- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Protocol for Preparing Mucoadhesive Emulsion Microgels and Assessing Their Mucoadhesion Properties In Vitro
Published: Vol 14, Iss 13, Jul 5, 2024 DOI: 10.21769/BioProtoc.5027 Views: 1228
Reviewed by: Olga KopachAmira S HanafyMathilde UllrichAnonymous reviewer(s)
Abstract
Intravesical instillation is an efficient therapeutic technique based on targeted administration of a drug directly into the lesion for the treatment of bladder diseases. This is an alternative to traditional systemic administration of drugs. However, this technique requires repeated procedures, which can lead to even greater inflammation and infection of the urethra. To date, novel systems that allow prolonged drug retention in the bladder cavity are actively being developed. We recently reported a targeted drug delivery system based on the mucoadhesive emulsion microgels consisting of the natural component whey protein isolate. Such micron-sized carriers possess high loading capacity, a prolonged drug release profile, and efficient mucoadhesive properties to the bladder urothelium. As a continuation of this work, we present a protocol for the synthesis of mucoadhesive emulsion microgels. Detailed procedures for preparing precursor solutions as well as studying the physico-chemical parameters of microgels (including loading capacity and drug release rate) and the mucoadhesive properties using the model of porcine bladder urothelium are discussed. Precautionary measures and nuances that are worth paying attention to during each experimental stage are given as well.
Key features
• The protocol for the synthesis of mucoadhesive emulsion microgels based on whey protein isolate is presented. The experimental conditions of emulsion microgels synthesis are discussed.
• Methods for studying the physico-chemical properties of mucoadhesive emulsion microgels (size of emulsion microgels particles, loading capacity, release kinetics) are described.
• The method for assessing mucoadhesive properties of emulsion microgels is demonstrated using the porcine bladder tissue model ex vivo.
Keywords: Emulsion microgels (EM)Graphical overview
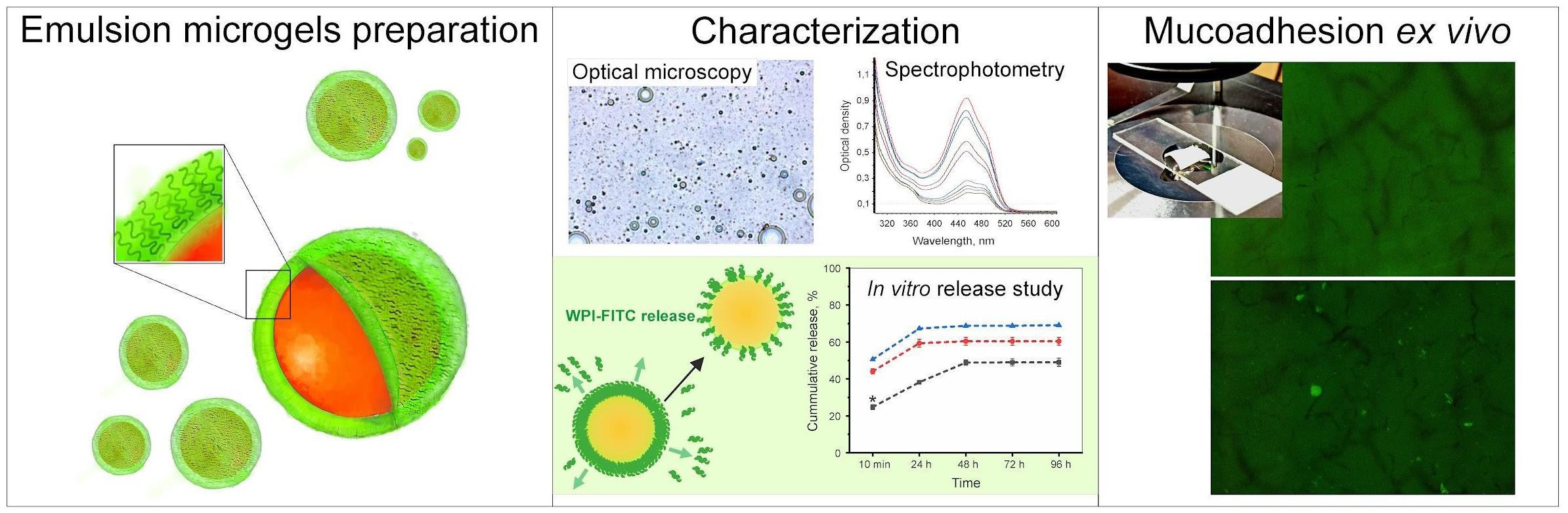
Background
Currently, for the treatment of urinary system diseases, such as cystitis or bladder cancer, targeted drug delivery using a catheter directly to the site of the disease is widely used. The intravesical instillation of antibacterial or cytostatic drugs has significant advantages over systemic administration; particularly, allowing the reduction of side effects on healthy organs (namely, inducing liver function disorders, undesirable effects on the central nervous system, and blood pressure, as well as delayed mutagenic, teratogenic, and carcinogenic effects of drugs) [1,2]. However, this delivery method has a number of disadvantages as well, which are conditioned primarily by the structure and basic physiological functions of the urinary system. The inner surface of the urinary bladder wall—the so-called urothelium—is represented by umbrella cells, whose size varies depending on their stretching degree. The surface of umbrella cells is covered with glycoproteins and proteoglycans, which form the glycosaminoglycan layer. This layer acts as an efficient barrier against the penetration of substances in the urine. However, at the same time, this layer prevents the penetration of drugs instilled to the bladder. Also, the constant flow of urine inside the bladder reduces the therapeutic effect and washes the drug out of the organ. Thus, it becomes necessary to repeat this procedure several times. According to feedback from patients receiving medications through intravesical instillation, such manipulation is quite painful. At the same time, there is a high risk of infection and inflammation of the urethra if catheterization is performed incorrectly [3].
The development of drug carriers that are able to retain drugs at the urothelium surface of the bladder for a long time might overcome these limitations. In this regard, the development of micro- and nano-sized carriers for intravesical drug delivery is of great interest. The most prominent strategies described in the literature include various types of carriers including thermosensitive hydrogels capable of undergoing sol-gel transition at body temperature as well as site-specific targeted liposomes and nanoparticles. However, in the first case, the natural dilution of the gels in the bladder leads to the loss of their gel-forming properties [4,5]. In the second case, in order to obtain such micro-sized systems, synthetic hydrophobic polymers are used, which are often dissolved in organic solvents that are mostly non-biocompatible and have irritating effects on the tissues [6–8].
