- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Reversible Photoregulation of Cell–Cell Adhesions With Opto-E-cadherin
Published: Vol 14, Iss 10, May 20, 2024 DOI: 10.21769/BioProtoc.4995 Views: 2710
Reviewed by: Munenori IshibashiJamie A. Davies Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Transient Transfection-based Cell Adhesion Assay with 293T Cells
Rohit Singh and Beom K. Choi
Jan 5, 2021 7265 Views
A Novel Method to Make Polyacrylamide Gels with Mechanical Properties Resembling those of Biological Tissues
Katarzyna Pogoda [...] Paul A. Janmey
Aug 20, 2021 5424 Views

A Flow Cytometry–Based Method for Assessing CAR Cell Binding Kinetics Using Stable CAR Jurkat Cells
Alex Shepherd [...] Scott McComb
Jun 20, 2024 3048 Views
Abstract
The cell–cell adhesion molecule E-cadherin has been intensively studied due to its prevalence in tissue function and its spatiotemporal regulation during epithelial-to-mesenchymal cell transition. Nonetheless, regulating and studying the dynamics of it has proven challenging. We developed a photoswitchable version of E-cadherin, named opto-E-cadherin, which can be toggled OFF with blue light illumination and back ON in the dark. Herein, we describe easy-to-use methods to test and characterise opto-E-cadherin cell clones for downstream experiments.
Key features
• This protocol describes how to implement optogenetic cell–cell adhesion molecules effectively (described here on the basis of opto-E-cadherin), while highlighting possible pitfalls.
• Utilises equipment commonly found in most laboratories with high ease of use.
• Phenotype screening is easy and done within a few hours (comparison of cell clusters in the dark vs. blue light in an aggregation assay).
• Three different functionality assay systems are described.
• After the cell line is established, all experiments can be performed within three days.
Keywords: PhotoswitchableGraphical overview
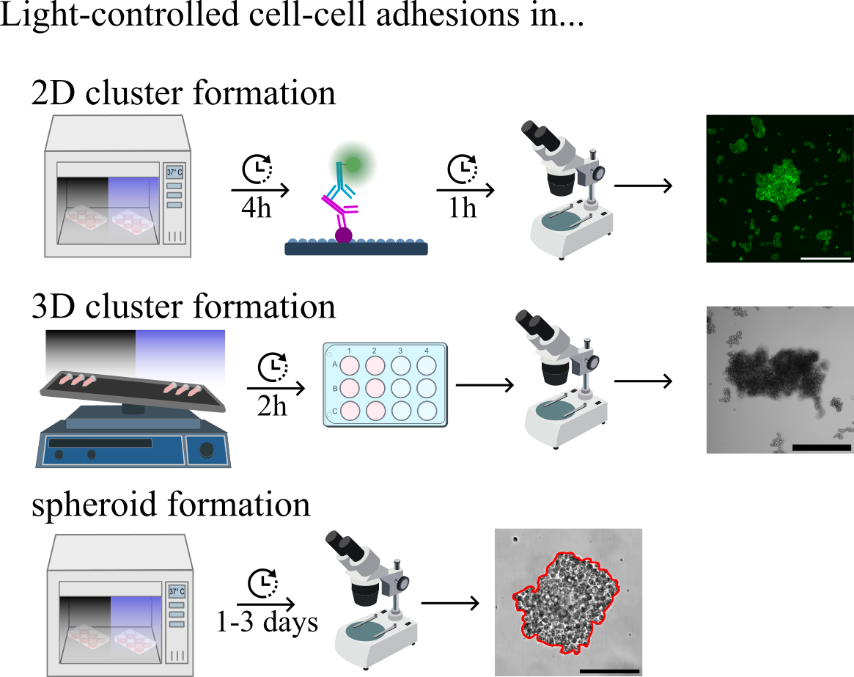
Analysis and characterisation of light-dependent opto-E-cadherin expressing cells
Background
In multicellular organisms, many essential biological processes such as embryonic development, tissue integrity, collective cell behaviour, and migration, are regulated by cell–cell adhesions [1], while aberrations play a key factor in diseases such as arthritis, neuronal dystrophy, and tumour progression [2–4]. Epithelial cadherin (E-cadherin) is a well-known cell-adhesion protein. Upon binding of two E-cadherin molecules on neighbouring cells, intracellular proteins are recruited to the cytoplasmic tail and connect directly to the cytoskeleton, affecting transcriptional regulation [5]. Its loss of expression or change in function may lead to epithelial-to-mesenchymal transition (EMT), tumour development, and metastasis [6,7]. In healthy tissues, EMT of cells is dynamically and locally regulated, which may be reversed by mesenchymal-to-epithelial transition (MET). At the same time, EMT plays a central role in gastrulation and wound healing in adults [8]. Attempts to study the underlying mechanisms have relied on gain- or loss-of-function experiments, inhibition with antibodies, or the removal of Ca2+ ions, necessary for the homophilic interaction between E-cadherins [9–11]. However, none of these methods fully captures the spatio-temporal regulation in cell–cell adhesion during EMT. Attempts to mimic cell–cell adhesions through chemical modifications of the cell surface with synthetic cell adhesion molecules were neither sustainable nor dynamic [12,13]. To gain spatiotemporal control over cell–cell adhesions, various chemo-optogenetic [14,15] and optogenetic tools that utilise light to dissociate cell adhesions [16] and create artificial cell–cell adhesions have been successfully developed [17–21].
