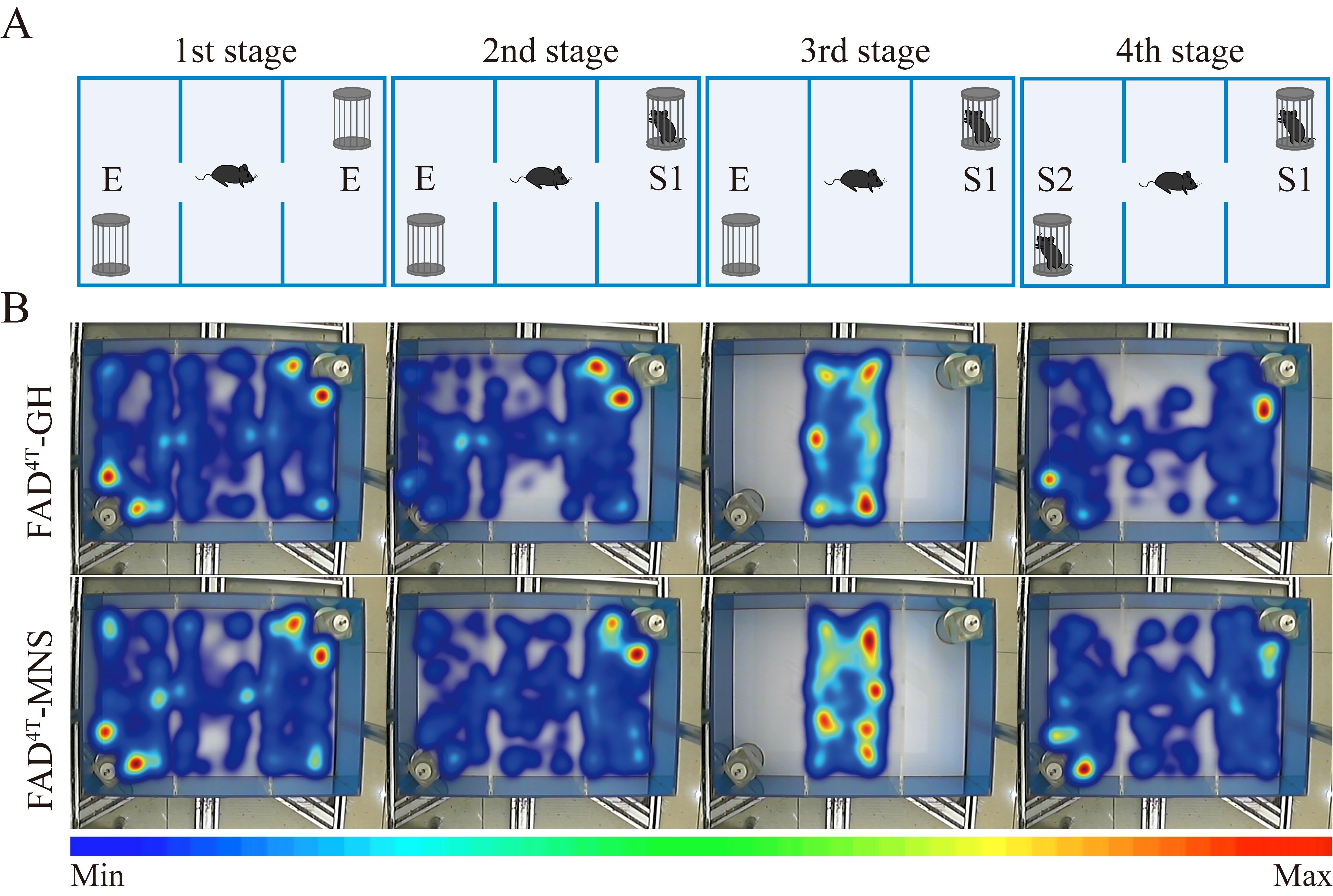
Figure 2. Multiple novel social (MNS) stimulation rescues social memory in FAD4T mice. A. Illustration of the different stages of the 3-chamber test. B. Heat map of mouse movement in the 3-chamber test. The two groups of mice showed normal social abilities, and MNS stimulation increased the exploration time of FAD4T mice toward S2 mice.