In our recent work, we developed biocompatible and biodegradable emulsion microgels based on whey isolate protein with sufficient mucoadhesive properties. Targeting of such micro-sized carriers into the bladder will extend the residence time of drugs, reducing the number of instillation procedures [9]. Thus, this approach will help to significantly improve the quality of life of patients suffering from diseases of the urinary system. However, it is necessary to carefully follow the protocol for the formation of emulsion microgels, since changing the ratios of precursors can lead to the formation of particles with different loading and mucoadhesive properties.
The protocol here presented describes in detail the procedure for the synthesis of fluorescently labeled emulsion microgels based on whey protein isolate. Calculations of the optimal oil-to-water ratios for obtaining the most stable emulsion systems are given. We consider the main ways to characterize the resulting particles and provide a step-by-step description of the methodologies and troubleshooting aspects involved in determining the loading capacity of emulsion microgels as well as their drug release rates. In addition, we provide precise guidance on how to perform qualitative and quantitative analysis of the mucoadhesive ability of microgels on porcine bladder tissue ex vivo.
Materials and reagents
Reagents
Linseed oil OLEOS (Russia) (unrefined cold pressed linseed oil, refractive index n20/D 1.4795, density 0.93 g/mL at 25 °C), https://oleos-info.ru/product/lnyanoe-maslo/
Whey protein isolate (WPI) California Gold Nutrition (USA), https://www.californiagoldnutrition.com/products/california-gold-nutrition-sport-whey-protein-isolate-unflavored-5-lb-2-27-kg-76479 (27 g protein, 6.2 g BCAAs, 4.7 g glutamic acid, low lactose. Lactose is used as an excipient, which is not involved in the formation of emulsion microgels. No additives or flavor enhancers)
Phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: P4417)
Tetramethylrhodamine isothiocyanate (TRITC) (Sigma-Aldrich, catalog number: T0820)
Fluorescein isothiocyanate isomer I (FITC) (Sigma-Aldrich, catalog number: F7250)
Rhodamine B isothiocyanate (RITC) (Sigma-Aldrich, catalog number: 283924)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C4901)
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S6014)
Dimethyl sulfoxide (DMSO) (EcoChemAnalyt, Russia, CAS: 67-68-5)
Urea (CH4N2O) (ReaChem, Russia, State Standard 2081-92, CAS: 57-13-6)
Ammonium chloride (NH4Cl) (ReaChem, Russia, State Standard 3773-72, CAS: 12125-02-9)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (ReaChem, Russia, State Standard 4523-77, CAS: 10034-99-8)
Sodium sulfate (Na2SO4) (ReaChem, Russia, State Standard 4166-76, CAS: 7757-82-6)
Sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O) (ReaChem, Russia, State Standard 245-76, CAS: 13472-35-0)
Sodium hydrogen phosphate (Na2HPO4) (ReaChem, Russia, State Standard 11773-76, CAS: 7558-79-4)
Hydrochloric acid (HCl) (EcoChemAnalyt, Russia, State Standard 14261-77, CAS: 7647-01-0)
Sodium hydroxide (NaOH) (EcoChemAnalyt, Russia, State Standard 4328-77, CAS: 1310-73-2)
Trypsin (Sigma-Aldrich, catalog number: T4799)
Milli-Q water (Merck Millipore, model: Milli-QTM Advantage A10TM, Germany)
Note: It is possible to use linseed oil from another manufacturer. However, it is worth paying attention to its properties (the refractive index n20/D of 1.4795 and the density of 0.93 g/mL at 25 °C).
Solutions
Saline 0.9% NaCl (w/v) solution (see Recipes)
2.5% WPI solution (see Recipes)
5% WPI solution (see Recipes)
7.5% WPI solution (see Recipes)
5 mg/mL FITC solution (see Recipes)
1 M NaOH solution (see Recipes)
PBS buffer (0.1 M, pH 8.3) (see Recipes)
Artificial urine solution (see Recipes)
0.5 mg/mL trypsin in Tris-HCl buffer solution (20 mL) (see Recipes)
70% ethanol (see Recipes)
Recipes
Note: In all recipes, liquid is added to the dry sample until the final specified volume is reached (“total” volume) in order to obtain an accurate solution concentration. For example, in Recipe 1, H2O is added to the dry sample of NaCl until the final volume of 1 L is reached (instead of just adding 1 L of H2O to the dry sample of NaCl). The volume of liquid is indicated in tables as a necessary recommended volume (not as a precise volume).
Saline 0.9% NaCl (w/v) solution
*w/v: weight (g) per volume (100 mL).
Reagent Final concentration Amount NaCl 0.9 % (w/v) 9 g H2O n/a 1 L Total n/a 1 L 2.5% WPI (w/v) aqueous solution
Reagent Final concentration Amount WPI 2.5 % 250 mg Saline 0.9% NaCl solution 0.9% (w/v) 10 mL Total n/a 10 mL Caution: This solution should be mixed thoroughly. Before use, please wait until the foam at the surface of the solution disappears.
Note: For the FITC conjugation procedure, WPI solution is prepared as described above in Recipe 2, but instead of water the PBS (pH 8.3) is used for protein dissolution.
5% WPI (w/v) aqueous solution
Reagent Final concentration Amount WPI 5% 500 mg Saline 0.9% NaCl solution 0.9% (w/v) 10 mL Total n/a 10 mL Caution: This solution should be mixed thoroughly. Before use, please wait until the foam at the surface of the solution disappears.
Note: For the FITC conjugation procedure, WPI solution is prepared as described above in Recipe 3, but instead of water the PBS (pH 8.3) is used for protein dissolution.
7.5% WPI (w/v) aqueous solution
Reagent Final concentration Amount WPI 7.5% 750 mg Saline 0.9% NaCl solution 0.9% (w/v) 10 mL Total n/a 10 mL Caution: This solution should be mixed thoroughly. Before use, please wait until the foam at the surface of the solution disappears.
Note: For the FITC conjugation procedure, WPI solution is prepared as described above in Recipe 4, but instead of water the PBS (pH 8.3) is used for protein dissolution.