More recently, we developed a blue light switchable E-cadherin molecule (opto-E-cad), which allows us to reversibly and dynamically switch between ON and OFF states of E-cadherin [22]. In opto-E-cad, a light-oxygen-voltage sensing domain (LOV2) is embedded in the extracellular domain of E-cadherin such that, in the dark, cell–cell adhesion can form. Upon blue light absorption by its cofactor FMN or FAD, the LOV2 domain undergoes a conformational change, disturbing a critical Ca2+ binding site and turning OFF the opto-E-cad-based adhesions. In the dark, these conformational changes reverse, and the cell–cell adhesions can form again. Overall, opto-E-cad is fully genetically codable and provides bidirectional control over cell–cell adhesions with high spatiotemporal precision. At the same time, opto-E-cad allows us to link optogenetics to cellular signalling associated with E-cadherin-based cell–cell adhesions, providing a unique tool to suitably study EMT and MET processes and their impact on development and disease progression. As proof of concept, we have demonstrated the feasibility and ease-of-use of opto-E-cad in several invasion assays either with single cells and spheroids in vitro and in vivo, as well as its influence on transcriptional regulation similar to observed changes in gene expression during EMT via q-RT PCR [23]. When considering E-cadherin, varying expression levels and adhesion strengths are a determining factor in its function [24,25]. Thus, straightforward methods to characterise cells with different levels of opto-E-cad expression are necessary, which we describe here.
Materials and reagents
Biological materials
MDA-MB-231 human breast adenocarcinoma epithelial cells (American Type Culture Cells, catalog number: HTB-26)
L929 mouse fibroblast cells derivative of strain L (American Type Culture Cells, catalog number: CCL-1)
HeLa human cervix adenocarcinoma epithelial cells (American Type Culture Cells, catalog number: CCL-2)
A431D (Magaret J. Wheelock, origin: University of Toledo, Ohio [26])
Opto-E-cad-GFP plasmid (Addgene, catalog number: 203327)
Reagents
Dulbecco’s modified Eagle medium (DMEM) (PAN Biotech, catalog number: 04-03591)
Fetal bovine serum (FBS) (PAN Biotech, catalog number: P30-3031)
Penicillin-streptomycin (P/S) (Gibco, catalog number: 15140122)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H4304)
Lipofectamine 3000 (Thermo Fisher, catalog number: L300001)
Geneticin (G418) (Roche, catalog number: 4727878001)
Phosphate-buffered saline (PBS) (Gibco, catalog number 18912014)
StemPro Accutase (Thermo Fisher, catalog number: A1110501)
Flavin adenine dinucleotide (FAD) (Carl Roth, catalog number: 6833)
Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 158127)
Hank’s buffered salt solution (HBSS) (Gibco, catalog number: 12549069)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7030)
Calcium chloride (CaCl2) (Carl Roth, catalog number: A119.1)
Ethylenediaminetetraacetic acid (EDTA) (Carl Roth, catalog number: 80423)
Triton X-100 (PanReac AppliChem, catalog number: not available)
Hoechst 3342 (Invitrogen, catalog number: H3570)
Phalloidin iFluor-594 (Abcam, catalog number: ab176757)
Cell tracker Green BODIPY dye (Invitrogen, catalog number: C2102)
Fluoromount GTM (Invitrogen, catalog number: 00595802)
Methyl cellulose (viscosity 15cP) (Sigma-Aldrich, catalog number: M7027)
Recipes
Growth medium
Reagent Final concentration Quantity or Volume DMEM n/a 435 mL FBS 10% 50 mL P/S (10,000 U/mL) 1% 5 mL HEPES (625 mM) 12.5 mM 10 mL Total n/a 500 mL Spheroid medium
Reagent Final concentration Quantity or Volume DMEM n/a 470 mL FBS 3% 15 mL P/S (10,000 U/mL) 1% 5 mL HEPES (625 mM) 12.5 mM 10 mL Methyl cellulose (viscosity 15cP) 0.66% 3.3 g Total n/a 500 mL Note: Filter FBS through a 0.22 μm filter before adding it to DMEM for growth and spheroid medium.
