- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
An Experimental Protocol for the Boyden Chamber Invasion Assay With Absorbance Readout
Published: Vol 14, Iss 15, Aug 5, 2024 DOI: 10.21769/BioProtoc.5040 Views: 2756
Reviewed by: Valérian DORMOYChhuttan L MeenaSalah Boudjadi

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Analysis of Random Migration of Cancer Cells in 3D
Sai P. Visweshwaran and Alexis Gautreau
Jan 5, 2020 6592 Views

Studying Chemotactic Migration in Dunn Chamber: An Example Applied to Adherent Cancer Cells
Khedidja Benseddik and Kossay Zaoui
Feb 5, 2022 2937 Views
Spherical Invasion Assay: A Novel Method to Measure Invasion of Cancer Cells
Stephen D. Richbart [...] Piyali Dasgupta
Feb 20, 2022 5407 Views
Abstract
The phenomenon of cell invasion is an essential step in angiogenesis, embryonic development, immune responses, and cancer metastasis. In the course of cancer progression, the ability of neoplastic cells to degrade the basement membrane and penetrate neighboring tissue (or blood vessels and lymph nodes) is an early event of the metastatic cascade. The Boyden chamber assay is one of the most prevalent methods implemented to measure the pro- or anti-invasive effects of drugs, investigate signaling pathways that modulate cell invasion, and characterize the role of extracellular matrix proteins in metastasis. However, the traditional protocol of the Boyden chamber assay has some technical challenges and limitations. One such challenge is that the endpoint of the assay involves photographing and counting stained cells (in multiple fields) on porous filters. This process is very arduous, requires multiple observers, and is very time-consuming. Our improved protocol for the Boyden chamber assay involves lysis of the dye-stained cells and reading the absorbance using an ELISA reader to mitigate this challenge. We believe that our improved Boyden chamber methodology offers a standardized, high-throughput format to evaluate the efficacy of various drugs and test compounds in influencing cellular invasion in normal and diseased states. We believe that our protocol will be useful for researchers working in the fields of immunology, vascular biology, drug discovery, cancer biology, and developmental biology.
Key features
• Measurement of tumor invasion using human cancer cells.
• Ability to measure the pro-invasive/anti-invasive activity of small molecules and biological modifiers.
• Measurement of chemotaxis, chemokines, trafficking of immune cells, and proteolytic activity of matrix metalloproteinases, lysosomal hydrolysates, collagenases, and plasminogen activators in physiological and pathological conditions.
• Investigation of the role of extracellular matrix proteins in the crosstalk between endothelial, epithelial, muscle, or neuronal cells and their adjacent stroma.
Keywords: InvasionGraphical overview
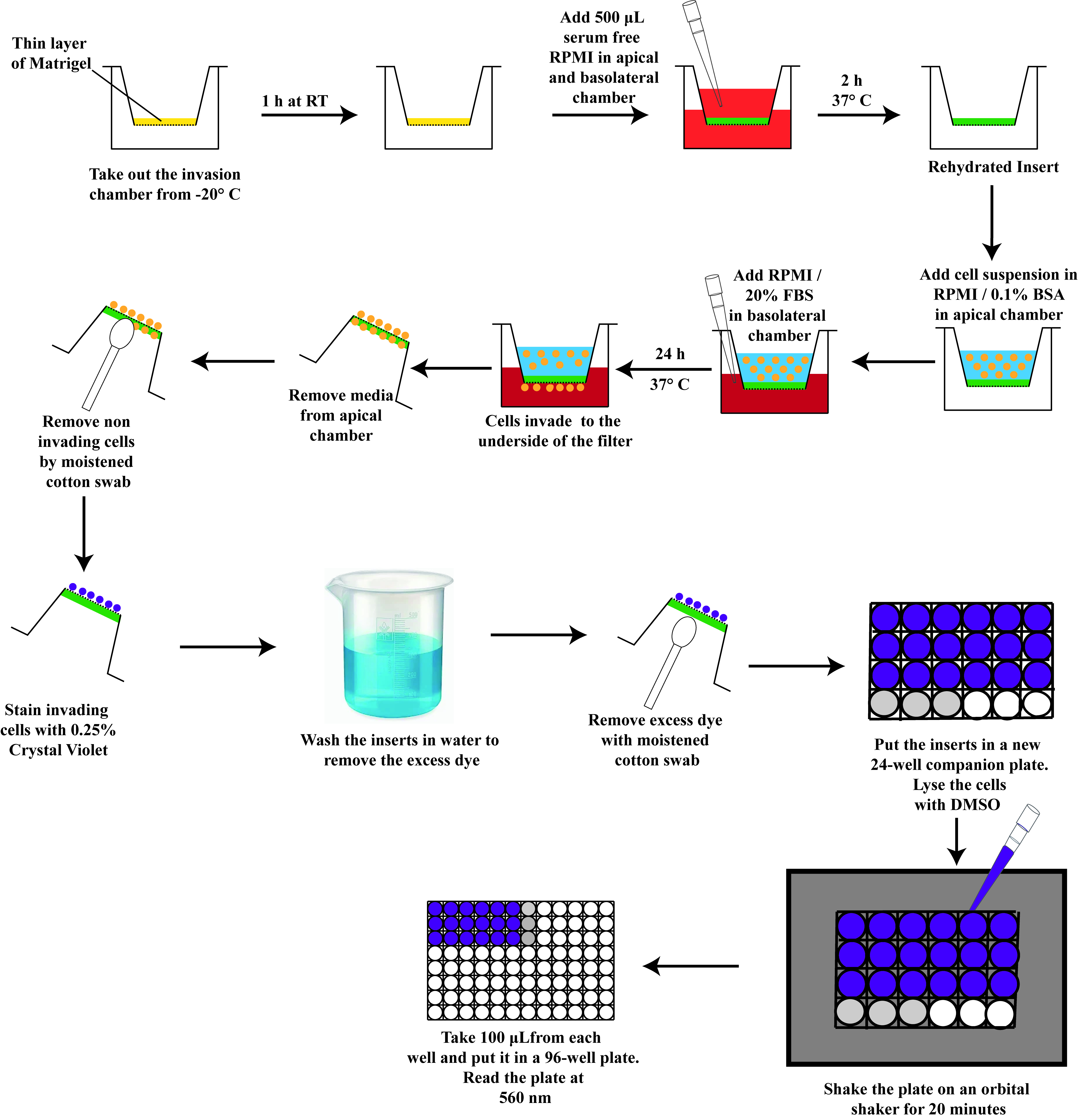
A schematic diagram depicting the steps of the Boyden chamber assay
Background
The process of metastasis is responsible for the majority of deaths in cancer patients [1]. Metastasis is a complex multi-step process comprised of primary tumor cell invasion into the surrounding tissue and extravasation into and intravasation out of the blood or lymphatic system, resulting in tumor cell colonization at distant sites and organs in the body [2,3]. Out of these processes, the process of tumor invasion has been the foundation of most drug-discovery studies. Invasion refers to the process by which cancer cells penetrate the basement membrane and launch themselves into circulation in the adjacent blood vessels or lymph nodes [4,5]. The Boyden chamber assay is the most prevalent method to measure this invasion capacity [6,7]. This assay requires two compartments separated by a microporous membrane coated with a Matrigel basement membrane matrix. In most versions of the Boyden chamber assay, cancer cells are placed in the upper compartment, and the lower compartment contains a robust chemoattractant. Under the influence of the chemoattractant, the cancer cells secrete proteases to degrade the Matrigel basement membrane, then migrate through the pores of the membrane into the lower compartment. A challenge to the traditional Boyden chamber assay is the arduous and time-consuming procedures of fixation and staining of the cells on the bottom surface of the porous membrane and subsequent photography and manual cell counting [6]. Our improved method of the Boyden chamber assay allows the results of the assay to be measured on a microplate reader. Therefore, our Boyden chamber assay protocol may be adapted to a high-throughput format. Currently, many research efforts are directed at improving our understanding of the metastatic cascade to advance our ability to design anti-cancer drugs, which will specifically target the process of metastasis. Out of all the events of metastasis, the process of tumor invasion has been the primary focus of drug discovery efforts [8,9]. The overarching goal of our improved Boyden chamber assay is to provide a simple, robust, and reproducible cell-based assay with a large-scale capability of evaluating and measuring the invasive ability of various cancer cells. We believe that our improved protocol of the Boyden chamber assay may pave the way for more rapid identification of novel anti-cancer drugs to combat metastasis.
Materials and reagents
Biological materials
DMS114 human small cell lung cancer (SCLC) cells [American Type Culture Collection (ATCC), catalog number: CRL-2066]. The cells should be grown to 70%–80% confluence in DMS114 culture media (see Recipe 3) and kept in a humidified environment at 37 °C (in the presence of 5% CO2) in a cell culture incubator [10]. Although we are describing the BCA protocol using DMS114 human SCLC cells, the pro-invasive activity of any relevant cancer cell line may be measured using this assay. Normal cells display pro-invasive activity under specific biological conditions. Such conditions may include treatment with growth factors, steroids, and carcinogenic chemicals. The BCA may be used to measure the effect of such chemicals on the invasion of normal cells.