5 mg/mL FITC solution
Reagent Final concentration Amount FITC 5 mg/mL 5 mg DMSO n/a 1 mL Total n/a 1 mL Note: Since a 5 mg sample is difficult to weigh, it is recommended to prepare a larger weight of FITC, which will require a larger volume of DMSO to prepare the final solution with the given FITC concentration of 5 mg/mL. For this, you need to proportionally increase FITC mass and DMSO volume. For example, weigh out 25 mg of FITC, then add DMSO until the volume reaches 5 mL. In this way, you will obtain 5 mL of a final FITC solution of 5 mg/mL.
1 M NaOH solution (100 mL)
Reagent Final concentration Amount NaOH 1 M 4 g H2O n/a 100 mL Total n/a 100 mL PBS solution (0.1 M, pH = 8.3)
Reagent Final concentration Amount PBS 1× 1 tablet H2O n/a 200 mL Total n/a 200 mL Note: Adjust pH of the resulting solution to 8.3 using 1 M NaOH and 1 M HCl solutions.
Artificial urine solution (pH 6.2)
Reagent Final concentration Amount Urea n/a 24.27 g NaCl n/a 6.34 g KCl n/a 4.50 g NH4Cl n/a 1.61 g CaCl2 n/a 0.67 g MgSO4·7H2O n/a 1.0 g NaHCO3 n/a 0.34 g Na2SO4 n/a 0.26 g NaH2PO4·H2O n/a 1.0 g Na2HPO4 n/a 0.11 g H2O n/a 2 L Total n/a 2 L Note: The dry weights and the liquid volume can be changed proportionally to each other depending on needs (for example, for 1 L, the masses of dry samples are correspondingly reduced by two times relative to the masses given in the table). It is recommended to add weights in the order of priority shown in the table. The solution should be stirred for 3 h at 22 °C. The prepared artificial urine solution should be stored at 2–8 .
0.5 mg/mL trypsin in Tris-HCl buffer solution (20 mL)
Reagent Final concentration Amount Trypsin 0.5 mg/mL 10 mg Tris base 1 M 2.42 g H2O n/a 20 mL Total n/a 20 mL Note: Adjust pH of the Tris solution to 7.5 using HCl 1 M. The solution should be stored at 2–8 °C.
Ethanol 70%
Reagent Final concentration Amount Ethanol 96% 70% 1 L H2O n/a 371 mL Total n/a 1,371 mL Note: When mixing these liquids, you must strictly add ethanol to water, not vice versa.
Laboratory supplies
Centrifuge tubes with flat cap, 15 mL (JetBioFil, catalog number: CFT550150)
Centrifuge tubes with flat cap, 50 mL (JetBioFil, catalog number: CFT500500)
Microcentrifuge tubes, 1.5 mL (JetBioFil, catalog number: CFT000015)
Microcentrifuge tubes, 2.0 mL (JetBioFil, catalog number: CFT000020)
Laboratory glass jar 100 mL, with divisions, with screw lid, dark glass (MiniMed, catalog number: 10007205)
Vial 2 mL, dark glass (ALWSCI Technologies, catalog number: C0001177)
Vial 10 mL, clear glass, 22 mm × 52 mm (ALWSCI Technologies, catalog number: C0000053)
Dialysis bag M-Cel, pore diameter 14 kDa (Viscase, catalog number: 2141-1425)
Dialysis bag clamp (Scienova GmbH, catalog number: 40329)
Laboratory beaker, clear glass, 2 L (MiniMed, catalog number:10003807)
Cellulose acetate membrane filter, 0.45 μm (Sartorius, catalog number: 11106-37-N)
Membrane filters PP, 10 μm (Gluvex, catalog number: MLPP1001000)
Glass for microslides (MiniMed, catalog number: 12003421)
Cover glass for microslides (MiniMed, catalog number: 12003309)
Syringe 1 mL (BD Micro-Fine Plus, catalog number: 320935)
96-well V-bottom plate, conical bottom, non-treated, no lid (Costar, catalog number: 3897)
Pipette microtips, 2–20 μL (JetBioFil, catalog number: PPT100020)
Pipette microtips, 10–200 μL (JetBioFil, catalog number: PPT000200)
Pipette microtips, 100–1,000 μL (JetBioFil, catalog number: PPT000000)
Petri dishes, D 9.0 cm (JetBioFil, catalog number: MCD000090)
Adson microsurgical tweezers, 130 mm (Medical Equipment, catalog number: MF-2000)
Tissue tweezers, 130 mm (Medical Equipment, catalog number: MF-2102)
Equipment
Ultrasonic homogenization and Bandelin Sonopuls HD 2070 homogenizer at a frequency of 20 kHz and a power density of 1 W/cm2 (Germany)
Amicon® Stirred Cell 50 mL (Merck Millipore)
Magnetic stirrer (IKA)
Magnetic stirring bar (IKA, model: IKAFLON® 15)
Multifunctional refrigerated centrifuge (Eppendorf, model: 5810R)
Microplate reader (BMG Labtech, model: CLARIO Star Plus)
Drybath thermo shaker (Thermo Scientific)
Inverted microscope with a 40× objective (Olympus IX73)
Water purification system for ultrapure water (Merck Millipore, model: Milli-QTM Advantage A10TM)
Single-channel variable volume dispenser 100–1,000 mL, 10–100 mL, 20–200 mL, 5–50 mL, and 1,000–5,000 mL (Thermo Fisher Scientific)
Software and datasets
ClarioStar MARS 4.01 R2 (BMG LabTech, Germany)
ImageJ software 1.51j8 (National Institutes of Health, USA) (the open-source version is available online for free for download)
OriginPro 2018 SR1 (OriginLab Corporation, USA)
Excel 2019 (Microsoft Cooperation, USA)
Procedure
Conjugation of WPI by FITC
Prepare a solution of 5% WPI in PBS (0.1 M, pH 8.3). Dissolve 1 g of WPI in 20 mL of PBS.
Prepare a 2.5 mL FITC solution in DMSO (concentration 5 mg/mL) in a dark glass bottle.
Mix WPI and FITC solutions in a dark glass bottle. Stir the resulting solution using an IKA magnetic stirrer for 24 h in a dark place at 4 °C.