Laboratory supplies
T-25 flasks (Sarstedt, catalog number: 83.3910.002)
6-well microplates (Greiner Bio-One, catalog number: 657160)
12-well microplates (Greiner Bio-One, catalog number: 665180)
96-well F-bottom microplates (Greiner Bio-One, catalog number: 655087)
96-well U-bottom suspension microplates (Greiner Bio-One, catalog number: 650185)
DNA LoBind microfuge tubes (Eppendorf, catalog number: 022431021)
24 mm × 24 mm glass coverslips (Roth, catalog number: HKE8.1)
Microscope slides (Thermo Scientific, catalog number: 15457544)
10 μL TipOne® pipette tips (Starlab, catalog number: S1111-3700-C)
200 μL TipOne® pipette tips (Starlab, catalog number: S1113-1706-C)
1,000 μL TipOne® pipette tips (Starlab, catalog number: S1111-6701-C)
Equipment
10 μL NeoLab pipette (Eppendorf, catalog number: A-0861)
100 μL NeoLab pipette (Eppendorf, catalog number: A-0864)
1,000 μL NeoLab pipette (Eppendorf, catalog number: A-0867)
CO2 incubator (PHCbi, model: MCO-170AICUVD-PE)
CO2 incubator with custom drilled cable inlets for LED panels (Thermo Fisher, model: Midi CO2 40L 3403)
Herasafe cabinet (Thermo Fisher, model: Herasafe 2030i)
Ultra centrifuge (Eppendorf, model: 5804)
Automated cell counter (Bio-Rad, model: TC20)
Cell sorter (BD Biosciences, model: FACSAria Fusion)
CLF flora LED (CLF Plant Climatics GmbH, model: not available)
Custom made LED panels and control modules. Equivalent self-made products can be found here [27]
Power meter (Thorlabs, catalog number: PM16-120)
3D orbital shaker (Grant-Bio, model: PSM3D)
Dmi8 brightfield microscope (Leica, model: Dmi8 automated)
Software and datasets
LAS X v.3.7.1.21655 (Leica, 25/01/2024)
ImageJ v.1.53f51 (ImageJ, 02/03/2023)
Procedure
Cell culture and generation of monoclonal opto-E-cad cell lines
The breast cancer cell lines MDA-MB-231, the skin cancer cell line A431D (A431, E-cadherin-deficient), HeLa, and the fibroblast cell line L929 were successfully tested and used with the opto-E-cad construct. The cells were cultured in DMEM without phenol red, supplemented with 10% FBS, 1% P/S (10,000 U/mL), and 12.5 mM HEPES at 37 °C and 5% CO2.
Transfect cells in a 24-well plate with 0.5 µg per well of opto-E-cad-GFP plasmid and Lipofectamine 3000 according to the manufacturer’s protocol.
Note: Transfection efficiency depends on the amount of DNA and transfection agent used and varies from cell line to cell line. Optimisation is recommended and efficiency can be visualised by fluorescent microscopy.
Two days after transfection, begin selecting cells with 1,800 μg/mL of G418 and maintain in the presence of selection medium for all further experiments.
Sort transfected cells into 96-well F-bottom plates as single clones after one week of cultivation with selection medium based on the fluorescent strength of GFP.
Note: When lifting cells for single-cell sorting, it is important to be gentle. Using Trypsin/EDTA is not recommended, as the E-cadherin molecules with the GFP-tag will be targets of proteolytic cleavage. Instead, Accutase as described below or 5 mM EDTA in PBS should be employed for lifting the cells.
