- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fast, Easy, and Comprehensive Techniques for Microscopic Observations of Fungal and Oomycete Organisms Inside the Roots of Herbaceous and Woody Plants
Published: Vol 14, Iss 11, Jun 5, 2024 DOI: 10.21769/BioProtoc.5013 Views: 2120
Reviewed by: Lucy XieAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth
Zeinab Fotoohiyan and Ali Salehi Sardoei
Oct 5, 2025 1269 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1452 Views
In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1560 Views
Abstract
The roots of herbaceous and woody plants growing in soil are complex structures that are affected by both natural and artificial fungal colonization to various extents. To obtain comprehensive information about the overall distribution of fungi or oomycetes inside a plant root system, rapid, effective, and reliable screening methods are required. To observe both fine roots, i.e., a common site for penetration of fungi and oomycetes, and mature roots, different techniques are required to overcome visual barriers, such as root browning or tissue thickening. In our protocol, we propose using fast, cost-effective, and non-harmful methods to localize fungal or oomycete structures inside plant roots. Root staining with a fluorescent dye provides a quick initial indication of the presence of fungal structures on the root surfaces. The protocol is followed by clearing and staining steps, resulting in a deeper insight into the root tissue positioning, abundance, and characteristic morphological/reproductive features of fungal or oomycete organisms. If required, the stained samples can be prepared by using freeze-drying for further observations, including advanced microscopic techniques.
Key features
• The protocol enhances tissue-clearing techniques employing KOH or NaOH and is applicable to a broad range of roots from different plant species.
• Hydroxides are mixed with hydrogen peroxide to obtain an efficient bleaching solution, which effectively clears roots without causing significant tissue damage.
• The protocol could also be used for staining of fungi or oomycetes localized both on the root surface or inside the root tissues.
• Simple combination of non-fluorescent methyl blue and fluorescent solophenyl flavine dyes allows the observation of fungal organisms in both brightfield and fluorescence microscopy.
Keywords: Fungi stainingGraphical overview
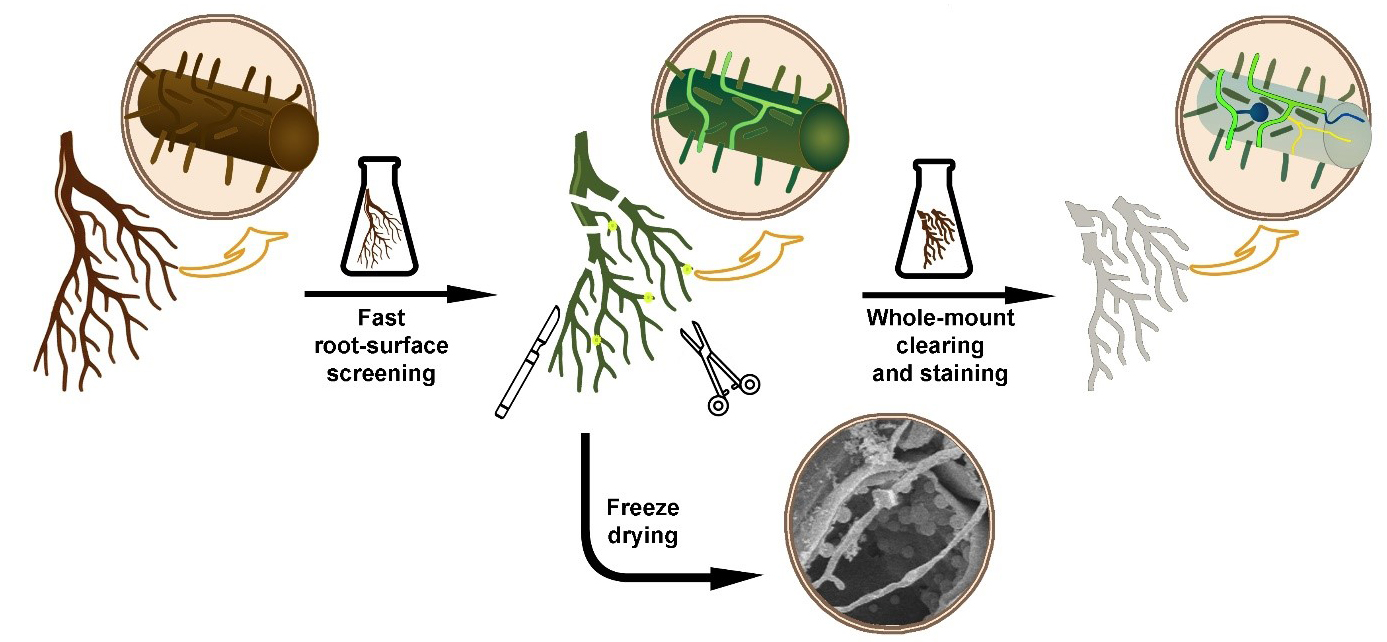
Background
Fungal and oomycete organisms, regardless of whether they are pathogenic or symbiotic, enter the roots of herbaceous or woody plants and grow inside their tissues. However, the minimal optical transparency of such roots is a key factor limiting direct microscopic observations of colonizing fungal organisms inside their root environment. Thin and relatively transparent young roots still do not allow efficient microscopic observation of their internal structures, including inside growing fungal organisms. In addition, older roots are much thicker and accumulate a lot of colorants, which deteriorates the observation at a microscopic level. Finally, when looking for fungi inside the root, they must be stained to contrast with the root tissues. To overcome these problems, diverse plant microtechniques allow different ways to visualize and effectively locate the fungal organism inside root tissues, for example, whole-mount root clearing and subsequent