It is highly important to wash the obtained conjugated FITC-WPI solution out from the unreacted FITC molecules. This can be performed by using various methods such as dialysis, gel-filtration, retraining, or chromatography. In this work, we used dialysis against water. For this, place the prepared FITC-WPI solution in a dialysis bag (pore size 12–14 kDa). Secure the dialysis bag tightly with clamps at both ends to avoid leakage of the solution. Immerse the dialysis bag with FITC-WPI solution in 2 L of deionized water and keep it under stirring for 48 h in a dark place at 4 °C.
Store the freshly prepared FITC-conjugated WPI in a refrigerator without access to light.
Caution: Fluorescent dyes are sensitive to light. Therefore, all procedures with fluorescently labeled protein are carried out in dark glass bottles or covered with aluminum foil.
Note: The reaction of FITC-WPI conjugation can proceed faster if the process takes place at room temperature. In this case, the conjugation takes 8 h. However, the WPI solution cannot be stored for a long time at temperatures above 8 °C. For this reason, we highly recommend performing the conjugation at 4 °C for 24 h. The use of magnetic stirrer IKA MINI is suitable for such a long stirring.

Figure 1. Dark glass bottle with FITC-WPI conjugate solution
Formation of mucoadhesive emulsion microgels
Place 2 mL of 5% WPI-FITC solution in a 20 mL glass beaker.
Add 0.3 mL of linseed oil to 2 mL of WPI-FITC solution. This corresponds to a 1:3 ratio of the WPI solution:oil (wt:wt). A detailed scheme for the synthesis of emulsion microgels is presented in Figure 2 (upper panel).
Caution: It is necessary to strictly observe the proportions of the WPI phase:oil phase, since the particle size of the resulting emulsion microgels will be strongly dependent on this ratio. The higher the water:oil ratio, the larger the resulting diameter of the emulsion microgels particles. A smaller amount of aqueous phase may result in a deficiency of WPI molecules in the system and, as a result, fewer protein-stabilized emulsions. The water:oil ratio also influences the loading capacity of emulsion microgels and their rates of degradation and release of encapsulated substances. To obtain larger emulsion microgels particles in their diameter and number, it is necessary to proportionally increase the volume of oil. If the concentration of the initial WPI solution is changing, the volume of oil also changes according to Table 1 below.
Table 1. Ratios of WPI solutions and oil (w:w) for preparing emulsion microgels
WPI concentration Ratio WPI solution:oil (w:w) 1:1 1:3 1:5 2.5% [V (WPI), mL:V (oil), mL] (2.0:0.05) (2.0:0.15) (2.0:0.25) 5% [V (WPI), mL:V (oil), mL] (2.0:0.1) (2.0:0.3) (2.0:0.5) 7.5% [V (WPI), mL:V (oil), mL] (2.0:0.15) (2.0:0.45) (2.0:0.75) Immerse the probe of the ultrasonic homogenizer into the resulting mixture of WPI solution and oil. Apply ultrasound at a frequency of 20 kHz and a power density of 1 W/cm2 for 1 min to obtain the emulsion microgel.
Note: During the process of ultrasonic homogenization, it is possible to use additional cooling measures to prevent localized heating. However, this is not a necessary step due to the short duration of exposure of the mixture to the ultrasound.
Filter the obtained emulsion microgels using a filtration cell Amicon® stirred cell 50 mL and a filter membrane with 0.45 μm pore size.
Note: You can learn how to work with a Amicon® stirred cell using the user guide on the official website of Amicon: https://www.merckmillipore.com/RU/ru/product/Amicon-Stirred-Cell-50mL,MM_NF-UFSC05001?ReferrerURL=https%3A%2F%2Fwww.google.com%2F#anchor_UG.
Wash the emulsion microgels using the filtration cell with 10 mL saline (0.9% NaCl) and a filter membrane surface with a 0.45 μm pore size using the 1,000 mL single-channel dispenser until the supernatants become clear.
After filtration, dilute the obtained sedimented emulsion microgels at the 0.45 μm filter membrane up to 5 mL with saline (0.9% NaCl). Then, carefully collect diluted microgels from the membrane surface using a pipette and place it in a separate tube for a while until the next step.
Pass the obtained emulsion microgels through the 10 μm filter membrane in order to separate the large oil droplets. Collect the filtered emulsion microgels in the glass vial.
Store the freshly prepared emulsion microgels in the capped glass vial in a refrigerator at 4 °C. Photographs of the obtained samples of emulsion microgels are presented in Figure 2A–2C (middle panel).