Cultivate cells until monoclonal lines are established for characterisation [22]. This procedure may take 4–6 weeks.
Note: Monoclonal lines express different levels of Opto-E-cad-GFP on the cell membrane. The experiments described in Procedure B–D help identify clones that are suitable for downstream applications where adhesion strength (summary of E-cadherin adhesions) and/or fold change in dark vs. blue light states are of importance.
Critical: For reproducible results, it is imperative that presentation of Opto-E-cad-GFP on the outer membrane of cells is the same, as the amount is a determining factor in its function. Thus, attempting experiments with polyclonal variants is less likely to succeed.
Light-controlled cell–cell adhesions in 2D culture
Prior to the experiment, monoclonal stable opto-E-cad cell lines were seeded into T25 flasks at 20%–30% confluence and cultivated for three days.
Wash the cells briefly with 1 mL of PBS, discard, and add 0.5 mL of Accutase diluted 1:4 in HBSS. It takes approximately 10 min for the cells to detach.
Use cell growth medium to suspend and collect cells in 15 mL tubes. Subsequently, centrifuge cells at 600× g for 5 min at room temperature. Resuspend the cells in growth medium.
Place 24 mm × 24 mm glass coverslips to the bottom of a 6-well plate. Wash twice briefly with 70% ethanol, remove the liquid, and let the well dry. Count the cells and seed 8,600/cm2 onto the coverslips in a total volume of 2 mL of growth medium supplemented with 0.5 μM FAD either under blue light (20.4 μW/cm2) or in the dark for 4 h in the incubator with 5% CO2 at 37 °C (see Figure 1A).
Notes:
The addition of the co-factor FAD is necessary for Opto-E-cadherin to work.
Distance between the light source and sample and general setup affects light intensity. A power meter is useful to determine absolute power and irradiance values if there are concerns about opto-E-cad activation or phototoxicity.
Wash the cells briefly with 1 mL of PBS three times and fix in 500 μL of 4% PFA in PBS for 10 min at room temperature.
Wash the cell briefly three times with 1 mL of PBS before permeabilizing the cell membrane with 0.1% Triton X-100 in PBS for 5 min.
Wash twice with 1 mL of PBS and stain the cells with 1 µg/mL in nuclear dye (Hoechst) and 0.1 µg/mL actin cytoskeleton (Phalloidin iFluor-594) dyes in 500 μL of PBS for 1 h at room temperature.