staining of fungal organisms [1], root hand sectioning followed by clearing and staining [2], or marking the fungi with fluorescent GFP [3]. The effectiveness of such techniques is limited by the time needed for their realization. Processing large root systems of herbs and especially woody plants requires a lot of time to find and visualize the fungal organisms in them. The main aim of this protocol is to combine the above-mentioned techniques of fluorescent marking and whole-mount clearing to offer a fast and easy way to observe the fungal and oomycete organisms inside the root tissues.
A key feature that enables staining at the root surface is the fact that fluorescent dye solophenyl flavine 7GFE 500 (syn. Direct Yellow 96) provides a fluorescent signal from the fungal structures but not from the root cells. Solophenyl flavine was introduced by Hoch et al. [4] as a new dye applicable for fungal cell-wall staining. Like calcofluor-white, a cellulose-specific fluorescent dye, solophenyl flavine also stains fungal structures. Moreover, the excitation and emission wavelengths of solophenyl flavine are shifted toward the red end of the spectrum, so the excitation is less dependent on UV or violet light. Moreover, the bright yellow–green signal from solophenyl flavine–stained fungi strongly contrasts against the background of a dark root. This makes screening effective for a quick inspection of the root surface. If deeper inspection of the root is required, clearing by removing cell protoplasm makes root tissues transparent. This is normally performed by using chemicals like NaOH or KOH [5]. If dark colorants still persist inside the root tissues, they need to be bleached before staining [6]. Hydrogen peroxide can bleach the roots and make them white. In this protocol, we use a mixture of NaOH (or KOH) with hydrogen peroxide as an efficient clearing/bleaching solution [7]. Metals that could be released from the samples, leading to reduced activity of the hydrogen peroxide, are chelated following the addition of sodium citrate. As hydrogen peroxide is extremely reactive, which results in bubble release, magnesium sulfate is added to prevent tissue damage caused by the air bubbles [8]. A key feature that allows staining inside the root is a mild clearing procedure that protects the root tissue structures and the fungal structures inside the root. Clearing also protects cell-wall binding sites, important for both cell wall–dye interaction and effective staining. Roots cleared this way can be stained again using solophenyl flavine. In some cases, however, there is a need for brightfield staining of fungal organisms. Dyes commonly used for non-fluorescent fungal staining, such as trypan blue [9], chlorazol black [10], or lactophenol cotton blue [11], are effective but harmful substances. In this protocol we use modified lactophenol cotton blue solution without phenol, which is harmful. Instead, methyl blue is mixed with glycerol and lactic acid to obtain blue staining of fungal organisms. If required, the protocol also offers a possibility of mixing methyl blue with solophenyl flavine and staining for both brightfield and fluorescence microscopy in one step. The proposed protocol improves the existing root-clearing techniques by using a shorter time and a lower clearing temperature. This improvement is achieved by combining clearing and bleaching into a single step and delicate sample handling.