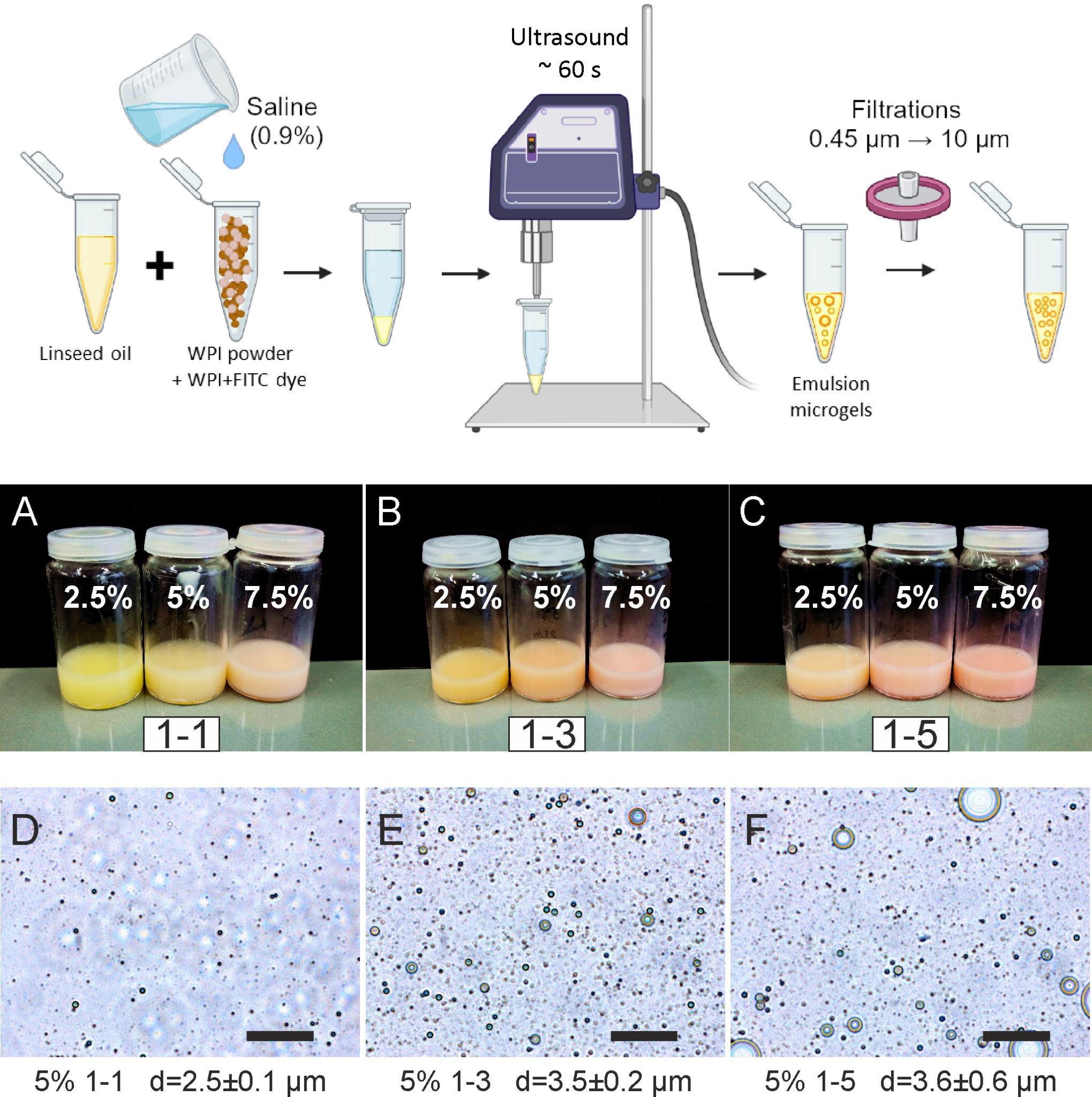
Figure 2. Preparation procedure and overview of the resulting emulsion microgels. Upper panel: Schematic process of emulsion microgels preparation. A–C: Photographs of prepared emulsion microgels with different ratios of WPI solution–oil and different concentrations of WPI solutions used for sample preparations. D–F: Optical images of emulsion microgels with different ratios of WPI solution–oil and fixed concentration of WPI solution (5%). The average diameters of particles of emulsion microgels are given as mean ± standard deviation.
Determination of emulsion microgel sizes
Diluted the initial emulsion microgel sample 1,000 times with saline (0.9% NaCl). Place 5 μL of diluted emulsion microgel onto a glass slide and cover with a coverslip.
Obtain optical images of emulsion microgels with the Olympus IX73 inverted microscope and a 40× objective (Figure 2D–2F).
Measure the diameters of emulsion microgels particles using optical images (Figure 2D–2F). Perform measurements using the ImageJ software. To calculate the average diameter of emulsion microgel particle size, at least 100 measurements and 10 images for each sample were analyzed. The average diameter of particles was presented as the mean ± standard deviation.
Note: The Olympus IX73 inverted microscope contains a fluorescent module. However, to calculate the number of particles in this work, brightfield images were used.
In vitro release study
Place 1 mL of emulsion microgel sample in a 2 mL Eppendorf tube. Add 1 mL of the artificial urine and mix using the vortex for 1 min. Prepare three independent samples for each time point of probing (24, 48, 72, 96, and 120 h). The whole route of release study is represented at Figure 3 (upper panel).
Place samples in a Drybath thermo shaker at 22 °C with continuous shaking (600 rpm).
At specific time points (10 min, 24, 48, 72, 96, and 120 h), take the samples from the Thermo shaker and centrifuge at 14,500× g for 3 min until the separation of oil and water phases occurs.
After centrifugation, the aqueous and oil phases should be separated (oil phase layer covers the aqueous phase on top). Probe the 1 mL of aqueous phase of the sample using a syringe needle.
Add 1 mL of fresh medium to the sample and mix it thoroughly with a vortex until the emulsion becomes homogeneous (usually it takes 3–5 min). Then, return it to the thermo shaker.
D1. Preliminary preparation of obtained probes for spectrophotometric analysisDilute 1 mL of obtained probes of aqueous phase with 1 mL of ethanol 70% in order to sediment the residual protein and dissolve the residual oil. Centrifuge the mixture at 14,500× g at room temperature for 3 min.
Separate the aqueous phase from sediment. The obtained probes are ready for spectrophotometric analysis.
Note: A dilution of obtained probes may be necessary before the spectrophotometric measurements. For this purpose, a stock solution of artificial urine is needed.
Spectrophotometric determination of FITC-WPI amount encapsulated and released from the emulsion microgels
Place 200 μL of each probe in triplicates (prepared as described above in section D) in a Costar® 96-cell microplate.
Analyze absorbance spectra in a range of 200–1,000 nm with the CLARIO Star Plus microplate reader (Figure 3A).
The absorbance maximum for FITC in artificial urine solution is registered at 478 nm at FITC concentration ranging from 5 to 50 μg/mL (Figure 3A).
The amount of FITC-WPI in probes is determined on the basis of optical density value at 478 nm using the corresponding calibration curves (Figure 3B). In order to obtain calibration curves, absorption spectra of solutions with various FITC concentrations in media were measured. For this, a solution of FITC in DMSO with a concentration of 1 mg/mL was prepared. Then, a series of solutions of known FITC concentrations ranging from 5 to 50 μg/mL were made (5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, and 50 μg/mL). These solutions were obtained by diluting a 1 mg/mL fluorescent dye solution with a stock solution of artificial urine (see step D1, note). Then, 200 μL of solutions of known concentrations were placed into the wells of the Costar® 96-cell microplate and measured spectrophotometrically. Based on the optical density at the fluorescent dye absorption maximum and known concentrations of FITC in solutions (Figure 3A), calibration curves were constructed (R2 = 0.998) (Figure 3B).