Data analysis
Heatmap is indeed a very effective data visualization tool, especially in behavioral and neuroscience research, as it can help researchers quickly and intuitively understand the activity patterns of mice in complex environments. Through heatmaps, researchers can observe the activity of mice in specific experimental settings, such as their interest in certain objects or the time spent exploring certain areas. In behavioral research, heatmaps are usually generated by superimposing the mouse’s movement trajectory data on the floor plan of the experimental environment. The color depth of each point or area represents the duration of the mouse’s stay at that location, or the frequency of the mouse passing through that location. In this way, researchers can quickly identify the areas that mice prefer to explore (usually shown in dark red or warm tones) and the areas that are hardly visited at all (usually shown in blue or cold tones). Therefore, by observing the color and depth of the heatmap, we can understand the exploration time and tendency of mice for different objects or unfamiliar mice, and judge their cognitive and social memory levels.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article:
Ren et al. [14]. Novel Social Stimulation Ameliorates Memory Deficit in Alzheimer's Disease Model through Activating α-Secretase. Journal of Neuroscience (Figure 5, panel A; Figure 6, panel F–L).
General notes and troubleshooting
After weaning (21 days after birth), the male and female mice are separated for breeding to prevent mating or pregnancy from affecting the experiment.
After the social stimulation program ends, the experimental mice and their cages are placed in the room where the behavioral tests are conducted to adapt to the new environment. Behavioral testing begins the following day.
The mouse breeding room and mouse behavioral testing room have a circadian rhythm with 12 h of light and 12 h of darkness (light: 7:00 AM–7:00 PM). Lighting intensity is around 100–200 lux, which is similar to the brightness of a typical office environment. The light quality should be white or cool-colored to avoid disrupting the mice’s circadian rhythms.
When moving mice from one location to another, use soft, padded forceps or gloves to avoid injuring the animals. Avoid sudden or jerky movements that can startle or distress the mice.
When conducting behavioral tests, such as novel object recognition or 3-chamber tests, ensure that the mice are placed gently into the testing apparatus and given time to acclimate before the experiment begins.
When observing the mice during the experiment, do so without causing them unnecessary stress. Avoid sudden movements or loud noises that can disrupt their behavior.
To minimize the stress and pressure on the mice during the stimulation or behavioral testing experiments, it is advisable to schedule these activities during periods of the day when the animals are known to exhibit lower corticosterone levels (8:00 AM–3:00 PM) [16,17]. This approach helps to ensure that the physiological responses of the mice to the experimental conditions are more representative of their baseline state and reduces the potential for stress-induced interference with the experimental outcomes.
If an animal demonstrates a pronounced bias or preference toward the right or left zone during the training phase or habituation phase, it is strongly advised to exclude it from the subsequent trials.
If during the novel object recognition phase, the total exploration time of the mouse for the objects does not exceed 8 s, the data for that mouse will be excluded.
The “stranger mice” in the 3-chamber test are not the same as the mice used for social stimulation. We use “stranger mice” and “novel mice” to distinguish between them.
In the 3-chamber test, the stages are conducted sequentially, one after the other. Each stage lasts 10 min, for a total of 40 consecutive minutes.
All the experiments in this research were completed in double-blind conditions.
Acknowledgments
This work was supported by grants from STI2030-Major Projects (2022ZD0205900, A.L.; 2021ZD0204000, W.X. and A.L.), Natural Science Foundation of China (NSFC 91632201, W.X.; NSFC 31970958, A.L.), Natural Science Foundation of Jiangsu Province (BK20211561, A.L.), Basic Research Project of Leading Technology of Jiangsu Province (BK20192004, A.L.), Shenzhen Science and Technology Innovation Foundation (2021Szvup028, A.L.), Guangdong Key Project (2018B030335001, W.X.). This protocol was adapted from the publication Ren et al. [14].
Competing interests
The authors declare that no competing interests exist.
Ethical considerations
All procedures involving mice were performed according to and approved by the Animal Care Committee at Southeast University, China.