Materials and reagents
Biological materials
Alnus glutinosa, Oryza sativa, or Arabidopsis thaliana roots or any other root material
Reagents
Potassium hydroxide (KOH) (Centralchem, CAS: 1310-58-3)
Sodium hydroxide (NaOH) (Centralchem, CAS: 1310-73-2)
Hydrogen peroxide, 50% (H2O2) (Centralchem, CAS: 7722-84-1)
Sodium citrate dihydrate [HOC(COONa)(CH2COONa)2·2H2O] (Sklochem-Agroekolab, CAS: 6132-04-3)
Magnesium sulphate heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, CAS: 10034-99-8)
Methyl blue (C37H27N3Na2O9S3) (Fisher Chemical, CAS: 28983-56-4) (please do not confuse with methylene blue)
Glycerol [HOCH2CH(OH)CH2OH] (Duchefa Biochemie, CAS: 56-81-5)
Lactic acid, 80% [CH3CH(OH)COOH] (Mikrochem, CAS: 50-21-5)
Solophenyl flavine 7GFE 500 (syn. Direct Yellow 96) (C39H34N10O13S4) (abcr Germany, CAS: 61725-08-4)
1% HCl
Solutions
1% NaOH bleaching solution—sample clearing and bleaching (see Recipes)
10% KOH bleaching solution—sample clearing and bleaching (see Recipes)
0.06% methyl blue—staining solution (stock) (see Recipes)
0.006% methyl blue—non-fluorescent fungi staining (working solution)
0.1% solophenyl flavine—fluorescent fungi staining (see Recipes)
Methyl blue + solophenyl flavine mixture—combined fluorescent and non-fluorescent fungi staining (see Recipes)
Recipes
1% NaOH bleaching solution (BS)
To prepare 100 mL of 1% NaOH BS, first dissolve 0.9 g of NaOH in 90 mL of distilled water. Add 1 g of sodium citrate dihydrate and 0.03 g of magnesium sulphate heptahydrate into 90 mL of 1% NaOH. Using a stirrer to dissolve all components. A large volume of prepared solution may be stored for months in a dark place. Mix thoroughly before using.
Prior to clearing, add 10 mL of 50% H2O2 into 90 mL of the prepared NaOH + sodium citrate dihydrate + magnesium sulphate heptahydrate solution. Stir shortly to minimize hydrogen peroxide reaction but be sure to mix the prepared solution well.
Reagent Final concentration Quantity or Volume NaOH 1% (w/v) 0.9 g Sodium citrate dihydrate 1% (w/v) 1 g Magnesium sulphate heptahydrate 0.03% (w/v) 0.03 g Hydrogen peroxide 5% (v/v) 10 mL H2O 90 mL Total 100 mL 10% KOH bleaching solution (BS)
Alternatively, for 10% potassium hydroxide bleaching solution (KOH BS), dissolve 9 g of KOH in 90 mL of distilled water. Other chemicals and steps are applied in the same order as for Recipe 1.
Reagent Final concentration Quantity or Volume KOH 10% (w/v) 9 g Sodium citrate dihydrate 1% (w/v) 1 g Magnesium sulphate heptahydrate 0.03% (w/v) 0.03 g Hydrogen peroxide 5% (v/v) 10 mL H2O 90 mL Total 100 mL Although hydrogen peroxide alone can bleach, mixing NaOH or KOH with hydrogen peroxide shortens the time required for clearing. Individual clearing with NaOH or KOH followed by hydrogen peroxide will work too, but mixed compounds work faster.
0.06% methyl blue stock solution
To make 0.06% methyl blue stock solution, dissolve methyl blue in water using a stirrer. Then, mix glycerol with lactic acid. Finally, add methyl blue solution into glycerol/lactic acid mixture and stir well. This solution can be stored in a cool, dark place for months. Before staining, prepare 0.006% working solution by diluting 5 mL of methyl blue stock solution in 50 mL of distilled water.
Reagent Final concentration Quantity or Volume Methyl blue 0.06% (w/v) 0.03 g Glycerol 50% (v/v) 25 mL Lactic acid 80% 25% (v/v) 12.5 mL H2O 12.5 mL Total 50 mL 0.1% solophenyl flavine staining solution
To prepare a 0.1% solophenyl flavine staining solution, dissolve 0.025 g of solophenyl flavine powder in 25 mL of distilled water and mix thoroughly. This solution can be stored in a cool, dark place for one month.
Reagent Final concentration Quantity or Volume Solophenyl flavine 0.1% (w/v) 0.025 g H2O 25 mL Total 25 mL Methyl blue + solophenyl flavine mixture
To obtain 50 mL of methyl blue + solophenyl flavine mixture, add 1 mL of 0.1% solophenyl flavine into 49 mL of 0.006% methyl blue working solution. Stir well.