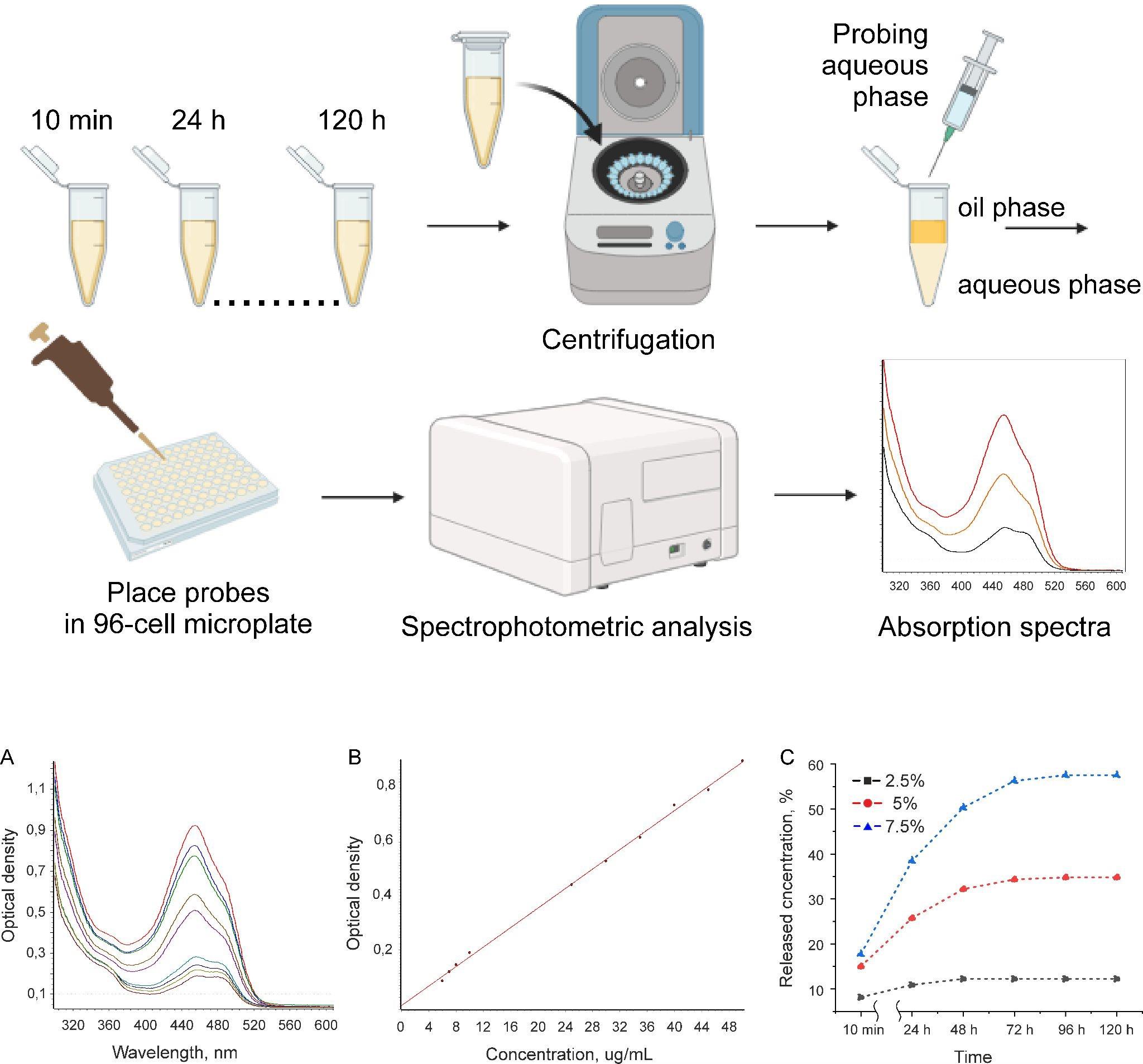
Figure 3. Overview of experiment on the model drug release from emulsion microgels. Upper panel: schematic process of release experiment. Bottom panel: (A) Characteristic adsorption spectra of FITC in artificial urine for different FITC amounts; (B) calibration curve of FITC in artificial urine; (C) kinetics of FITC-WPI release from emulsion microgels in artificial urine. Data are presented as the mean ± standard error calculated from three measurements for each sample.To obtain the release kinetics curves, the calculated amounts of FITC-WPI in probes received during the experiment at specific time points (10 min, 24, 48, 72, 96, and 120 h) (see section D) were plotted against the time of incubation of the emulsion microgels in model medium (artificial urine). The dependence of the released FITC-WPI concentration from the emulsion microgels on the incubation time is shown in Figure 3C. The most stable and prolonged release of WPI-FITC is observed for the sample (7.5%, 1–3). The maximum released amount is observed after 96 h and is ~58% of total FITC amount loaded in the microgels. In the case of the sample (5%, 1–3), the release kinetics profile reaches a maximum level after 48 h. In the case of the sample (2.5%, 1:3), a statistically significant increase in FITC-WPI concentration was detected after 10 min of incubation.
Quantification of FITC-WPI loading capacity in emulsion microgels
The amount of FITC-WPI in filtrates obtained during the process of sample preparation (Msolution) (as described in section D) was measured in order to determine the FITC-WPI loading capacity in emulsion microgels. The filtrates were processed as described in step D1. The amount of FITC in filtrates was determined spectrophotometrically as described in section E.
Note: In this case, the stock solution used in probes preparation and dilutions was the mixture of ethanol 70%: saline NaCl 0.9% (at a 1:1 ratio).
The loading capacity of FITC-WPI in emulsions was calculated as:
where Minitial is the amount of FITC in the initial conjugated FITC-WPI solutions, which were used in the preparation of emulsions; Msolution is the amount of FITC-WPI in solutions obtained after emulsion filtration.
Mucoadhesion properties and retention of the emulsion microgels on porcine urinary bladder tissues
Porcine bladder tissues were obtained from the Saratov State Vavilov Agrarian University (Saratov, Russia). The freshly extracted urinary bladders were immediately packed with dry ice and transported in a cold box. The experiment started immediately after the urinary bladder was received.
Carefully cut a bladder tissue to samples with size 1 cm × 1 cm and wash with 3 mL of artificial urine solution. Cut samples with meticulous precision and accuracy to keep the upper urothelial layer intact.
Perform background fluorescence microscopy imaging on each tissue sample before application of the emulsion microgels.