Mount the cells with 15 µL of Fluoromount GTM onto microscope slides and acquire fluorescence images at 10× for an area of 1 cm2 using a motorised stage on the Dmi8 microscope (see Figure 1B).
Determine the number of cells using the nucleus staining and cell cluster area by Phalloidin iFluor staining using the particle analysis tool in ImageJ. Consider areas larger than 500–1,500 μm2 as cells and areas larger than 10,000 μm2 as clusters. The average MDA-opto-E-cad cell size in 2D is 950 ± 390 μm2. The image analysis tool has been previously established [17].
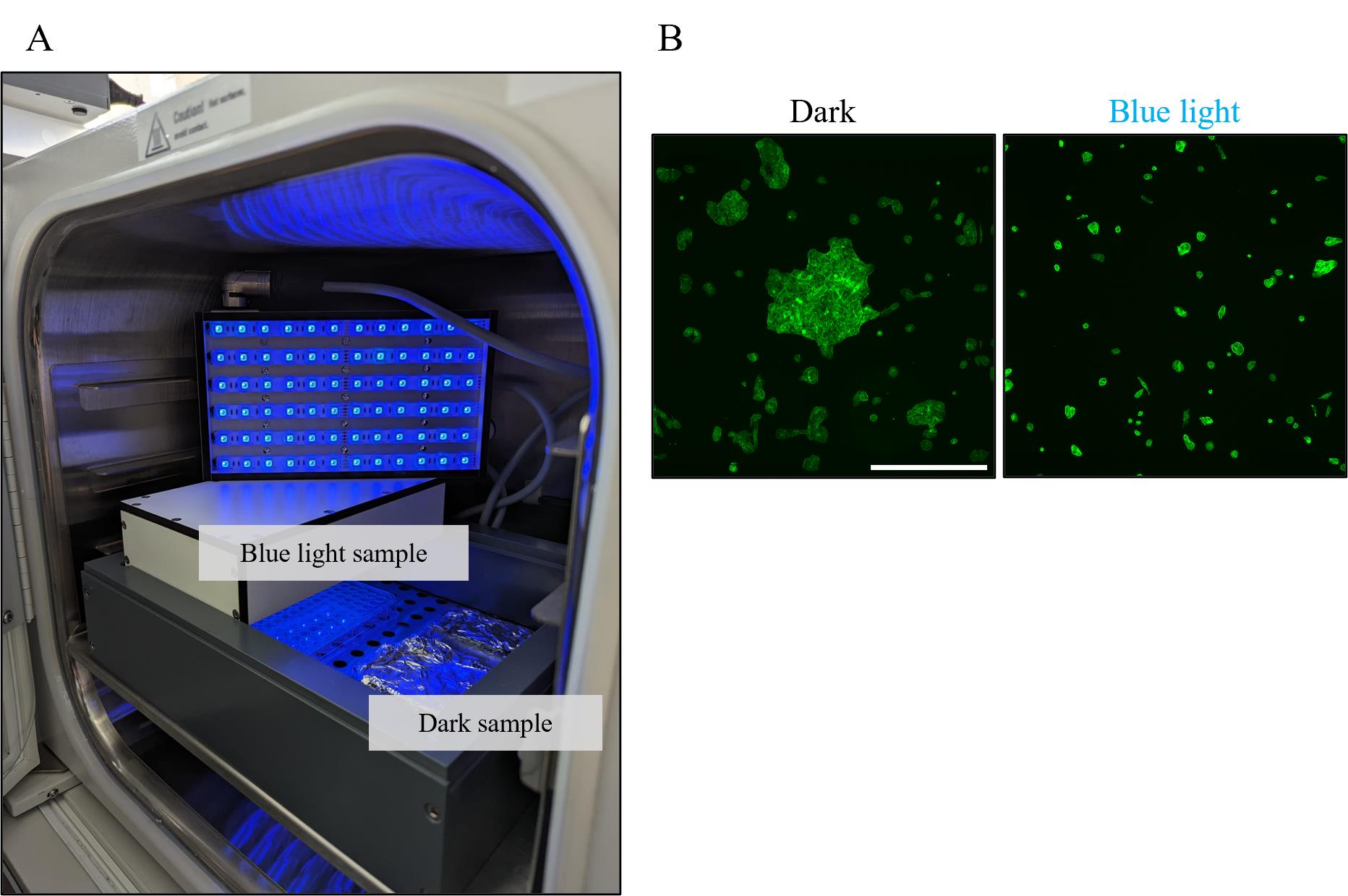
Figure 1. 2D cluster experiment setup. A. Light panel setup in the incubator. The panels are set on top of a 7 cm high frame above the cell samples, which are either kept under blue light or wrapped in aluminium foil. B. Example images of MDA opto-E-cad cells in the dark or under blue light for 4 h. Fixed and stained with Phalloidin iFluor-594. Scale bar is 500 µm.
3D clustering: Photoswitchable cell–cell adhesions in suspension culture
Prior to the experiment, cells were seeded into T25 flasks at 20%–30% confluence and cultivated for three days.
Remove growth medium from adherent cells in a T25 flask and wash twice briefly with 1 mL of PBS.
Add 0.5 mL of 5 mM EDTA and keep the flask in the incubator until cells are lifted. Depending on the cell confluency, this takes 5–10 min.
Add 5 mL of growth medium and subsequently transfer the cell suspension into a 15 mL Falcon tube.
Centrifuge the cells at 600× g for 5 min at room temperature.
Remove the supernatant and gently resuspend the cells in 3 mL of HBSS supplemented with 1% BSA and 2 mM Ca2+.
Determine the cell density in the suspension and verify if cells are indeed present as a single-cell suspension; otherwise, gently resuspend the cells some more.
Adjust the concentration to 5 × 104 cells/mL in HBSS supplemented with 1% BSA and 2 mM CaCl2 and add 0.5 µM (final conc.) of the FAD cofactor.
Aliquot the cell suspension to 1 mL per low-binding 1.5 mL microfuge tube and run every condition in duplicates.
On a 3D orbital shaker, place the tubes laterally and run the experiment for 1–6 h at room temperature (see Figure 2A). Use approximately 270 μW/cm light (light sample) or protect the dark sample from the light by wrapping in aluminium foil. Light conditions are typically changed every 30 min when performing experiments that rely on reversibility and bidirectional switching.