Laboratory supplies
Petri dishes of various size (it depends on the size of the examined root system) for root processing and staining:
Petri dish Ø150 mm (Fisher Slovakia, catalog number: 1215.1525)
Petri dish Ø120 mm (Fisher Slovakia, catalog number: 1215.1220)
Petri dish Ø50 mm (Fisher Slovakia, catalog number: 1215.0512)
Petri dish Ø40 mm (Fisher Slovakia, catalog number: 1215.0412)
Crystallizing dish 500 mL (Fisher Slovakia, catalog number: 1212.0115)
Beaker 250 mL (Fisher Slovakia, catalog number: 1112.0250)
Beaker 150 mL (Fisher Slovakia, catalog number: 1112.0150)
Beaker 50 mL (Fisher Slovakia, catalog number: 1112.0050)
Glass slide 76 mm × 26 mm (Fisher Slovakia, catalog number: 1820.1100)
Cover glass 24 mm × 50 mm (Fisher Slovakia, catalog number: 1820.1250)
Parafilm M 50 mm × 50 mm (Fisher Slovakia, catalog number: 2105.6001)
Razor blade (Agar Scientific, catalog number: AGT5115)
Laboratory scissors (Fisher Slovakia, catalog number: 2305.7711)
Laboratory needle (Fisher Slovakia, catalog number: 2305.4822)
Laboratory tweezer (Fisher Slovakia, catalog number: 2305.4143)
Scalpel (Fisher Slovakia, catalog number: 2305.9004)
Disposable plastic Pasteur pipette (Fisher Slovakia, catalog number: 2101.6460)
Pasteur glass pipette (Fisher Slovakia, catalog number: 1780.0150)
Magnetic stir bar (Fisher Slovakia, catalog number: 6115.4008)
Micropipette 1,000 µL (Fisher Slovakia, catalog number: 4052.0050)
Flat paint brush
Equipment
Heating plate with regulated temperature (30–70 °C)
Light (brightfield) and fluorescent microscope (Leica, model: DM4000) equipped with a Leica fluorescence filter A, D, I3 or any other filter with the following characteristics: excitation 400 nm and emission 470 nm
Freeze dryer (Christ Alpha 1-2 LD plus) (or any other freeze-drying machine), if freeze-drying is considered to be carried out
Procedure
Fast root-surface screening using solophenyl flavine staining
Gently rinse the root system in water to wash out the fixatives (if used) and remove soil particles or debris by using brush, needles, or forceps. Be careful as some fungal organisms are only slightly attached to the root surface; also, soil particles or debris may contain some fungal organisms. Sometimes, it is not possible to remove all soil particles; however, that can be advantageous and provide additional information about the interaction between the fungus, root, and soil.
Submerge the roots into a minimum of 5 mL of 0.1% solophenyl flavine solution for 5–7 min at laboratory temperature (20–25 °C). If necessary (if roots are mature or thick), keep in warm solution (30–40 °C) on a heating plate without stirring (stirring can damage fragile roots) for 10–30 min. According to the root system size, use enough solophenyl flavine dye and be sure that all roots are submerged.
Wash samples briefly in distilled water, using an approximately 50–100 times larger volume of water than the volume of used dye.
Transfer roots into a drop of water on a glass slide. If the roots are too large to be observed as a one piece, use scissors, scalpel, or tweezers and divide the root into appropriately smaller pieces.
Observe samples under the fluorescence light using the blue filter set (e.g., Leica A or D filter set) with excitation around 400 nm and emission around 470 nm. For more information, please see solophenyl flavine fluorescence characteristics.
Stained roots are suitable for whole-mount clearing, sectioning (e.g., cryosectioning), or freeze-drying.
Root whole-mount clearing and staining