Reagents
CorningTM BioCoatTM MatrigelTM invasion chamber inserts coated with Matrigel matrix. These invasion chambers are sold in two formats. The first is a 6-well format (Fisher Scientific, catalog number: 08-774-121). The second is a 24-well format (Fisher Scientific, catalog number: 08-774-122). Store at -20 °C. We use the 24-well format for the protocol described in the present manuscript
Sterile bovine serum albumin (BSA) solution at a concentration of 30% (w/v) in DPBS (Millipore-Sigma, catalog number: A7596)
Roswell Park Memorial Institute (RPMI)-1640 medium (ATCC, catalog number: 30-2001). Store at 4 °C, 1 M HEPES (Thermo Fisher, catalog number: 15630080)
Fetal bovine serum (FBS) (ATCC, catalog number: 30-2020). Long-term storage for FBS is at -70 °C. In our laboratory, FBS is aliquoted into 50 mL centrifuge tubes and stored at -20 °C. The FBS aliquot is thawed and stored at 4 °C for immediate use
Trypsin-EDTA (0.25% Trypsin, 0.53 mM EDTA) (ATCC, catalog number: 30-2101). Long-term storage for Trypsin-EDTA is at -20 °C. However, Trypsin-EDTA is aliquoted and stored at 4 °C for immediate use
1 M HEPES (Thermo Fisher Scientific, catalog number 15630080). Long-term storage for 1 M HEPES is at -20 °C. However, HEPES is aliquoted and stored at 4 °C for immediate use
Penicillin-streptomycin solution (ATCC, catalog number: 30-2300)
Dulbecco’s phosphate buffered saline (PBS) without calcium and magnesium (Corning, Fisher, catalog number: 21-031-CM). Store at 4 °C
Corning cell counting chamber (Fisher Scientific, catalog number: 07-200-988)
Capsaicin (Sigma-Aldrich, catalog number: M2028-50MG). Capsaicin is the hot and spicy ingredient of chili peppers. It is a potent agonist of transient receptor potential cation channel subfamily V member 1 (TRPV1). Store at 4 °C
PP2 is a potent and selective inhibitor of the Src family tyrosine kinases [11]. PP2 was manufactured by Enzo Biosciences (Enzo Biosciences, catalog number: 50-201-0680) and obtained from Thermo Fisher Scientific. The PP2 is delivered as powder and stored at -20 °C. The PP2 is dissolved in DMSO (Corning DMSO, Fisher, catalog number: MT-25950CQC) at a final concentration of 10 mM. The PP2 solution is aliquoted into microfuge tubes and stored at -20 °C
Dimethyl sulfoxide (DMSO), ACS certified (Fisher BioReagents, catalog number: D128-500). Store at room temperature
Aqua Solutions crystal violet 1% w/v aqueous (Fisher Scientific, catalog number: NC9002731). Store at room temperature
Solutions
Stock capsaicin (100 mM) (see Recipes)
WS-CAP-100X (2 mM) (see Recipes)
VEH-CAP-100X (see Recipes)
Stock PP2 (10 mM) (see Recipes)
WS-PP2-100X (500 µM) (see Recipes)
VEH-PP2 (see Recipes)
Crystal violet staining solution (see Recipes)
DMS114 culture medium (see Recipes)
RPMI-BSA (see Recipes)
Recipes
| Reagent | Final concentration | Solvent | Volume of each aliquot | Total volume | Storage |
|---|---|---|---|---|---|
| Stock capsaicin | 100 mM | DMSO | 25 µL | 100 µL | -20 °C |
| WS-CAP-100X | 2 mM | RPMI/0.1% BSA | Made fresh before use | 100 µL | On ice until use |
| VEH-CAP-100X | 2% DMSO (v/v) | Serum-free RPMI | NA | 100 µL | Made fresh before use |
| Stock PP2 | 10 mM | DMSO | 25 µL | 333 µL | -20 °C |
| WS-PP2-100X | 500 µM | RPMI/0.1% BSA | Made fresh before use | 100 µL | Made fresh before use |
| VEH-PP2-100X | 5% DMSO (v/v) | Serum-free RPMI | NA | 100 µL | On ice until use |
| Crystal violet staining solution | 0.25% crystal violet | Milli Q Water | NA | 20 mL | Room temperature |
Stock capsaicin (100 mM), 1 mL
Weigh 30.5 mg of capsaicin in a sterile autoclaved amber-colored 1.5 mL microfuge tube. In a laminar flow hood, add 1 mL of DMSO to the tube containing capsaicin. Vortex to mix. Aliquot in sterile amber-colored 1.5 mL microfuge tubes. We typically put 25 µL of capsaicin stock solution in each aliquot. Store the aliquots at -20 °C. The aliquots are stable for one month at -20 °C. Laboratory personnel should wear gloves, mask, and face goggles when working with powdered capsaicin. Capsaicin can cause intense irritation if it comes in contact with the skin, face, or eyes.
WS-CAP-100X (2 mM), 100 µL
Take out an aliquot of 100 mM capsaicin solution from -20 °C. Put the tube in a 37 °C water bath for 5 min (until the entire solution is thawed). Vortex briefly to mix. Add 2 µL of 100 mM stock capsaicin to 98 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) in a laminar flow hood. Vortex to mix. Keep the solution on ice until used in the experiment. We make a fresh solution of WS-CAP before every assay and discard any leftover solution.
VEH-CAP-100X, 100 µL
Add 2 µL of DMSO to 98 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) in a laminar flow hood. Vortex to mix. We do not store the VEH-CAP-100X solution. We make a fresh solution of VEH-CAP before every assay and discard any leftover solution.
Stock PP2 (10 mM), 333 µL
Add 333 µL of DMSO to the bottle of 1 mg of PP2 in a laminar flow hood. Vortex to mix. Aliquot in autoclaved amber-colored 1.5 mL microfuge tubes (we put 25 µL of PP2 solution in each aliquot). Store the aliquots in -20 °C. The aliquots are stable for one month at -20 °C.
WS-PP2-100X (500 µM), 1 mL
Take out an aliquot of 10 mM PP2 solution from -20 °C. Put the tube in a 37 °C water bath for 5 min (until the entire solution is thawed). Vortex briefly to mix. Add 5 µL of stock solution of DMSO to 95 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) in a laminar flow hood. Vortex to mix. Keep the solution on ice until it is used for the experiment. We make a fresh solution of WS-PP2 before every assay and throw away the leftover solution.
VEH-PP2, 1 mL
Add 5 µL of DMSO to 95 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) laminar flow hood. Vortex to mix. We make a fresh solution of vehicle before every assay and do not store it.
Crystal violet staining solution, 20 mL
Add 5 mL of 1% crystal violet solution to 15 mL of Milli Q water. Vortex to mix. Store the solution at room temperature.
DMS114 culture medium
DMS114 cells were cultured following the published protocols [10]. All procedures are performed in a laminar flow hood. The DMS114 culture medium consists of RPMI-1640 with 1% Penicillin streptomycin solution (containing 100 units/mL penicillin, 50 µg/mL streptomycin), 25 mM HEPES, and 10% FBS. ATCC RPMI-1640 (30-2001 formulation) comes formulated with 10 mM HEPES. We then add an additional 7.5 mL of 1 M HEPES to 500 mL of RPMI-1640 to obtain a 25 mM concentration of HEPES. Store the media at 4 °C. To note, many laboratories add 50 mL of FBS to the full bottle of media to obtain 10% (v/v). We do not typically add FBS to the entire RPMI bottle. The rationale for this is to facilitate the most efficient use of each RPMI bottle. Many of our experiments require serum-free media, so we add the FBS as needed. The cells should be grown to 70%–80% confluence using the media described above and kept in a sterile cell culture incubator with a humidified environment, set at 37 °C with 5% CO2.
DMS114 culture medium Final concentration in media Quantity or Volume
added to 500 mL media
RPMI-1640 NA 500 mL Penicillin-streptomycin solution 100 units/mL penicillin, 50 µg/mL streptomycin 5 mL HEPES 25 mM 7.5 mL FBS 10% v/v 50 mL RPMI-BSA
Add 100 µL of BSA from the bottle containing 30% BSA solution to 30 mL of serum-free RPMI-1640 (at room temperature) in a sterile 50 mL tube. Mix gently to avoid air bubbles. All these procedures should be performed in the laminar flow hood. We make fresh RPMI-BSA before each experiment. Store at room temperature for the duration of the assay.
DMS114 culture medium Final concentration in media Quantity or Volume RPMI-1640 NA 30 mL 30% BSA solution 0.1% v/v 50 µL
Laboratory supplies
NuncTM EasYFlaskTM T-75 cell culture flasks (Thermo Scientific, catalog number: 156499)
Sterile Corning 24-well polystyrene tissue culture insert companion plate with lid (Corning Life Sciences, catalog number: 353504)
96-well plate, non-treated surface, pack of 25 (Thermo Scientific, catalog number: 12-566-202)
PuritanTM sterile DNA-free standard cotton swab, wood handle (Fisherbrand, catalog number: 22-025-201)
Sterile 15 mL polypropylene centrifuge tubes (Thermo Scientific, Nunc, catalog number: 12-556-017)
1.5 mL natural microcentrifuge tubes (Fisherbrand Premium, catalog number: 05-408-129). Autoclave before use
1.5 mL amber-colored microcentrifuge tubes (Fisherbrand Premium, catalog number: 05-408-134). Autoclave before use
Nunc 15 mL conical sterile polypropylene centrifuge tubes with plastic racks (Thermo Scientific, catalog number: 12-565-269)
Nunc 50 mL conical sterile polypropylene centrifuge tubes with plastic racks (Thermo Scientific, catalog number: 12-565-271)
Low-retention 2 mL microcentrifuge tubes (Fisherbrand, catalog number: 02-681-332)
Curved medium point stainless-steel general-purpose forceps (Fisherbrand, catalog number: 16-100-110). Autoclave the forceps before use with the Boyden chamber assay
Fine point high-precision forceps (Fisherbrand, catalog number: 22-327379). Autoclave the forceps before use with the Boyden chamber assay
Equipment
NU-540 laminar-flow biosafety cabinet (NuAire, Plymouth, MN, model: LabGard® ES NU-540 Class II, Type A2)
Cell culture incubator maintained at 37 °C and 5% CO2 (Thermo Scientific, Waltham, MA, model: Heracell VIOS 150i)
Microplate reader (Agilent, model: BioTek Gen 5)
Benchtop centrifuge (ThermoElectron Corporation, model: IEC Centra CL2)
Gilson PIPETMAN Pipettes [Marshall Scientific, catalog number: F144059M (P1000), F144058M (P200), F144056M (P10), F144054M (P2)]
Software and datasets
Agilent BioTek Gen 5 microplate reader and imager software
GraphPad Prism version 10.2.1
Procedure
Culture of DMS114 cells
The DMS114 human SCLC cell line was obtained from ATCC (Manassas, Virginia).
DMS114 cells were grown to 70%–80% confluence in DMS114 culture medium supplemented with 10% FBS (for instructions on making the media, see Recipe 8). Cells are cultured in T-75 tissue culture flasks. The flasks are kept in a cell culture incubator maintained in a humidified environment at 37 °C and 5% CO2 [10].
Working with Corning BioCoat invasion chambers (containing Matrigel matrix–coated inserts)
We will use the invasion chambers in the 24-well format to perform the Boyden Chamber Assay. A box of Corning BioCoatTM MatrigelTM invasion chamber is comprised of two packets containing two sterile 24-well plates loaded with the invasion chambers. The Corning BioCoatTM MatrigelTM invasion chambers are stored at -20 °C. We require one packet out of this box for the Boyden chamber assay. Keep the packet on the laboratory bench (at room temperature) for approximately 1 h so that the contents of the packet warm up to room temperature (Figure 1).