Apply 100 μL of emulsion microgels solution to the mucosal surface of urinary bladder samples with a pipette, maintaining its uniform spreading on the bladder surface, and keep for 10 min. After that, remove non-absorbed emulsion microgels by washing with 6 mL of the artificial urine solution three times. For this, intensively pour the artificial urine from the pipette tip on the surface of the bladder tissue sample, which is placed in the Petri dish (9 cm in diameter). After that, collect spent artificial urine from the Petri dish. The experiment was conducted in triplicate for each type of emulsion microgels.
Place porcine bladder tissues on the microscope stage of inverted microscope Olympus IX73 with a 40× objective (Figure 4A). The fluorescence of WPI-FITC was excited at the wavelength range 470–495 nm, and the emission was detected at the wavelength range 510–550 nm.
Caution: It is important to begin the experiment promptly after obtaining a fresh porcine bladder since the mucosal layer of the urothelium begins to degrade almost immediately.
Figure 4B shows a fluorescent image of the surface of porcine bladder tissue treated with the emulsion microgels sample (5%, 1:3) for 10 min and after a single wash with artificial urine.
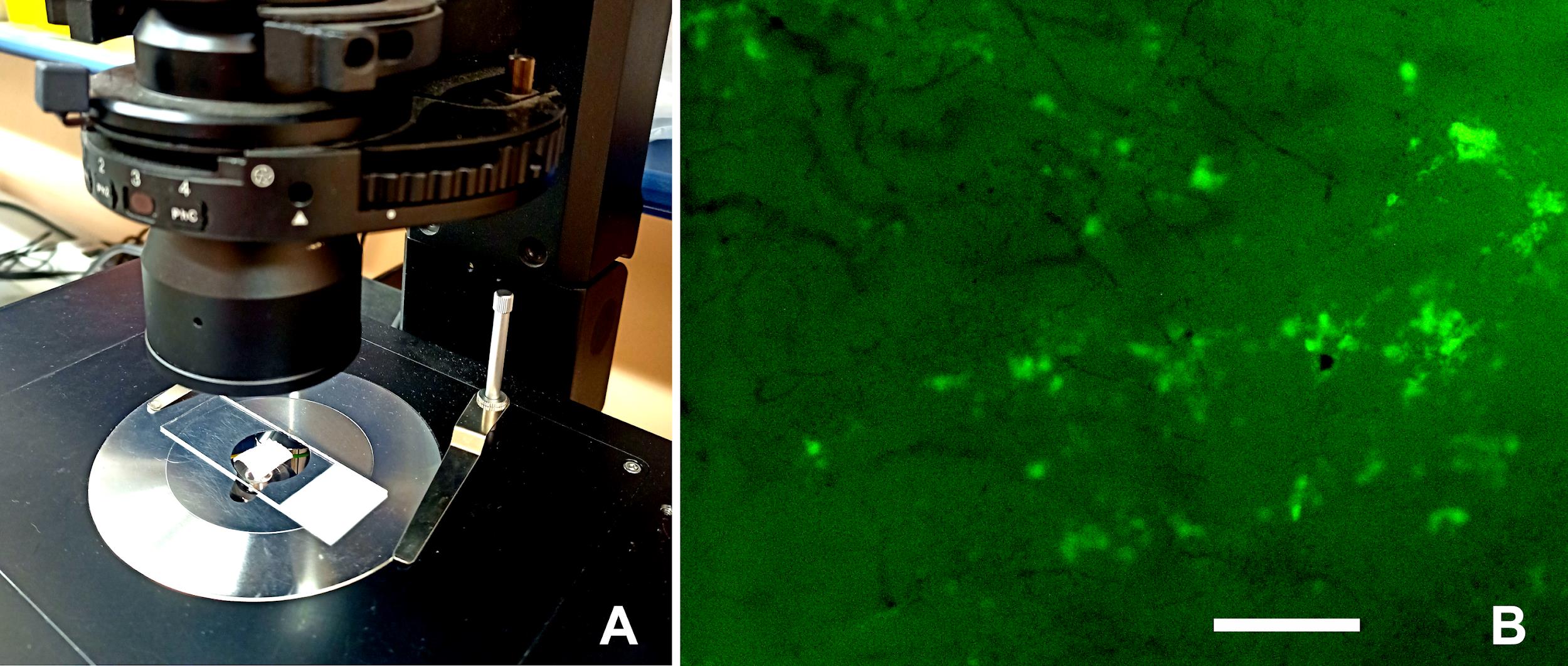
Figure 4. Study of fluorescence signals from FITC-labeled emulsion microgels adsorbed on porcine urinary bladder tissue. (A) Porcine urinary bladder tissue on the microscope stage. (B) Optical fluorescent images of the urothelium surface of porcine bladder after its incubation with emulsion microgels samples and after its one-time washing with artificial urine. Exposure time, 50 ms. Scale bar: 100 μm.
Quantitative estimation of WPI-FITC content in bladder mucosa
Incubate urinary bladder tissues in 1 mL of trypsin solution in Tris-HCl buffer for 60 min at 37 °C and continuous shaking at 500 rpm. After that, centrifuge obtained homogenates at 400× g for 1 min at room temperature. After that, collect the liquid phase with a pipette.
Add 200 μL of obtained liquid phase to the Costar® 96-cell microplate (in triplicate). Emission spectra (475 nm excitation, 490–510 nm emission range with 1 nm step) were recorded with the CLARIOstar Plus microplate reader.
Intact urinary bladder tissues homogenates mixed with known FITC amounts (40, 30, 20, 10, 5, and 2.5 μg/mL) were used as solutions for obtaining the calibration curve and prepared in the same approach.
The emission intensity at 500 nm was used in the calibration and calculation of the WPI-FITC content in bladder tissues.
Data analysis
Calculation of droplet sizes of emulsion microgels
For calculating the average emulsion droplet size, at least 100 measurements and 10 images for each sample were analyzed. The ImageJ software was used for image processing and statistics. At the first step, upload the optical image of the emulsion microgels into the ImageJ software. Before measurement, calibrate the scale of this image in length units (set scale). Then, measure the diameter of an emulsion droplet directly at the image by means of available tools of measuring linear dimensions in this program (for example, a segment). Based on 100 measurements, calculate the average particle diameter as mean ± standard deviation using statistical methods in Excel. ANOVA can be used to compare average diameters of particles of different types of emulsion microgels. A p-value of < 0.05 is considered as statistically significant.