For whole-mount clearing, we recommend using surface-stained roots from previously described steps. Nevertheless, non-stained roots are also good (Figure 1A).
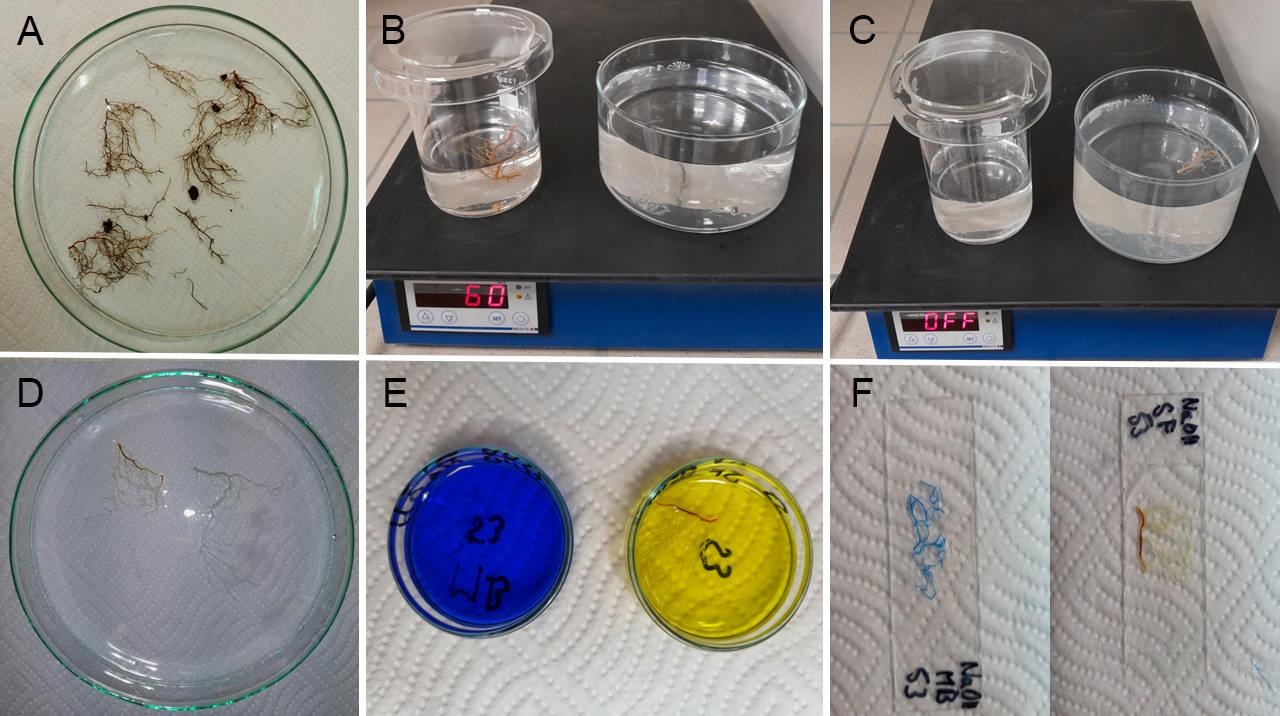
Figure 1. Laboratory steps for root clearing and staining. A. Overview of roots in a Petri dish before clearing. B. Clearing and bleaching of roots in bleaching solutions on a heating plate. C. Washing roots after clearing. D. Cleared roots in a Petri dish before staining. E. Staining roots with solophenyl flavine (yellow color) and methyl blue (blue color) in small Petri dishes. F. Completed root specimens prepared for microscopic observations.Transfer the roots to a bleaching solution (NaOH BS or KOH BS). Keep solution warm (60 °C) on a heating plate without stirring (stirring can damage fragile roots) for 1 h. A volume of 50–100 mL of solution should be enough, but it strongly depends on the root tissue mass and discoloration. If the roots are too dark, use more bleaching solution or prolong time (Figure 1B).
Put the cleared roots into the preheated dH2O (60 °C, at least 500 mL) and let them cool down at laboratory temperature for an appropriate time (approximately 30–60 min) (Figure 1C).
Roots are now prepared for staining (Figure 1D).
Gently transfer cleared roots into the staining solution (e.g., on the tip of a laboratory needle or tweezer, avoiding pressurizing roots with a tweezer jaw).
Depending on the desired outcome, stain roots with methyl blue for brightfield observations, with solophenyl flavine for fluorescence observations, or with methyl blue + solophenyl flavine mixture for observations in both types of microscopies in parallel (Figure 1E).
Methyl blue staining: Add a minimum of 5 mL of methyl blue working solution into a small Petri dish and insert the cleared roots. Stain at laboratory temperature (20–25 °C) for 10–15 min (enough for most of the roots) (Figure 1E). Shortly wash in distilled water (0.5 min, as prolonged washing may remove dye from roots). Methyl blue–stained samples should be acidified. Put a drop of 1% HCl on a glass slide, submerge the stained samples into it, and observe covered with a cover glass (Figure 1F). Use brightfield microscopy. Fungal and oomycetes structures are stained blue.
Solophenyl flavine staining: Add a minimum of 5 mL of solophenyl flavine into a small Petri dish and insert cleared roots. Stain at laboratory temperature (20–25 °C) for 5 min (Figure 1E). Wash in distilled water until all free dye is released from the stained roots (5–7 min; prolonged washing does not decrease staining intensity of roots), put on a glass slide in a drop of distilled water, and observe it under a cover glass (Figure 1F). Use fluorescence microscopy. Fungal and oomycetes structures are stained blue, green, or yellow.
Methyl blue + solophenyl flavine mixture staining: Add a minimum of 5 mL of solophenyl flavine + methyl blue mixture into a small Petri dish and insert cleared roots. Timing and temperature depend on the root origin and characteristics such as thickness and age. We suggest submerging for 5 min at laboratory temperature (20–25 °C) for soft and thin roots (in vitro–cultivated plants, small herbaceous species). On the other hand, we suggest submerging for 30 min at elevated temperature (50 °C) for thicker and older roots (woody plants). After staining, wash samples in distilled water for 5 min, put a drop of 1% HCl on a glass slide, and cover with cover glass. Use brightfield and/or fluorescence microscopy for observation (Figure 2A–F).