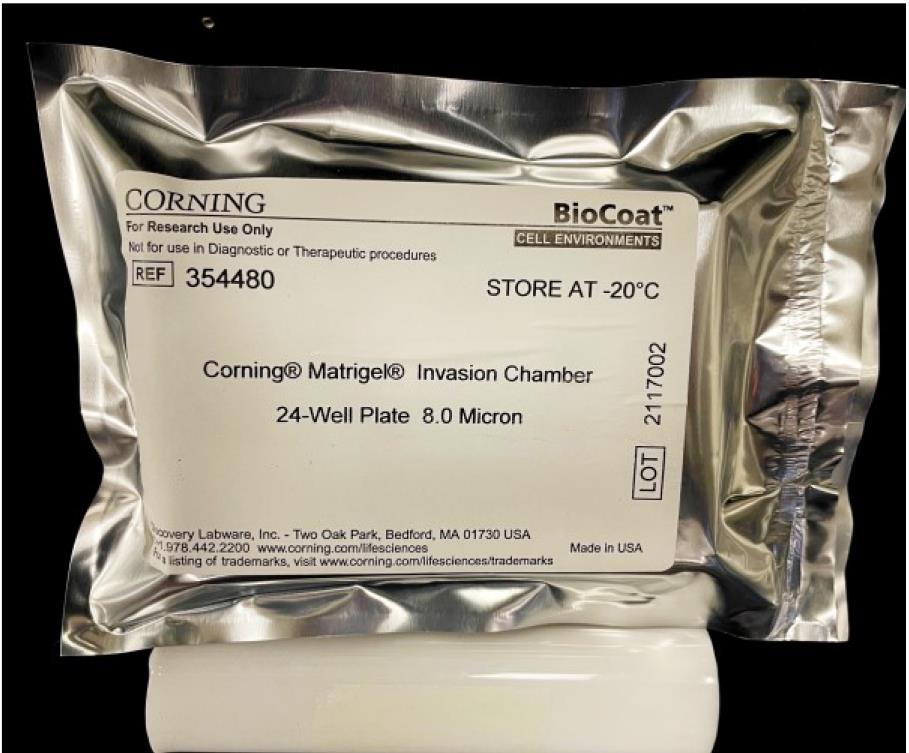
Figure 1. Packet of Matrigel-coated invasion chambers (in 24-well format)Note: If you do not plan to use all of the inserts (inside a 24-well tissue culture plate) for your experiment, do not allow them to thaw, as repeated freeze-thaws will damage the Matrigel matrix barrier. Open the package (Figure 1) in a laminar flow hood (under aseptic conditions). With the help of sterile curved stainless-steel forceps, transfer the required number of invasion chamber inserts to a separate 24-well tissue culture companion plate to thaw. Wrap the unused inserts in the original packaging, tape them securely, and quickly return them to the -20 °C freezer.
Take the packet (containing one sterile 24-well plate loaded with the invasion chambers) and open it inside the laminar flow hood. The Corning BioCoat Matrigel invasion chambers consist of a tissue culture–treated sterile 24-well plate containing a total of 12 Matrigel-coated invasion chambers in the middle two rows of the plate (six invasion chamber inserts per row). The picture of the invasion chambers inside the 24-well companion plate is shown in Figure 2A. Figure 2B depicts the sideways view of the Matrigel-coated invasion chambers inside the 24-well plate.
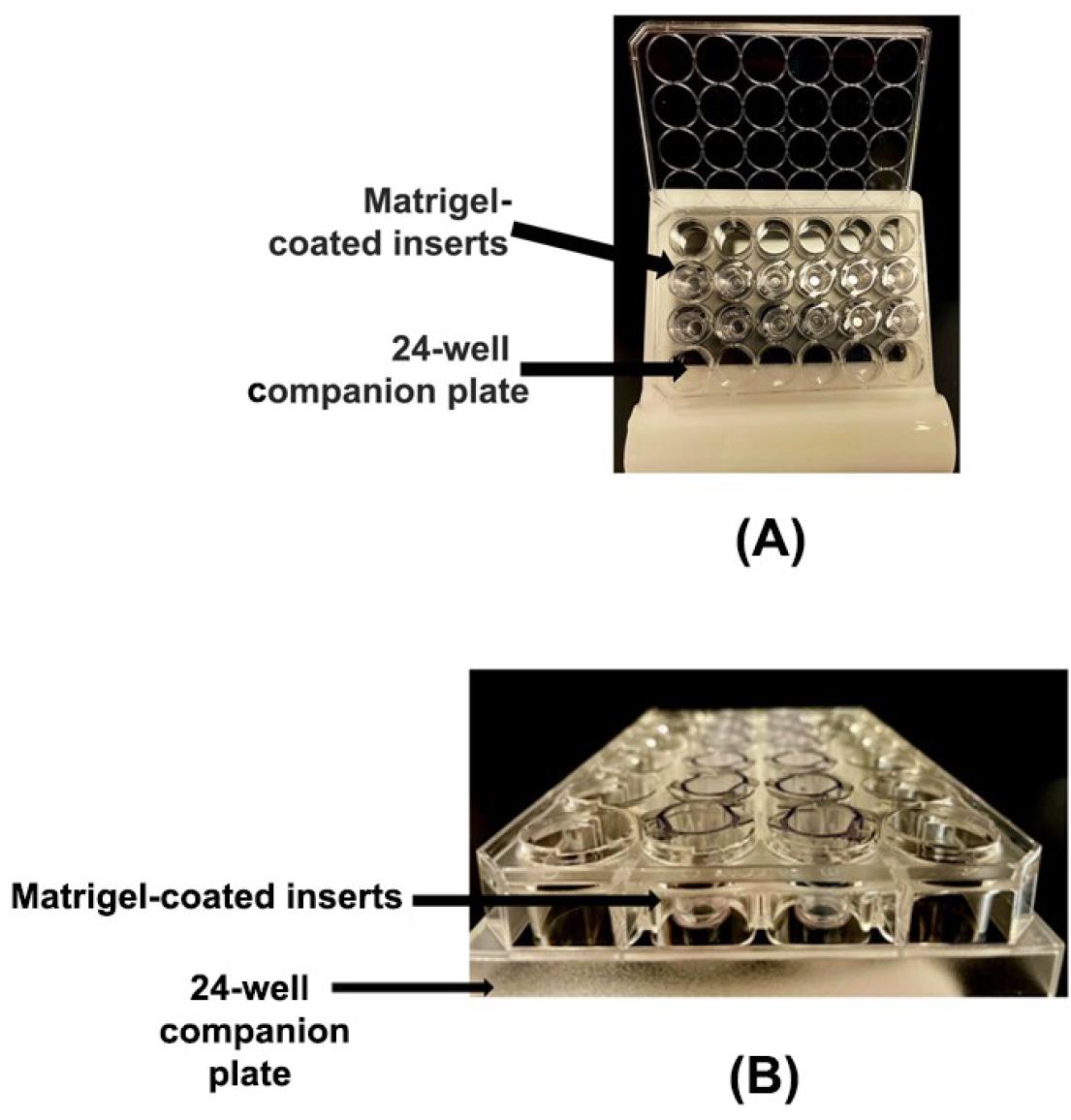
Figure 2. Invasion chambers in a 24-well format. (A) Photograph of Matrigel-coated invasion chambers arranged in a 24-well companion plate. (B) Side-view image of the Matrigel-coated invasion chambers arranged in a 24-well companion plate.The invasion chamber inserts have a polyethylene terephthalate (PET) porous mesh membrane at the bottom of the inserts. This PET membrane has a pore size of 8 μm. The PET membrane is coated with a thin layer of Matrigel basement membrane matrix (Figure 3).

Figure 3. Matrigel-coated invasion chamber inserts. The bottom of the insert comprises a polyethylene terephthalate (PET) membrane whose pore size is 8 μm and is coated with a thin layer of Matrigel.Before starting the assay, draw the schema of the assay. Each sample is tested in duplicate (Figure 4).
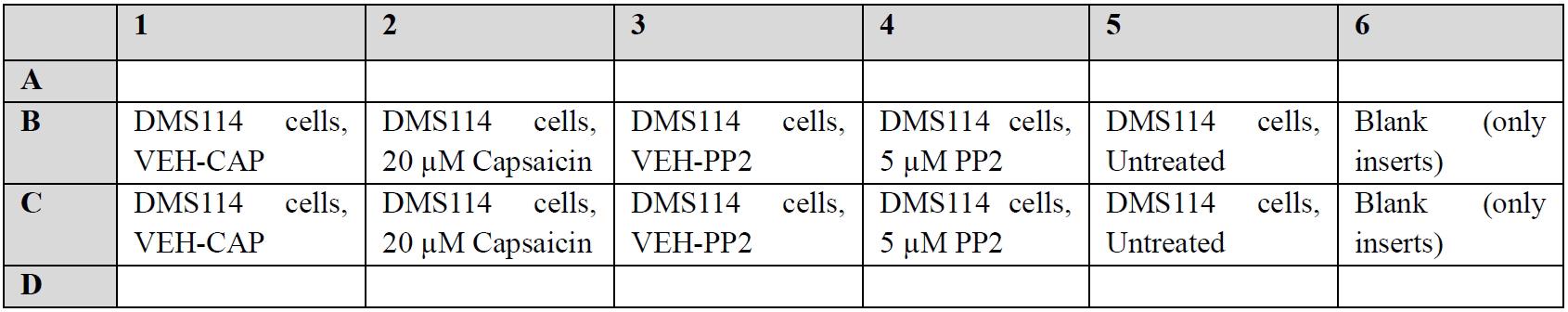
Figure 4. Schema of the Boyden chamber assayThe inserts should be handled with autoclaved curved stainless-steel forceps, as shown in Figure 5. Do not touch the mesh at the bottom of the insert to avoid damaging the porous membrane or the Matrigel.

Figure 5. The Matrigel-coated invasion chamber insert should be handled with a pair of forcepsThe inserts rest on the rims of each well and divide each well into two regions: the apical chamber, which is within the insert, and the basal chamber, which surrounds the outside of the insert (Figure 6).
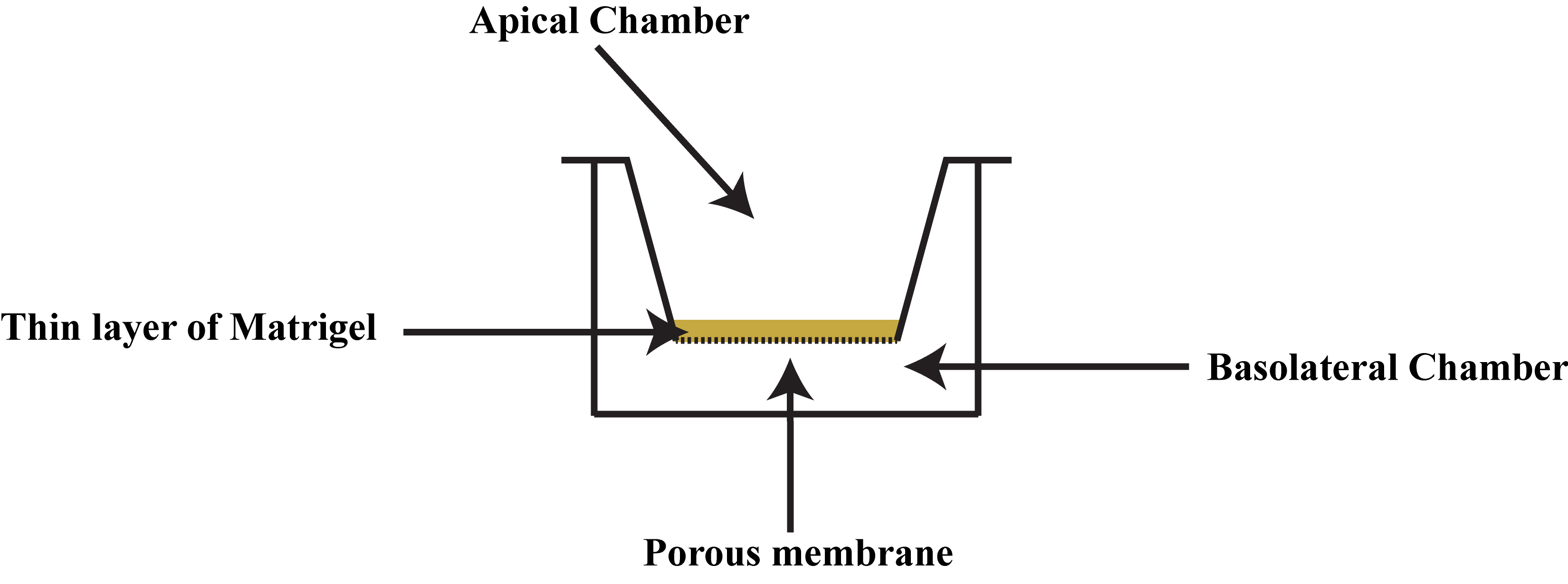
Figure 6. The apical and basolateral chambers in the Boyden chamber assayEnsure that the inserts align properly with the positioning notches of the companion 24-well plate. The outline of the rims on the companion plate has been indicated by black marker to better show the location of the notches (Figure 7). The black arrows indicate the positioning notches of the companion 24-well plate.