References
- Alzheimer's Association. (2023). 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 19(4):1598–1695.
- Scheltens, P., De Strooper, B., Kivipelto, M., et al. (2021). Alzheimer's disease. Lancet. 397(10284): 1577–1590.
- Goedert, M. (2015). Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 349(6248): e1255555.
- Dhillon, S. (2021). Aducanumab: First Approval. Drugs. 81(12): 1437–1443.
- Hoy, S. M. (2023). Lecanemab: First Approval. Drugs. 83(4): 359–365.
- Reardon, S. (2023). Alzheimer’s drug donanemab: what promising trial means for treatments. Nature. 617(7960): 232–233.
- Cai, S. (2021). Does social participation improve cognitive abilities of the elderly? J Popul Econ. 35(2): 591–619.
- Fratiglioni, L., Wang, H. X., Ericsson, K., Maytan, M. and Winblad, B. (2000). Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 355(9212): 1315–1319.
- Sommerlad, A., Kivimäki, M., Larson, E. B., Röhr, S., Shirai, K., Singh-Manoux, A. and Livingston, G. (2023). Social participation and risk of developing dementia. Nat Aging. 3(5): 532–545.
- Sommerlad, A., Sabia, S., Singh-Manoux, A., Lewis, G. and Livingston, G. (2019). Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLoS Med. 16(8): e1002862.
- Zhou, Z., Wang, P. and Fang, Y. (2018). Social Engagement and Its Change are Associated with Dementia Risk among Chinese Older Adults: A Longitudinal Study. Sci Rep. 8(1): 1551.
- Muntsant, A. and Giménez-Llort, L. (2020). Impact of Social Isolation on the Behavioral, Functional Profiles, and Hippocampal Atrophy Asymmetry in Dementia in Times of Coronavirus Pandemic (COVID-19): A Translational Neuroscience Approach. Front Psychiatry. 11: e572583.
- Coleman, M. E., Roessler, M. E. H., Peng, S., Roth, A. R., Risacher, S. L., Saykin, A. J., Apostolova, L. G. and Perry, B. L. (2023). Social enrichment on the job: Complex work with people improves episodic memory, promotes brain reserve, and reduces the risk of dementia. Alzheimers Dement. 19(6): 2655–2665.
- Ren, Q., Wang, S., Li, J., Cao, K., Zhuang, M., Wu, M., Geng, J., Jia, Z., Xie, W., Liu, A., et al. (2024). Novel social stimulation ameliorates memory deficit in Alzheimer's disease model through activating α-secretase. J Neurosci.: e1689232024.
- Wu, X., Morishita, W., Beier, K. T., Heifets, B. D. and Malenka, R. C. (2021). 5-HT modulation of a medial septal circuit tunes social memory stability. Nature. 599(7883): 96–101.
- Barriga, C., Martín, M. I., Tabla, R., Ortega, E. and Rodríguez, A. B. (2001). Circadian rhythm of melatonin, corticosterone and phagocytosis: effect of stress. J Pineal Res. 30(3): 180–187.
- Gong, S., Miao, Y. L., Jiao, G. Z., Sun, M. J., Li, H., Lin, J., Luo, M. J. and Tan, J. H. (2015). Dynamics and Correlation of Serum Cortisol and Corticosterone under Different Physiological or Stressful Conditions in Mice. PLoS One. 10(2): e0117503.
Article Information
Publication history
Received: May 2, 2024
Accepted: Jul 3, 2024
Available online: Jul 19, 2024
Published: Aug 5, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ren, Q., Wang, S., Xie, W. and Liu, A. (2024). A Social Stimulation Paradigm to Ameliorate Memory Deficit in Alzheimer's Disease. Bio-protocol 14(15): e5046. DOI: 10.21769/BioProtoc.5046.
- Ren, Q., Wang, S., Li, J., Cao, K., Zhuang, M., Wu, M., Geng, J., Jia, Z., Xie, W., Liu, A., et al. (2024). Novel social stimulation ameliorates memory deficit in Alzheimer's disease model through activating α-secretase. J Neurosci.: e1689232024.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link