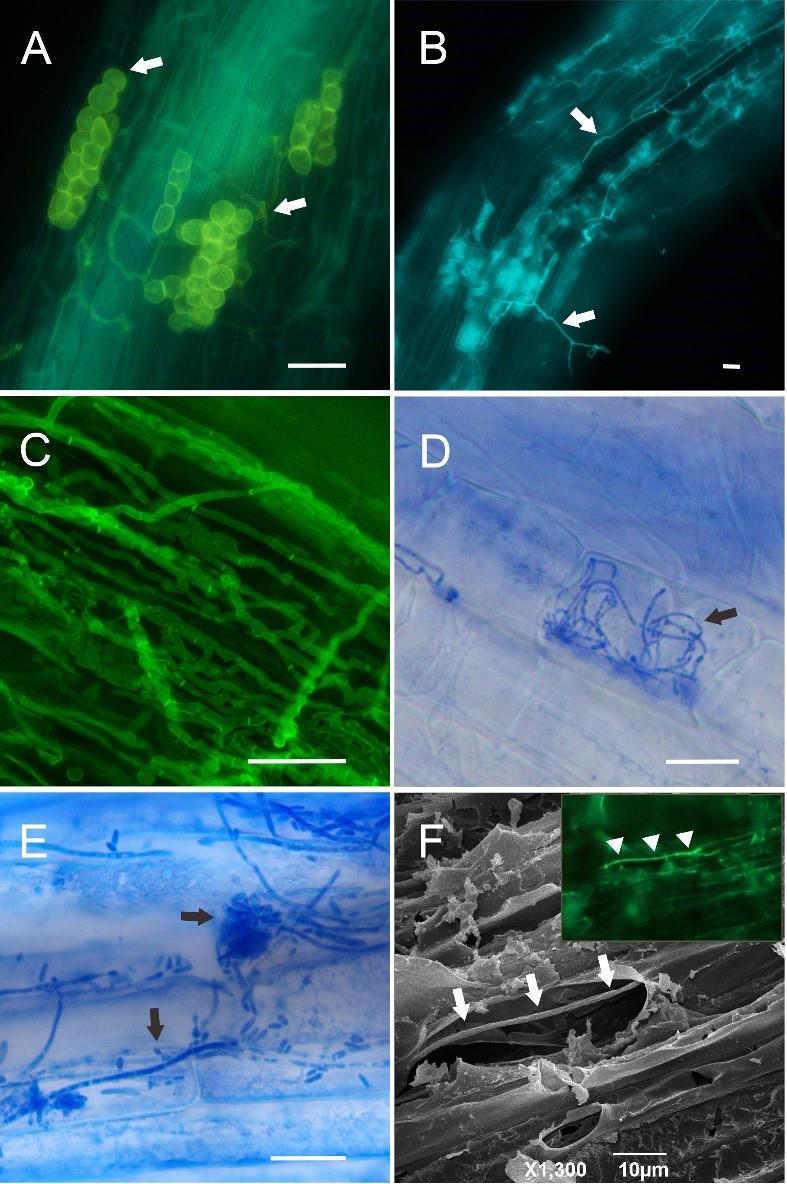
Figure 2. Microscopy images of fungi and oomycetes inside the roots of selected plant species (Alnus glutinosa, Arabidopsis thaliana, Oryza sativa) after the clearing and staining steps described in the protocol. A. Oomycetes (arrows) inside Alnus glutinosa roots, KOH BS clearing, methyl blue + solophenyl flavine mixture staining, fluorescence microscopy. B. Oomycetes (arrows) inside Alnus glutinosa roots, NaOH BS clearing, solophenyl flavine staining, fluorescence microscopy. C. Fungal hyphae inside Oryza sativa roots, KOH BS clearing, methyl blue + solophenyl flavine mixture staining, fluorescence microscopy. D. Fungal hyphae (arrow) inside Oryza sativa roots, NaOH BS clearing, methyl blue staining, brightfield microscopy. E. Endophytic fungus (arrows) on the Arabidopsis thaliana root surface, non-cleared, methyl blue staining, brightfield microscopy. F. Oomycete hypha (arrows) inside Alnus glutinosa root, non-cleared, solophenyl flavine staining, scanning electron microscopy. The inset shows the identical hypha (arrows) in fluorescence microscopy before freeze-drying and SEM observation. Scale bars: A–E = 20 µm, F = 10 µm.
Freeze-drying of stained samples
The following steps are optional and may follow after step A6. The majority of surface-stained samples (without clearing and bleaching) are suitable for the subsequent assessment using scanning electron microscopy (SEM).