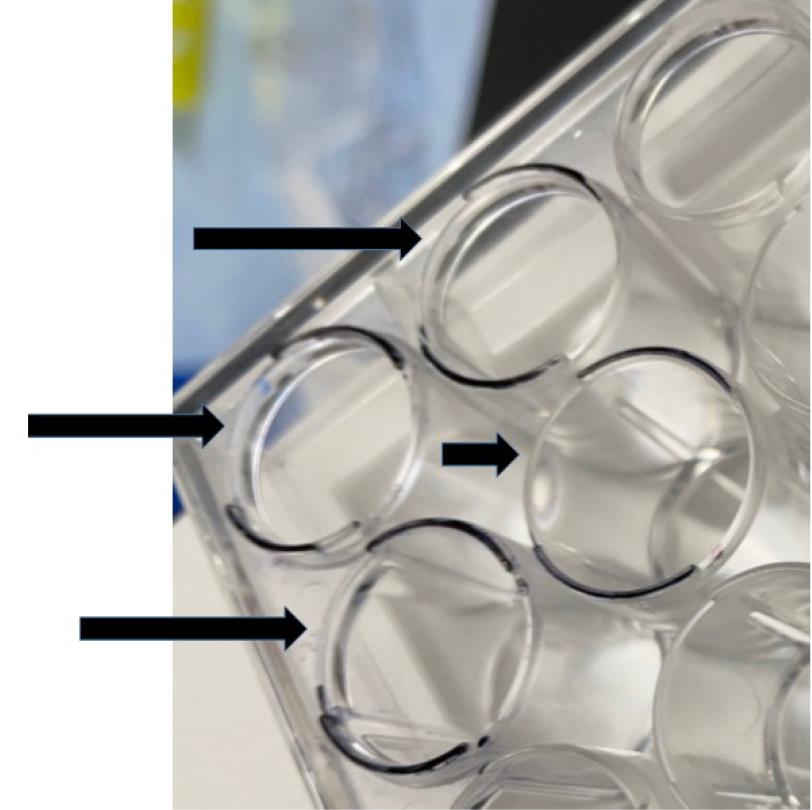
Figure 7. The positioning notches of the companion 24-well plate are indicated by black arrowsAdd 500 µL of serum-free RPMI (at a temperature of 37 °C) to the apical chamber of the inserts using a P1000 Pipetman (Figure 8A and B). Take care not to touch the membrane with the tip or puncture it. Avoid generating air bubbles while adding the media to the apical chamber.
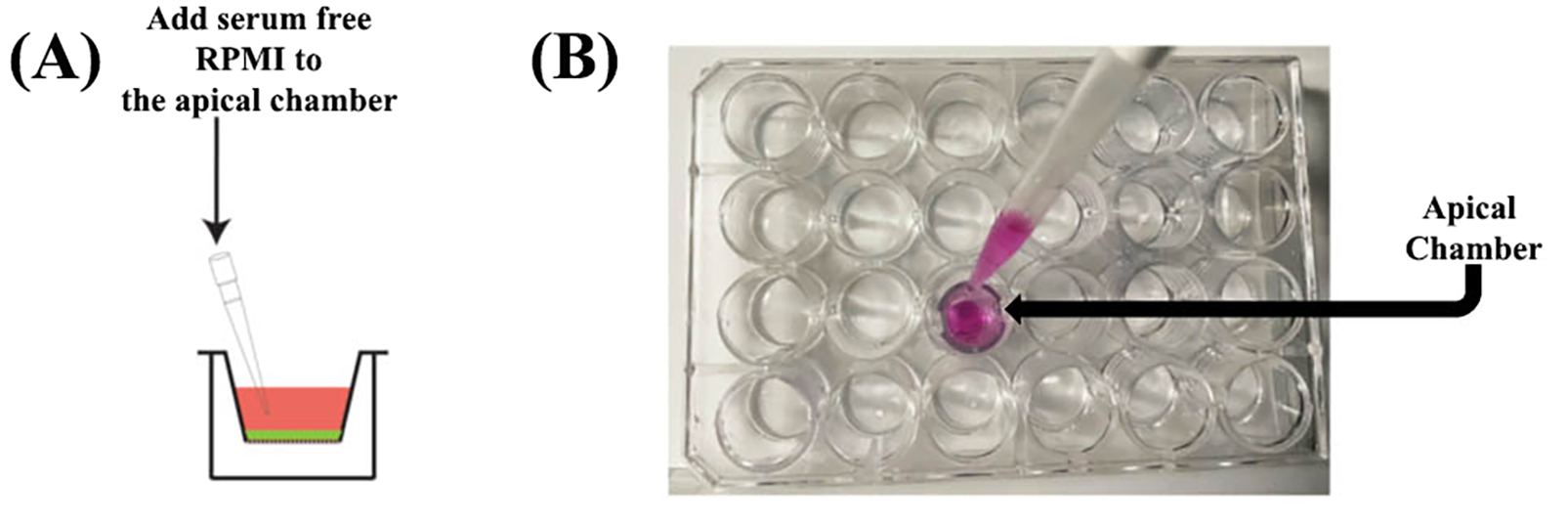
Figure 8. Rehydrating the apical chamber of the insertAdd 500 µL of serum-free RPMI (at a temperature of 37 °C) to the basal chamber (Figure 9). Place the P1000 tip in the empty space beside the insert (between the notches on the insert) and gently dispense the warm serum-free RPMI (Figure 9A–C). Avoid generating air bubbles while adding the media to the basolateral chamber.
Allow the inserts to rehydrate for 2 h in a humidified environment at 37 °C and 5% CO2 in a cell culture incubator (Figure 10). The rehydrated insert should be used immediately after the 2-h rehydration period. Do not wait to use the rehydrated inserts on the next day.
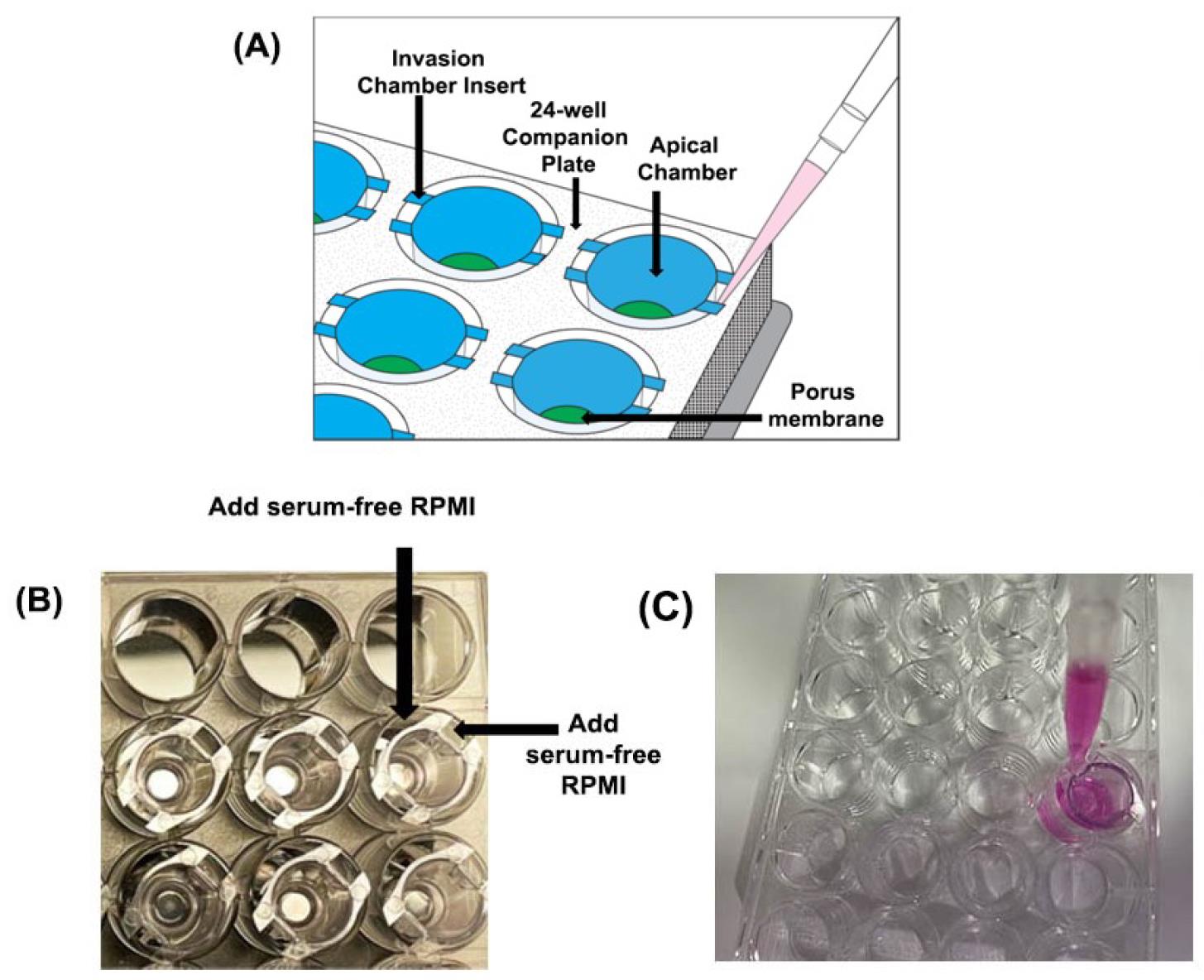
Figure 9. Addition of medium in the basolateral chamber of the invasion chamber. (A) Serum-free RPMI should be added in the space outside the insert or within the notches to avoid air bubbles. (B) Invasion chambers inside the companion plate. (C) Rehydrating the basolateral chamber of the insert.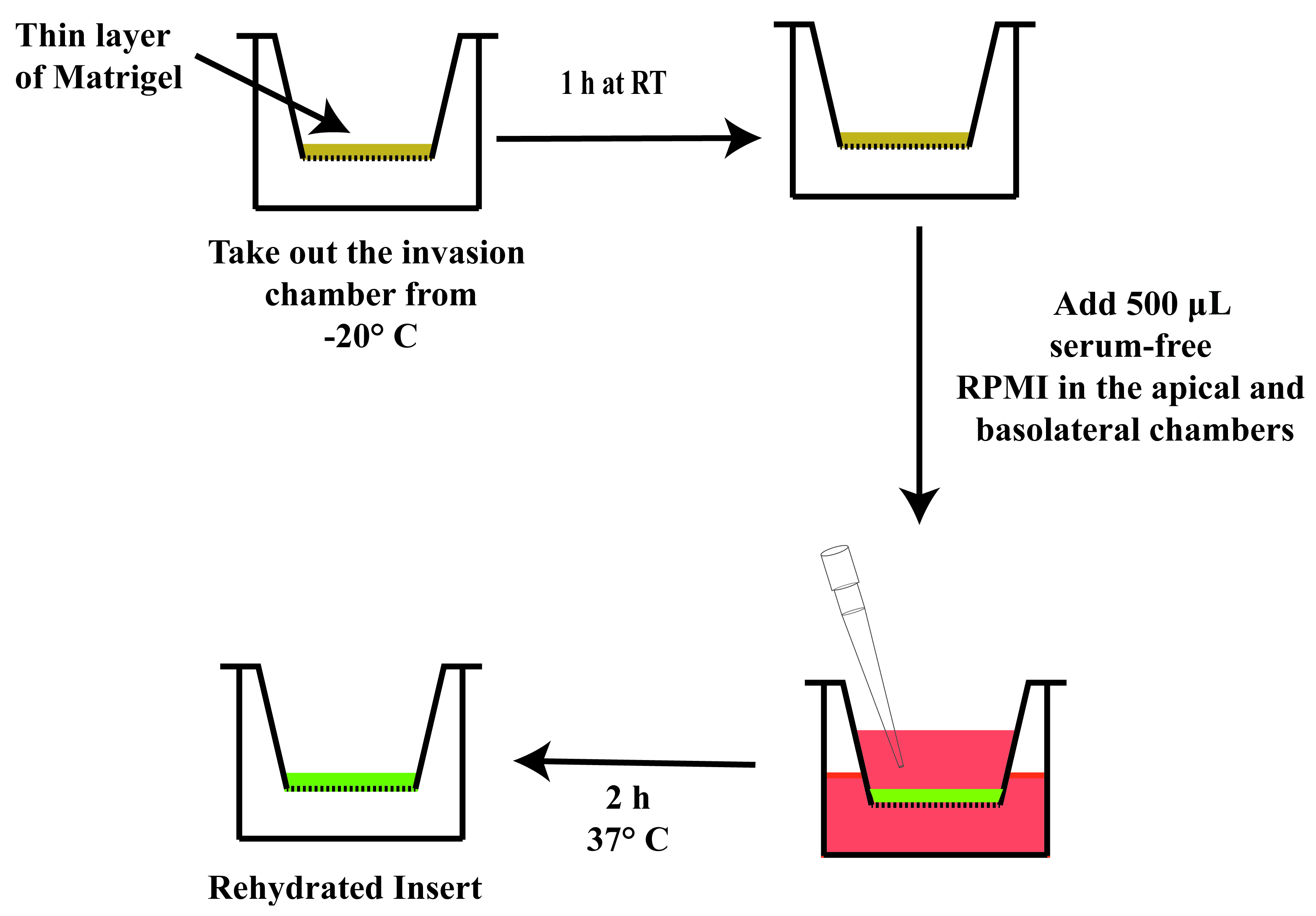
Figure 10. Schematic diagram outlining the steps for rehydrating the insertIt is vital that there are no air bubbles trapped underneath the PET membrane in the basolateral chamber (Figure 11). If there are air bubbles, gently tap the side of the plate to dislodge the bubbles. Another strategy to remove air bubbles is to tilt the insert chamber at a slight angle to dislodge the bubbles from below the insert.
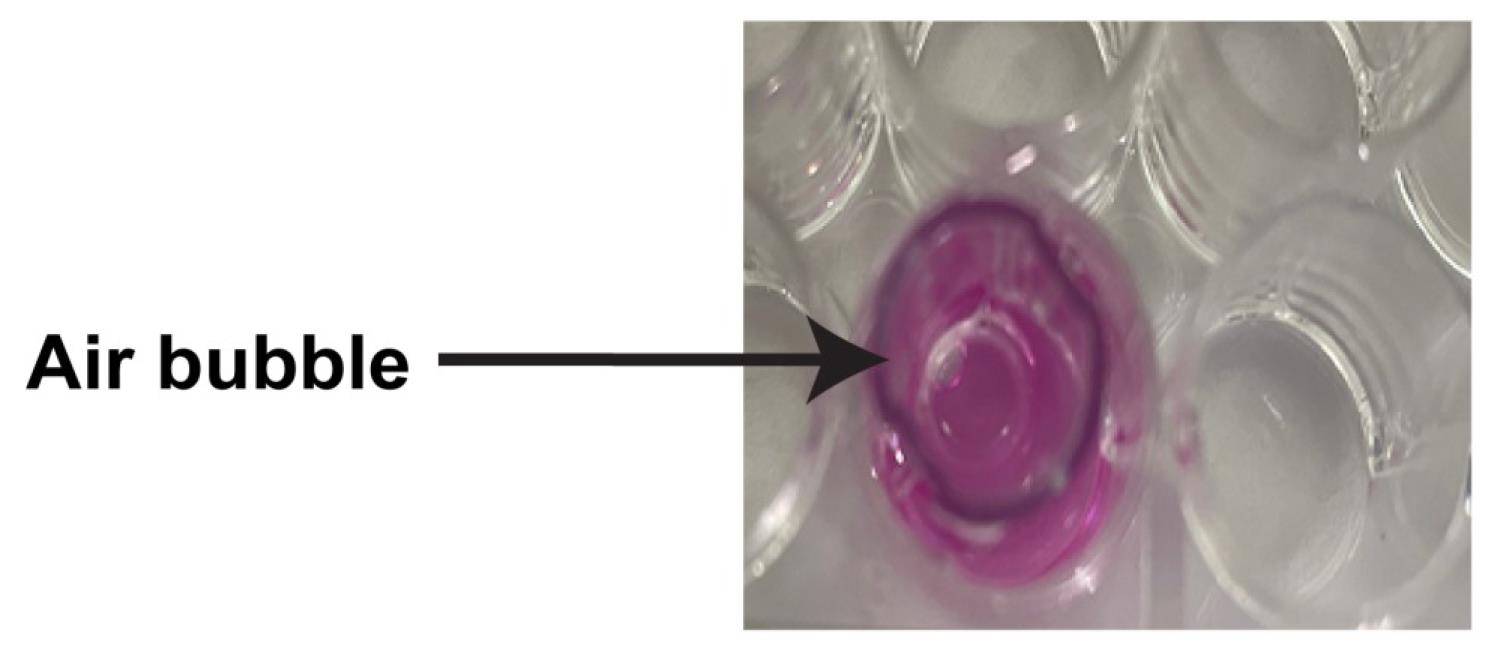
Figure 11. An air bubble trapped underneath the insert
Performing the Boyden chamber invasion assay: DAY 1
DMS114 cells were cultured in T-75 tissue culture flasks in DMS114 culture medium (see section A). The cells should be grown to 70%–80% confluence in a humidified environment at 37 °C (in the presence of 5% CO2) in a cell culture incubator [10].