Quantitative measurement of FITC-WPI amount
The amounts of FITC-WPI, which were (i) encapsulated in emulsion microgels, (ii) released during incubation model media, and (iii) retained on the urothelium of the porcine bladder, were measured spectrophotometrically based on their absorption spectra.
All experiments were carried out in triplicate to obtain statistically significant results. Based on three measurements, average amounts of FITC-WPI were calculated and presented as mean ± standard deviation.
ClarioStar MARS software was used to obtain calibration lines and calculate the FITC-WPI average amount.
The Origin software was used to obtain the release kinetics curves.
Validation of protocol
The whole procedure is validated in our recent work and supplemental information.
Saveleva et al. [9]. Mucoadhesive Emulsion Microgels for Intravesical Drug Delivery: Preparation, Retention at Urothelium, and Biodistribution Study. ACS Applied Materials and Interfaces (Figure 1, panels B–D, H–J; Figure 3, panel D, Figure S-3, panel “2.5% 1:3, 1 wash”).
General notes and troubleshooting
General notes
We assume that the presented protocols for characterizing and testing WPI-based emulsion microgels can also be adapted (with minor modifications) to various other types of emulsion-based carriers containing mucoadhesive components (e.g., polysaccharides, proteins, and biopolymers).
This protocol, with minimal adaptive modifications, could also be applied to the studies on other model mucous tissues containing a mucosal layer, for example, mucous membranes of the intestines, eyes, nose, and throat, to study the mucoadhesive ability of various drug carriers (considering the fluorescent labeling of these carriers).
Troubleshooting
To maintain better stability and long-term storage of emulsion microgels, it is recommended to add a preserving agent (for example, sodium azide) to the WPI solution at the preparation stage. Store the prepared emulsion microgels in the refrigerator at 4 °C.
During long-term storage, slight phase separation and the formation of a layer of large oil droplets on the surface are possible. To bring samples of emulsion microgels to their initial condition, subject them to ultrasonication (frequency of 20 kHz and a power density of 1 W/cm2) with the ultrasound probe for 1 min. This will allow re-homogenization of the emulsion microgels.
The absence of a fluorescent signal from emulsion microgels after application to the surface of the bladder is possible at a low concentration of the encapsulated fluorescent dye. In this case, it is necessary to optimize the settings of the fluorescent microscope. It is worth considering that living tissues have their own autofluorescence, in which case it is necessary to adjust the maximum level of fluorescent signal for samples with the maximum dye content.
Acknowledgments
This study was supported by the Russian Science Foundation (project No. 21-75-10042), https://rscf.ru/project/21-75-10042. This protocol was adapted from Saveleva et al. [9]. We also acknowledge all contributors to prior work [9] in which this protocol is based.
Competing interests
The authors declare no competing interests.
Ethical considerations
No human subjects are involved in this work.
References
- Kolawole, O. M., Lau, W. M., Mostafid, H. and Khutoryanskiy, V. V. (2017). Advances in intravesical drug delivery systems to treat bladder cancer. Int J Pharm. 532(1): 105–117.
- GuhaSarkar, S. and R Banerjee, R. (2010). Intravesical Drug Delivery: Challenges, current status, opportunities and novel strategies. J Control Release. 148(2): 147–159.
- Ognenovska, S., Mukerjee, C., Sanderson-Smith, M., Moore, K. H. and Mansfield, K. J. (2022). Virulence Mechanisms of Common Uropathogens and Their Intracellular Localisation within Urothelial Cells. Pathogens. 11(8): 926.
- Karavana, S., Ay Şenyiğit, Z., Çalışkan, Ã., Sevin, G., İlem Özdemir, D., Erzurumlu, Y., Şen, S. and Baloğlu, E. (2018). Gemcitabine hydrochloride microspheres used for intravesical treatment of superficial bladder cancer: a comprehensive in vitro/ex vivo/in vivo evaluation. Drug Des Devel Ther. 1959–1975.
- Tamura, K., Kikuchi, E., Konno, T., Ishihara, K., Matsumoto, K., Miyajima, A. and Oya, M. (2015). Therapeutic effect of intravesical administration of paclitaxel solubilized with poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate) in an orthotopic bladder cancer model. BMC Cancer. 15(1): 317.
- Frangos, D. N., Killion, J. J., Fan, D., Fishbeck, R., Von Eschenbach, A. c. and Fidler, I. J. (1990). The Development of Liposomes Containing Interferon Alpha for the Intravesical Therapy of Human Superficial Bladder Cancer. J Urology. 143(6): 1252–1256.
- Rajaganapathy, B. R., Chancellor, M. B., Nirmal, J., Dang, L. and Tyagi, P. (2015). Bladder Uptake of Liposomes after Intravesical Administration Occurs by Endocytosis. PLoS One. 10(3): e0122766.
- Tyagi, P., Chancellor, M. B., Li, Z., de Groat, W. C., Yoshimura, N., Fraser, M. O. and Huang, L. (2004). Urodynamic and Immunohistochemical Evaluation of Intravesical Capsaicin Delivery Using Thermosensitive Hydrogel and Liposomes. J Urology. 171(1): 483–489.
- Saveleva, M. S., Lobanov, M. E., Gusliakova, O. I., Plastun, V. O., Prikhozhdenko, E. S., Sindeeva, O. A., Gorin, D. A. and Mayorova, O. A. (2023). Mucoadhesive Emulsion Microgels for Intravesical Drug Delivery: Preparation, Retention at Urothelium, and Biodistribution Study. ACS Appl Mater Interfaces. 15(21): 25354–25368.
Article Information
Publication history
Received: Mar 21, 2024
Accepted: Jun 4, 2024
Available online: Jun 24, 2024
Published: Jul 5, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Saveleva, M. S., Lobanov, M. E. and Mayorova, O. A. (2024). A Protocol for Preparing Mucoadhesive Emulsion Microgels and Assessing Their Mucoadhesion Properties In Vitro. Bio-protocol 14(13): e5027. DOI: 10.21769/BioProtoc.5027.
Category
Biological Engineering > Biomedical engineering
Medicine
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