Insert two parafilm strips between the glass slide and the border of the cover glass to prevent root squeezing (Figure 3).

Figure 3. Example of inserting the parafilm between the glass slide and cover glassFind the desired object (fungal structure) on a root surface using fluorescence microscopy.
Put samples on the glass slide into a freeze-drying machine and freeze it. Start vacuuming and drying the samples.
When the samples are dry (time of drying strongly depends on both the size of sample and the volume of water inside the sample; approximately 2 h), gently remove the cover glass and transfer samples onto the adhesive carbon tape mounted on the SEM specimen tub.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article:
Corcobado et al. [13]. Metabolomic and Physiological Changes in Fagus sylvatica Seedlings Infected with Phytophthora plurivora and the A1 and A2 Mating Types of P. ×cambivora. Journal of Fungi 8(3): 298, Figure 2.
Moreover, our procedure is validated by Figure 2 shown in this protocol.
General notes and troubleshooting
General notes
It is not easy to determine the exact volume needed for individual solutions. Examined roots can have various sizes. The volume of clearing/bleaching solution should be 10 times higher than the volume of the examined root system. To wash in distilled water, we recommend using a 4–5 times larger volume than the volume of the clearing/bleaching solution. If solophenyl flavine or methyl blue are used as staining dye, the stained roots should be fully submerged into the staining solution. It is not necessary to add the staining solution in excess. A drop (approximately 50 µL) of mounting medium (water or 1% HCl) is used for microscopic observations.
If better-quality staining of surface or sub-surface fungal organisms is required, it is recommended to use solophenyl flavine dissolved in ClearSee solution. The examined samples are incubated at laboratory temperature for days or weeks. This procedure completely protects root tissues against the damage that is caused by clearing and bleaching. The same procedure is also suitable for subsequent sectioning or freeze-drying. However, ClearSee staining is a time-consuming process. We recommend using a 0.1% solution of solophenyl flavine in ClearSee. For more details about ClearSee solution, see Ursache et al. [12].
Be sure that all baths for sample washing or bleaching are preheated and samples are transferred to the next step without any rapid change in temperature (e.g., during the clearing/bleaching step at 60 °C in the bleaching solution, distilled water prepared for washing should be heated to the same temperature as the bleaching solution).
Except for staining with methyl blue + solophenyl flavine mixture, there is also a possibility to stain with solophenyl flavine at first, then wash the samples in distilled water, and subsequently stain with methyl blue. This double-staining helps to quench undesirable fluorescence of cell walls that is caused by solophenyl flavine. However, as this staining procedure is complementary, some fungal structures become stained blue with methyl blue and may lose their fluorescence from solophenyl flavine staining.
Troubleshooting
Clearing and bleaching methods described above are suitable for a broad range of roots that are sampled from soil. Bleaching time and temperature for agar- or hydroponics-cultivated fine roots should be adequately adjusted. For example, bleaching for agar-cultivated A. thaliana seedlings should be 30 min at 40–50 °C. Fine roots that are sampled from soil can also be cleared in a shorter time than 30 min. If the roots remain white-brown or white-yellow after 1 h of clearing and bleaching, we recommend extending the clearing/bleaching step for another 10 or 15 min. However, if the root system consists of roots with a large diameter variation, some thin roots can be already white, whereas thick roots still remain partially discolored.
After clearing and bleaching, fungal structures show some weak fluorescence if excited with violet/blue light. However, this signal is much more stable, and excitation is shifted toward the red (blue-green) spectrum, after staining with methyl blue + solophenyl flavine mixture.
Methyl blue is prone to nonspecific staining that has to be carefully recognized. If the roots are not cleared properly, cell nuclei or cytoplasm may be stained. Cell nucleuses are evenly organized inside root tissues: one nucleus in one cell. Fungal structures are distributed randomly. If stained non-specifically, other structures in roots can be recognized and distinguished from fungal structures according to their shape and morphology.
For certain unknown reasons, some samples cannot be stained with methyl blue. There may be a problem due to the clearing or the nature of the sample. In some samples, both fungal structures and root tissues are intensively stained with solophenyl flavine, resulting in a weak contrast. In both cases, double-staining with solophenyl flavine and methyl blue dyes or staining with methyl blue + solophenyl flavine mixture helps.
Stopping point
A stopping point is relevant mainly for section B. At step B4, if the roots are prepared for staining, they can be stored before staining in a refrigerator (1–2 days, 4 °C). At step B8, there is also a possible stopping point. If the SF-stained samples are placed on a glass slide, not in a drop of water but in a drop of water:glycerol mixture (1:1), they can be stored for several days in the refrigerator. However, ensure that the mounting medium does not evaporate from the glass slide and the specimen is still mounted. If not, add a little of the water/glycerol mixture again until the sample is completely mounted.