Wash cells once with DPBS. Add the relevant volume of pre-warmed trypsin-EDTA solution (see Reagents) to the side wall of the flask. In our laboratory, we add 0.4 mL of trypsin-EDTA for every 10 cm2 of culture area in the flask. Therefore, we will add 3 mL of trypsin-EDTA to a T-75 flask. Gently rock the flask to ensure the trypsin solution covers the entire cell monolayer. Incubate the flask at 37 °C. The flasks should be placed flat (horizontally) in the cell culture incubator. Within 1–2 min, the cells will start rounding up and detaching from the bottom of the flask. Observe the cells using an inverted microscope to monitor the cell detachment process. Gently tap the side of the flask to fully detach the cells if necessary. Almost 90% of the cells should be detached and rounded up within 2 min of treatment with trypsin-EDTA.
Note: Prevent excessive exposure of the cells to trypsin solution (≥ 10 min).
Once cells appear detached, add 1 mL of FBS to inactivate the trypsin. Gently disperse the solution by pipetting up and down (several times) over the cell monolayer surface. Collect the cells in a 15 or 50 mL tube.
Note: Excessive forceful pipetting can cause cell damage.
Gently spin down the cells at 800× g for 5 min. Aspirate the media. Flick the tube to resuspend the cells. This usually loosens the pellet enough to resuspend the cells. Add 10 mL of DPBS to wash the cells and remove any traces of FBS. Centrifuge the cells at 800× g for 5 min. Aspirate the supernatant. Flick the tube to loosen the cell pellet and gently resuspend the cells in 1 mL of RPMI-BSA (Recipe 9).
Note: Do not vortex the cell suspension.
Count the cells using the Corning cell counting chamber. Adjust the concentration of the cells to 5 × 105 cells/mL in a 15 mL centrifuge tube using RPMI-BSA. For performing the assay as described in Figure 4, we will need 10 mL of cell suspension at a density of 5 × 105 cells/mL in RPMI-BSA. This tube will be referred to as TUBE-DMS114-FINAL.
Arrange six autoclaved 2 mL microcentrifuge tubes, labeled A–F, on a rack in the laminar flow hood.
Add the following drugs to the microcentrifuge tubes from step C6, as described in Figure 12 (columns 1–3, colored in green).
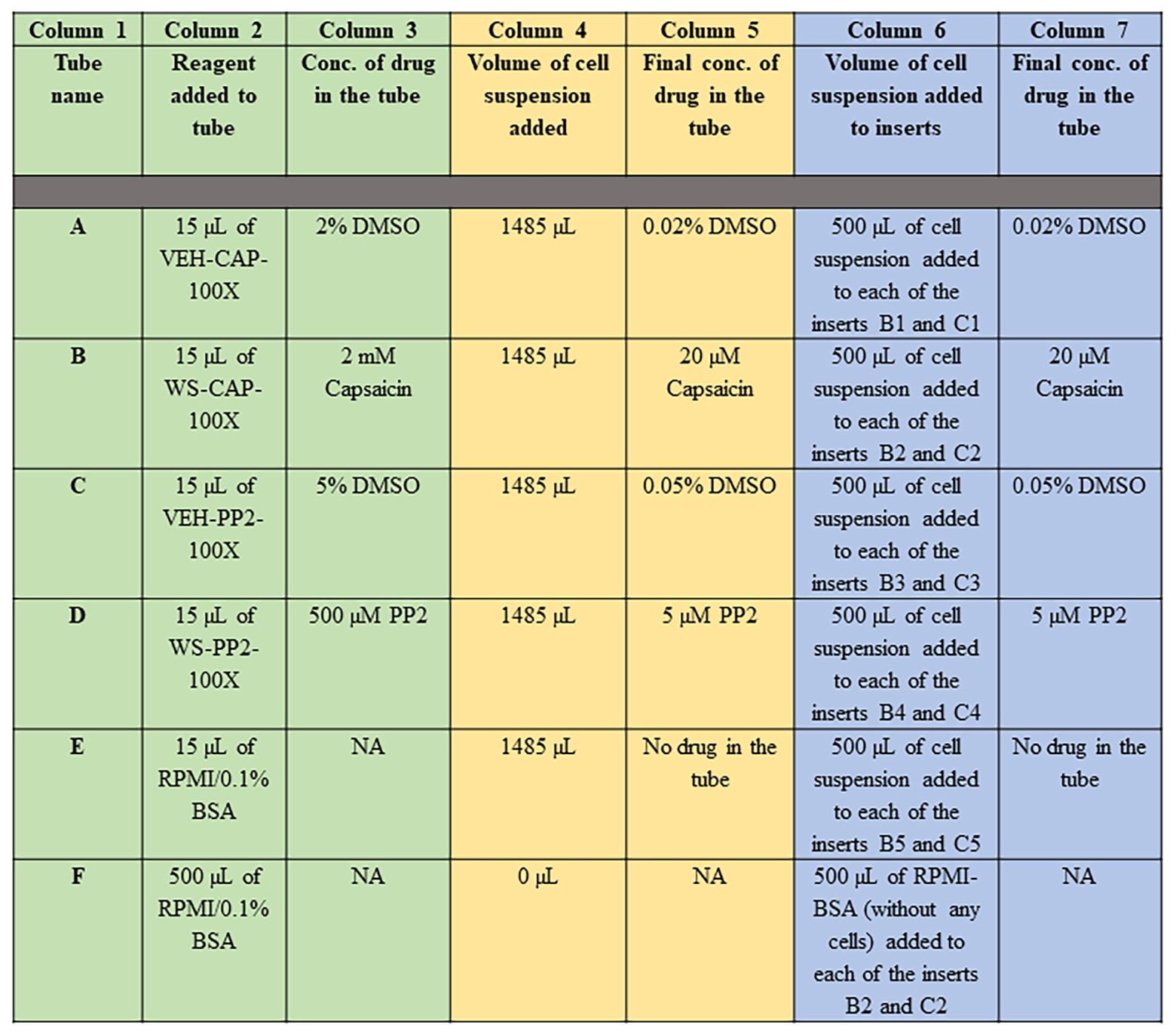
Figure 12. Protocol for treating DMS114 human SCLC cells to the indicated drugs (in 2 mL microfuge tubes labeled A–F) is described in Columns 1–3 (colored in green). The final concentrations of the drugs (in the 2 mL microfuge tubes labeled A–F) after the addition of DMS114 cell suspension is represented in Columns 4–5 (colored in yellow). The protocol for the addition of DMS114 cells (treated with/without the indicated drug) into the apical chamber of the Matrigel-coated inserts (fitted in the 24-well companion plate) is depicted in Columns 6–7 (colored in blue). Conc.= Concentration.Add 1485 µL of DMS114 cell suspension (from TUBE-DMS114-FINAL) to each of the tubes A–E. Mix the cells with the drugs inside the tube by holding the tubes between your thumb and index finger and inverting the tubes 2–3 times. Do not vortex. Figure 12 describes the final concentrations of the drugs in the tubes (columns 4 and 5, colored in yellow).
Remove the rehydrated invasion chambers (along with the 24-well companion plate) from the 37 °C cell culture incubator. Gently remove the media from the apical and basal chamber using a Pipetman. DO NOT aspirate/puncture membrane.
Following the schema of the assay shown in Figure 4, take 500 µL of the cell suspension from TUBE A and add it to the apical chamber of the insert in well B1. Next, take 500 µL of the cell suspension from TUBE A and add it to the apical chamber of the insert in well C1. Continue loading the remaining inserts as described in Figure 12 (columns 6–7, colored in blue).
Add 500 µL of RPMI supplemented with 20% FBS (as the chemoattractant) in the basolateral chamber. Ensure that no air bubbles are trapped underneath the PET membrane in the basolateral chamber (refer to step B11). If there are air bubbles, gently tap the side of the plate to dislodge the bubbles. Another strategy to remove air bubbles is to tilt the insert chamber at a slight angle to dislodge the bubbles from below the insert.
Development of the Boyden chamber invasion assay: DAY 2
After 24 h, take the plate out of the cell culture incubator and place it on the laboratory bench.
Take out the inserts with a pair of forceps (Figure 13A) and gently place them upside down on a paper towel so that the solution inside the apical chamber is drained out on the paper towel (Figure 13B). Use caution not to touch or disrupt the basolateral side of the insert.

Figure 13. After 24 h, the insert is taken out with a pair of forceps (A) and drained on a Kimwipe (B)Take the sterile DNA-free cotton swab and moisten it with warm serum-free RPMI medium (Figure 14A).
Insert the moistened cotton swab and remove all the cells (and the Matrigel matrix) from the upper surface of the membrane (apical surface) by applying gentle but firm pressure while moving the tip over the membrane. Hold the insert at an angle so the bottom of the membrane is not touching a flat surface and gently swab the upper side of the membrane, taking care to remove the cells but not detaching the insert membrane from the housing. This process is termed as “scrubbing” the membrane. Repeat the scrubbing with a second swab moistened with medium (Figure 14B).
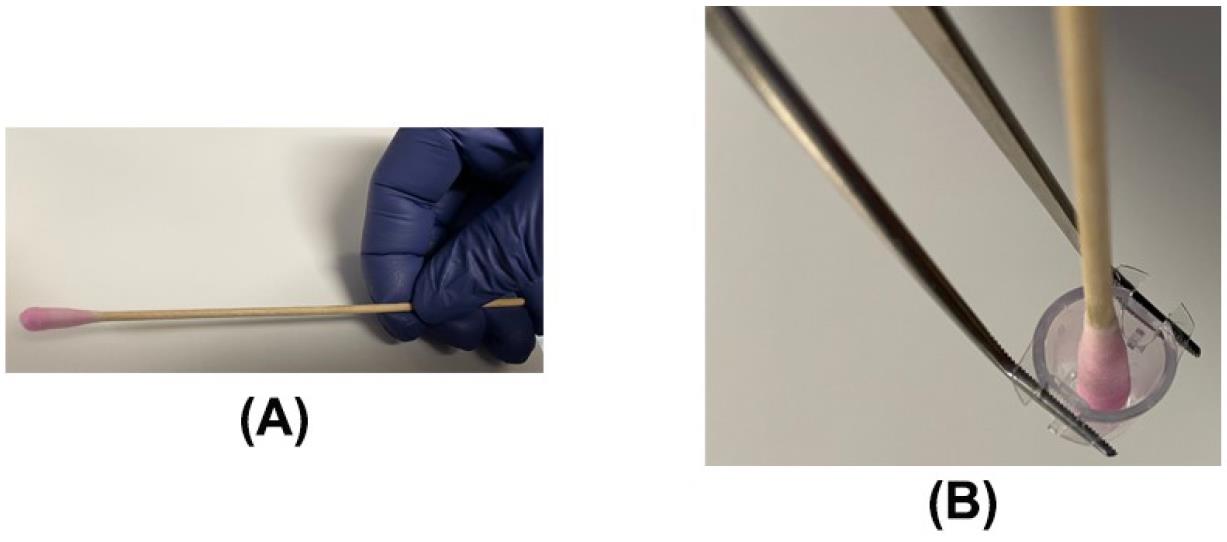
Figure 14. Removing the non-invading cells from the invasion chamber using a moistened cotton swab. (A) A cotton swab moistened with serum-free RPMI. (B) Scrubbing the insert with a moistened cotton swab.
Figure 15. Staining the invasion chambers with crystal violet. (A) A solution of 0.25% crystal violet was added to each well of the companion plate. (B) The inserts were gently lowered into the crystal violet solution for staining.Take a new companion plate. Add 400 µL of 0.25% crystal violet solution to each well of rows B1–B6 and C1–C6 of the companion plate (Figure 15A). Using a pair of forceps, gently transfer the scrubbed invasion chamber inserts into the companion plate in their corresponding location of the schematic (Figure 4) and incubate the inserts with the dye for 20 min at room temperature (Figure 15B).
Add distilled water (~750 mL) to three 1 L beakers. Using a pair of forceps, lift the insert (Figure 16A) and dip the insert in and out of water (in the first beaker) for about 2 min. Lightly invert the inserts and tap the corner of the inserts gently on a Kimwipe to remove any remaining liquid inside the insert. After this, dip the insert in and out of water (in the second beaker) for about 2 min. Lightly invert the inserts and tap the corner of the inserts gently on a Kimwipe to remove any remaining liquid inside the insert. After this, dip the insert in and out of water (in the third beaker) for about 2 min. Lightly invert the inserts and tap the corner of the inserts gently on a Kimwipe to remove any remaining liquid inside the insert.
Use a moistened sterile cotton swab to scrub the excess dye from the apical surface of the membrane and the walls of the insert (Figure 16B). Step D4 describes the correct procedure to scrub the membrane and the walls of the insert.