Extra caution
Caution is needed when mixing NaOH or KOH bleaching solutions. These reactions are exothermic, and heat is released. One should also be careful with a volume of hydrogen peroxide. If too much hydrogen peroxide is added, extreme bubbling during clearing/bleaching may damage root samples. Always protect the heating device (heating place) against small drops coming from the bleaching solution or bleached samples during a transfer. They may contaminate or damage the surface coating of your device. If using HCl for methyl blue staining, be careful as it may also etch the mechanical stage of your microscope.
Acknowledgments
This work was supported by the Slovak scientific grant agency VEGA (1/0108/23) and the TUZVO internal grant agency IPA (1/2023). A part of this protocol was published by Corcobado et al. [13].
Competing interests
The authors declare no conflicts of interest.
References
- Kowal, J., Arrigoni, E. and Lane, S. (2020). Acidified Blue Ink-staining Procedure for the Observation of Fungal Structures Inside Roots of Two Disparate Plant Lineages. Bio Protoc. 10(20): e3786.
- Brundrett, M. C. and Tedersoo, L. (2020). Resolving the mycorrhizal status of important northern hemisphere trees. Plant Soil. 454: 3–34.
- Nonomura, T., Tajima, H., Kitagawa, Y., Sekiya, N., Shitomi, K., Tanaka, M., Maeda, K., Matsuda, Y. and Toyoda, H. (2003). Distinguishable staining with neutral red for GFP-marked and GFP-nonmarked Fusarium oxysporum strains simultaneously colonizing root surfaces. J Gen Plant Pathol. 69(1): 45–48.
- Hoch, H., Galvani, C., Szarowski, D. and Turner, J. (2005). Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia. 97(3): 580–588.
- Lux, A., Vaculík, M. and Kováč, J. (2015). Improved methods for clearing and staining of plant samples. In: Yeung, E. C. T., Stasolla, C., Sumner, M. J. and Huang, B. Q. (Eds.). Plant Microtechniques and Protocols. Springer International Publishing, Cham, Switzerland, pp. 167–178.
- Ruzin, S. E. (1999). Plant Microtechnique and Microscopy. Oxford University Press, New York.
- Li, Y., Fu, Q., Rojas, R., Yan, M., Lawoko, M. and Berglund, L. (2017). Lignin‐Retaining Transparent Wood. ChemSusChem. 10(17): 3445–3451.
- Gladyshev, N. F., Dvoretskii, S. I., Zhdanov, D. V., Ul'yanova, M. A. and Ferapontov, Y. A. (2003). Choice of a Stabilizer for the Reaction of KOH with Hydrogen Peroxide to Produce Potassium Superoxide. Russ J Appl Chem. 76(11): 1858–1859.
- Wilkes, T. I., Warner, D. J., Edmonds-Brown, V., Davies, K. G. and Denholm, I. (2020). A comparison of methodologies for the staining and quantification of intracellular components of arbuscular mycorrhizal fungi in the root cortex of two varieties of winter wheat. Access Microbiol. 2(2): e000083.
- Chenchouni, H., Mekahlia, M. N. and Beddiar, A. (2020). Effect of inoculation with native and commercial arbuscular mycorrhizal fungi on growth and mycorrhizal colonization of olive (Olea europaea L.). Sci Hortic. 261: 108969.
- Marques, J. P. R. and Nuevo, L. G. (2022). Double-Staining Method to Detect Pectin in Plant-Fungus Interaction. J Visualized Exp.: e63432.
- Ursache, R., Andersen, T. G., Marhavý, P. and Geldner, N. (2018). A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. 93(2): 399–412.
- Corcobado, T., Milenković, I., Saiz-Fernández, I., Kudláček, T., Plichta, R., Májek, T., Bačová, A., Ďatková, H., Dálya, L. B., Trifković, M., et al. (2022). Metabolomic and Physiological Changes in Fagus sylvatica Seedlings Infected with Phytophthora plurivora and the A1 and A2 Mating Types of P. ×cambivora. J Fungi. 8(3): 298.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Toma, T., Kováč, J. and Ďurkovič, J. (2024). Fast, Easy, and Comprehensive Techniques for Microscopic Observations of Fungal and Oomycete Organisms Inside the Roots of Herbaceous and Woody Plants. Bio-protocol 14(11): e5013. DOI: 10.21769/BioProtoc.5013.
Category
Plant Science > Plant immunity > Host-microbe interactions
Plant Science > Plant cell biology > Cell staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