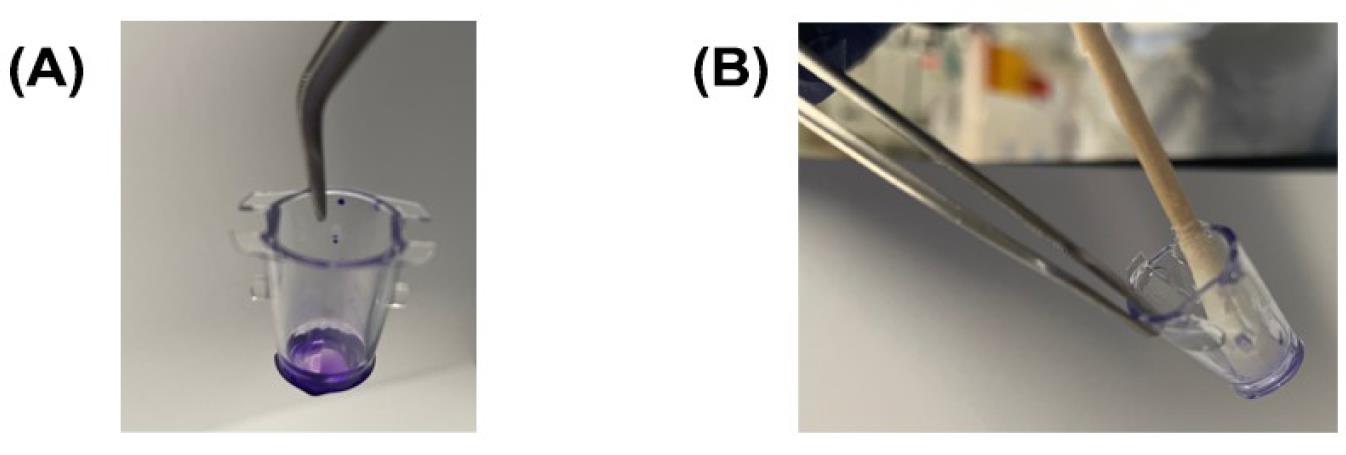
Figure 16. Removing the excess dye from the crystal violet-stained insert. (A) The invasion chamber insert stained with crystal violet solution. (B) Scrubbing the insert to remove the excess dye and remaining non-invading cells.Take a new companion plate. Add 400 µL of DMSO to each well of the companion plate. Using a pair of forceps, gently transfer the invasion chamber inserts into the companion plate in rows B1–B6 and C1–C6. and incubate the inserts with the DMSO on an orbital shaker for 20 min at room temperature (Figure 17). The DMSO will lyse crystal violet–stained cells and a blue color will be observed.
Figure 17. Crystal-violet stained cells (on the underside of the filter) are lysed with DMSO to give a blue colorRemove the inserts from the plate. The old companion plate contains a blue-colored solution. Transfer 100 µL from each sample (in the companion plate) to a 96-well plate (each sample is tested in duplicate) and read the absorbance of the plate at 560 nm. Note that some ELISA readers have the capability of reading 24-well plates. In that case, the absorbance can be measured using a 24-well plate. In our laboratory, we measure the absorbance of the plates immediately after the transfer of the solution to the 96-well plate. We have no knowledge about how long we can wait (after the completion of the assay) to measure the absorbance of the plates at 560 nm.
Data analysis
Each sample/treatment was tested in duplicate in the Boyden chamber assay. The entire assay was repeated six independent times. Subtract the absorbance of the blank wells from all the samples. The absorbance obtained in DMS114 cells treated with the vehicle control (VEH-CAP wells) was taken as 1 and the absorbance obtained in the DMS114 cells treated with 20 µM capsaicin was represented as fold-change of vehicle control. A similar analysis was performed for the PP2-treated DMS114 cells, which represents the positive control for the assay.
Open GraphPad Prism (version 10.1.2). Using the numbers obtained in step 1 (described above), create a column graph of the data.
All data are plotted as mean ± standard deviation (SD). The data should be analyzed by performing a one-way ANOVA followed by Tukey’s post-hoc test. All analyses should be completed using a 95% confidence interval. Data is considered significant when P ≤ 0.05 (Figure 18).
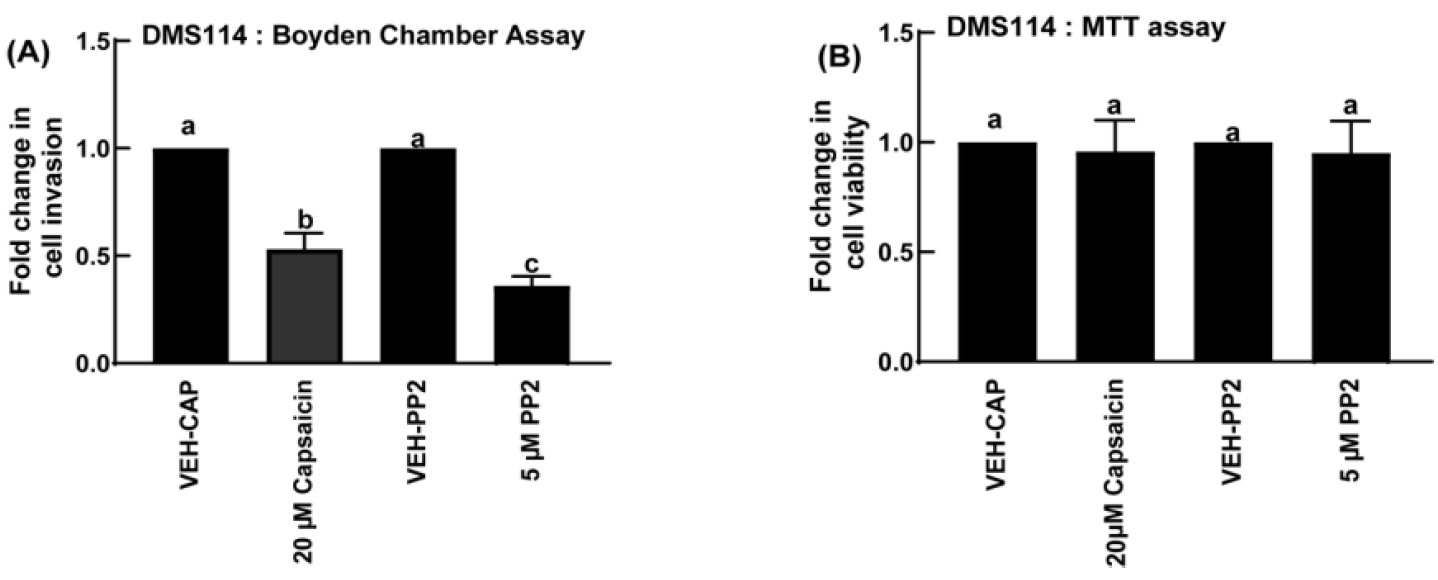
Figure 18. Treatment of DMS114 cells with 20 µM capsaicin potently inhibits invasion as measured in the Boyden chamber assay. (A) The Src inhibitor PP2 was used as the positive control for the assay. (B) An MTT assay was done to demonstrate that none of these compounds inhibited the viability of DMS114 cells. Values represented by the same letter are not statistically significantly different from each other (P ≤ 0.05).Validation of protocol
This protocol or parts of it have been used and validated in the following research article:
• Hurley et al. [12]. Non-pungent long chain capsaicin-analogs arvanil and olvanil display better anti-invasive activity than capsaicin in human small cell lung cancers. Cell Adhesion and Migration 11: 80–97.
General notes and troubleshooting
General notes
The Boyden chamber experiment is one of the most frequently used experimental techniques to evaluate cell invasion [6,7]. Apart from analyzing the pro-invasive activity of human cancer cells and biological agents, the Boyden chamber assay may be used to assess cell migration, haplotaxis [a directional movement of cells in response to signaling molecules that mediate cell adhesion like the extracellular matrix (ECM)], chemotaxis, cell motility, and chemokinesis (cell migration in response to soluble signaling molecules in the absence of a gradient, usually involving a change in migration speed or cytoskeletal reorganization) [6,7]. In our protocol, the endpoint of the Boyden chamber assay involved colorimetric detection using an ELISA reader. Other methods of endpoint detection (in the Boyden chamber assay) include fluorometric detection, chemiluminescence detection, X-gal staining, cell staining and counting (using microscopy techniques), and measurement of transepithelial electrical resistance (TEER). The Agilent xCELLigence RTCA DP analyzer evaluates cell invasion by the measurement of cellular impedance [13]. A few of these abovementioned Boyden chamber assay modalities have the capacity to be adapted for automation and high-throughput screening strategies.
It is always important to incorporate relevant positive and negative controls while performing a Boyden chamber assay [7,14]. For example, our Boyden chamber assay protocol evaluates the anti-invasive activity of capsaicin (at a concentration of 20 µM). It is possible that the treatment of DMS114 cells with 20 µM capsaicin may induce cell cycle arrest or kill off a substantial number of cells. If cells are quiescent (in G0) phase) they will not travel across the membrane of the invasion chambers. Similarly, if the cells are dying (from treatment with the test drug), they will be unable to invade across the membrane. It must be remembered that such factors may yield false results in the assay. Therefore, when determining the anti-invasive activity of drugs using the Boyden chamber method, an MTT and a proliferation assay must be done in parallel to make sure that the cells being treated remain healthy and viable throughout the assay. It is important to include positive controls in the experiment. For example, we have included the drug PP2, which is known to inhibit the invasion of cancer cells. Another control for the experiment may be primary normal cells, which undergo very little invasion. But it must be remembered that all cells (normal or cancerous) can acquire pro-invasive activity under the relevant physiological conditions.
Lastly, we discuss a few limitations of the Boyden chamber assay. The physiological relevance of transwell inserts to recapitulate invasion in vivo is poor [14,15]. The pore size of the membrane highly influences the number of cells that invade the membrane [14]. In its original format, the assay requires a large number of cells to obtain a sufficient signal, and the movement of cells through the filter cannot be visualized [6]. It can be difficult to control the concentration of the chemokine gradient, and this may produce aberrant or inconsistent results [14]. It is not possible to perform a dynamic determination of cell invasion since it is impossible to visualize the cells while they are migrating across the membrane. The data obtained from the Boyden chamber assay does not shed light on cell–cell and cell–matrix interactions [7,14,15]. An alternate method to measure cell invasion is the spherical invasion assay (SIA). The SIA analyzes the movement of human cancer cells as they migrate from a primary Matrigel layer, across the interface, and travel into a secondary Matrigel layer [16]. The SIA more accurately mirrors the actual process of invasion under physiological conditions [12]. The cells that grow in an ECM retain biological characteristics of tumors, such as responsiveness to diffusion gradient of oxygen, nutrients, and pH [17]. The growth of cells inside the ECM allows for complex cell–cell and cell–matrix interactions. The SIA can be adapted to organoids, spheroids [18], retinal angiogenic sprouts [19], tumor stem cells [20], neurospheres [21], and cells grown on polymeric scaffolds [22]. Such considerations reinforce the idea that the phenomenon of cell invasion should be studied (and validated) by using multiple experimental models. For example, when researchers are evaluating the anti-invasive activity of a drug, they should perform multiple independent assays like the Boyden chamber assay, microfluidic invasion assay, or spherical invasion assay and evaluate whether all these assays yield similar results, in order to infer that the drug has anti-invasive activity in vitro. Finally, the results obtained from the invasion experiments should be validated in an appropriate animal model to arrive at a definitive conclusion regarding the anti-invasive capacity of a drug or test compound.
Troubleshooting
Problem Possible cause Solution Very low signal or no signal in the assay •Cells not seeded densely enough, resulting in too few cells migrating across the porous membrane •It is a good idea to standardize the optimal seeding density of cell numbers for the Boyden chamber assay. The optimal cell number may vary from cell line to cell line. Consider setting up a calibration assay varying the number of cells in a range of 20,000 cells/mL to 2 million cells/mL and perform the assay. Choose the cell number that yields the highest signal/background ratio. • Insufficient incubation time. We measured the anti-invasive activity of capsaicin over 24 h. It may be possible that some compounds take longer to inhibit the invasion of cancer cells. Therefore, the optimal incubation time of the assay should be determined by performing a time-kinetics of the experiment. •Increase the incubation times for the assay. • Insufficient concentration of crystal violet • We use 0.25% crystal violet for the Boyden chamber assay. You can increase the concentration of crystal violet to 0.5%. • Air bubbles underneath the membrane • It is critical that the BioCoat Matrigel invasion chambers are properly seated in the notches. Make sure that there are no air bubbles underneath the membrane because the cells will not migrate through the dry patches. • Membrane is damaged • Do not puncture the membrane with a pipette tip or cotton swab
• Incorporate positive and negative controls for the assay (see General notes). Such controls may involve the use of known anti-invasive drugs and normal human cells (which invade minimally through the membrane).• Variability in FBS • The chemoattractant in the bottom chamber is media containing 20% FBS. Make sure FBS is sourced from a reliable company. There is inherent lot-to-lot variability in FBS. Aliquot the FBS and store at -70 °C and utilize the same batch of FBS for all assay replicates. Avoid excessive freeze/thaw cycles of the FBS. • Adding excessive FBS in the apical chamber • The chemoattractant (media with 20% FBS) should be placed in the lower, or basolateral, chamber. It slowly diffuses into the upper, or apical, chamber setting up a chemotactic gradient for the cells to move towards the membrane, degrade the Matrigel, and travel to the underside of the membrane.
If too much FBS is present in the apical compartment, the chemotactic gradient will be compromised. Resuspend the cells (to be seeded in the apical chamber) in media containing 0.1% BSA. Do not exceed 0.4% FBS in the apical chamber.• Cells have died or undergone cell cycle arrest during the assay • The treatment of cells with various drugs (or test agents) may significantly reduce cell viability or cell cycle arrest. In that case, the cells may lack the capacity to invade across the membrane. Performing an MTT and a proliferation assay in parallel to the Boyden chamber assay will ensure that the cells are not quiescent/dying during the assay (see General notes).
• Incorporate positive and negative controls for the assay (see General notes). Such controls may involve the use of known anti-invasive drugs and normal human cells (which invade minimally through the membrane).
• Before lysing the cells with DMSO, look at the invasion chamber with a microscope to ensure that there are cells on the underside of the membraneHigh background signal in the assay • Insufficient washing of the inserts
• Refer to step D4 and D7for directions to efficiently wash the inserts • Inefficient scrubbing of inserts to remove non-invading cells. • Refer to step D4 and D7for directions to efficiently scrub the inserts • Impurities on the porous membrane • Avoid touching the membrane of the insert by hand or setting the invasion chamber on a dirty surface. • Debris in cell culture flask or the cells are stressed out • Make sure that the cells are healthy, and the culture is free of bacterial contamination, debris, and foreign particles. It may be necessary to start a new culture from seed stocks or order new cells. Variation between replicates • Pipetting errors, insufficient replicates, improper washing of the inserts
• Use calibrated pipettes and avoid air bubbles
• Measure each sample in duplicate/triplicate
• Ensure that the membrane of the invasion chamber is not damaged. Damage to the membrane by forceps or cotton swabs can contribute to unexpected variation.
• Do not allow the membrane to dry out at any step.
• Refer to step D4 and D7 for directions to efficiently wash the inserts.Acknowledgments
We acknowledge Dr. S. Chellappan and his laboratory for their continuous support. This work was supported in part by the WV-INBRE Administrative T3 Supplements for Research on Women’s Health in the IDeA States (Grant #. 3P20GM103434-23W1). The women’s Health T3 grant was a supplement of the West Virginia IDeA Network of Biomedical Research Excellence (WV-INBRE) grant (NIH grant P20GM103434; PI: Dr. G. Rankin). P.D. and M.A.V. are supported by a National Institutes of Health R15 Academic Research Enhancement Award (Grants 1R15CA161491-01A1 and 2R15CA161491-02). M.A.V. is also supported by NIH R15AI15197-01 and R15HL145573-01. S.D.R. is a recipient of NSF-SURE and WV-NASA Space Consortium undergraduate fellowships respectively.
Our improved protocol of the Boyden chamber assay was based on the following publications [23–25].
Competing interests
The authors declare no competing interests.
References
- Fares, J., Fares, M. Y., Khachfe, H. H., Salhab, H. A. and Fares, Y. (2020). Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduction Targeted Ther. 5(1): 28.
- Gerstberger, S., Jiang, Q. and Ganesh, K. (2023). Metastasis. Cell 186(8): 1564–1579.
- Liu, M., Yang, J., Xu, B. and Zhang, X. (2021). Tumor metastasis: Mechanistic insights and therapeutic interventions. MedComm. 2(4): 587–617.
- Krakhmal, N. V., Zavyalova, M. V., Denisov, E. V., Vtorushin, S. V. and Perelmuter, V. M. (2015). Cancer Invasion: Patterns and Mechanisms. Acta Naturae. 7(2): 17–28.
- Novikov, N. M., Zolotaryova, S. Y., Gautreau, A. M. and Denisov, E. V. (2021). Mutational drivers of cancer cell migration and invasion. Br J Cancer. 124(1): 102–114.
- Boyden, S. (1962).The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 115(3): 453–466.
- Chen, H. C. (2005). Boyden Chamber Assay. In: Guan, J. L. (Ed.). Cell Migration. Methods in Molecular Biology, vol 294. Humana Press.
- Anderson, R. L., Balasas, T., Callaghan, J., Coombes, R. C., Evans, J., Hall, J. A., Kinrade, S., Jones, D., Jones, P. S., Jones, R., et al. (2019). A framework for the development of effective anti-metastatic agents. Nat Rev Clin Oncol. 16(3): 185–204.
- Stoletov, K., Beatty, P. H. and Lewis, J. D. (2020). Novel therapeutic targets for cancer metastasis. Expert Rev Anticancer Ther. 20(2): 97–109.
- Improgo, M. R. D., Johnson, C. W., Tapper, A. R. and Gardner, P. D. (2011). Bioluminescence-Based High-Throughput Screen Identifies Pharmacological Agents That Target Neurotransmitter Signaling in Small Cell Lung Carcinoma. PLoS One. 6(9): e24132.
- Hanke, J. H., Gardner, J. P., Dow, R. L., Changelian, P. S., Brissette, W. H., Weringer, E. J., Pollok, B. A. and Connelly, P. A. (1996). Discovery of a Novel, Potent, and Src Family-selective Tyrosine Kinase Inhibitor. J Biol Chem. 271(2): 695–701.
- Hurley, J. D., Akers, A. T., Friedman, J. R., Nolan, N. A., Brown, K. C. and Dasgupta, P. (2017). Non-pungent long chain capsaicin-analogs arvanil and olvanil display better anti-invasive activity than capsaicin in human small cell lung cancers. Cell Adhes Migr. 11(1): 80–97.
- Xu, J., Wang, H., Li, W., Liu, K., Zhang, T., He, Z. and Guo, F. (2020). E3 ubiquitin ligase CHIP attenuates cellular proliferation and invasion abilities in triple-negative breast cancer cells. Clin Exp Med. 20(1): 109–119.
- Guy, J. B., Espenel, S., Vallard, A., Battiston-Montagne, P., Wozny, A. S., Ardail, D., Alphonse, G., Rancoule, C., Rodriguez-Lafrasse, C., Magne, N., et al. (2017). Evaluation of the Cell Invasion and Migration Process: A Comparison of the Video Microscope-based Scratch Wound Assay and the Boyden Chamber Assay. J Visualized Exp.: 56337.
- Ritch, S. J., Brandhagen, B. N., Goyeneche, A. A. and Telleria, C. M. (2019). Advanced assessment of migration and invasion of cancer cells in response to mifepristone therapy using double fluorescence cytochemical labeling. BMC Cancer. 19(1): 376.
- Richbart, S., Merritt, J., Moles, E., Brown, K., Adeluola, A., Finch, P., Hess, J., Tirona, M., Miles, S., Valentovic, M., et al. (2022). Spherical Invasion Assay: A Novel Method to Measure Invasion of Cancer Cells. Bio Protoc. 12(4): e4320.
- Pampaloni, F., Reynaud, E. G. and Stelzer, E. H. K. (2007). The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 8(10): 839–845.
- Gunti, S., Hoke, A. T., Vu, K. P. and London, N. R. (2021). Organoid and Spheroid Tumor Models: Techniques and Applications.Cancers.13(4): 874.
- Stitt, A. W., McGoldrick, C., Rice-McCaldin, A., McCance, D. R., Glenn, J. V., Hsu, D. K., Liu, F. T., Thorpe, S. R. and Gardiner, T. A. (2005). Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes 54(3): 785–794.
- Atashzar, M. R., Baharlou, R., Karami, J., Abdollahi, H., Rezaei, R., Pourramezan, F. and Zoljalali Moghaddam, S. H. (2020). Cancer stem cells: A review from origin to therapeutic implications. J Cell Physiol. 235(2): 790–803.
- da Silva Siqueira, L., Majolo, F., da Silva, A. P. B., da Costa, J. C. and Marinowic, D. R. (2021). Neurospheres: a potential in vitro model for the study of central nervous system disorders. Mol Biol Rep. 48(4): 3649–3663.
- Stratton, S., Shelke, N. B., Hoshino, K., Rudraiah, S. and Kumbar, S. G. (2016). Bioactive polymeric scaffolds for tissue engineering. Bioact Mater. 1(2): 93–108.
- Nogueira, C., Kim, K. H., Sung, H., Paraiso, K. H. T., Dannenberg, J. H., Bosenberg, M., Chin, L. and Kim, M. (2010). Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 29(47): 6222–6232.
- Ogasawara, M., Matsubara, T. and Suzuki, H. (2001). Inhibitory Effects of Evodiamine on in Vitro Invasion and Experimental Lung Metastasis of Murine Colon Cancer Cells. Biol Pharm Bull. 24(8): 917–920.
- Shen, J., Xu, L., Owonikoko, T. K., Sun, S. Y., Khuri, F. R., Curran, W. J. and Deng, X. (2012). NNK promotes migration and invasion of lung cancer cells through activation of c-Src/PKCι/FAK loop. Cancer Lett. 318(1): 106–113.
Article Information
Publication history
Received: Apr 10, 2024
Accepted: Jun 30, 2024
Available online: Aug 2, 2024
Published: Aug 5, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Brown, K. C., Sugrue, A. M., Modi, K. J., Light, R. S., Conley, K. B., Cox, A. J., Bender, C. R., Miles, S. L., Valentovic, M. A. and Dasgupta, P. (2024). An Experimental Protocol for the Boyden Chamber Invasion Assay With Absorbance Readout. Bio-protocol 14(15): e5040. DOI: 10.21769/BioProtoc.5040.
Category
Cancer Biology > Invasion & metastasis > Cell biology assays > Cell invasion
Cell Biology > Cell-based analysis > Enzymatic assay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