Note: The clustering results are affected by the orbital shaker settings; generally, an angle of 5° and 30 rpm works best in our hands.
Pipette 500 μL of 4% PFA into a 12-well cell culture plate per well. Use a cut 1,000 μL pipette tip to carefully transfer the cluster suspension from the tube into the PFA.
Note: The aim is to slightly increase the pipette tip opening to prevent potential clusters from being disrupted during the transfer into the 12-well plate.
Critical: Remove any bubbles from the wells with a pipette to ensure good image quality and easy analysis.
Acquire brightfield images of the entire well. Ideally, use a microscope with a scanning platform and subsequently use a stitching tool to merge single image files. Typically, low magnification objectives (4×) are sufficient to retain the sharp contrast between cell cluster and background during analysis, while keeping the number of acquired images to a minimum (see Figure 2C).
Analyse the clusters with ImageJ. As the cell clusters are 3D objects, the two-dimensional projected area of 5,000 μm equals approximately 20 MDA-MB-231 cells. Anything equal or larger is considered a cell cluster. A step-by-step analysis has been previously described [19].
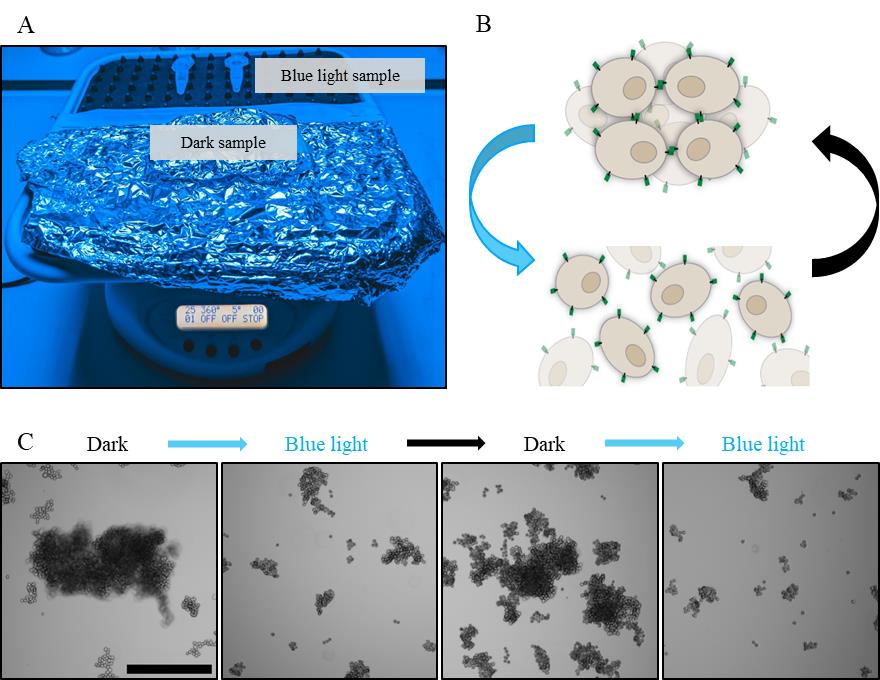
Figure 2. 3D cluster experiment setup. A. Cell samples are placed on an orbital shaker that rotates 25× per minute at a 5° angle, under blue light or wrapped in aluminium foil. B. Schematic of opto-E-cad reversibility. C. MDA opto-E-cad cells alternating between dark conditions and blue light incubation every 30 min. Scale bar is 500 µm.
Light-controlled spheroid formation
Grow cells to 80% confluence in a T25 flask. Remove growth medium and wash cells twice briefly with 1 mL of PBS.
Dilute Cell Tracker Green BODIPY to a final concentration of 5 µM in 5 mL of serum-free medium and add to the cells. Incubate for 45 min in the incubator.
Wash cells twice briefly with 1 mL of PBS to remove excess dye. Detach the cells using 0.5 mL of Accutase diluted 1:4 in HBSS. Keep the cells in the incubator at 37 °C and 5% CO2. The cells lift from the flask within 5–10 min. If necessary, tapping the flask will facilitate the process.
Harvest cells with 5 mL of growth medium by pipetting, centrifuge at 600× g for 5 min at room temperature, remove the supernatant, and resuspend cells in 1 mL of growth medium.
Determine the concentration of the cell suspension and add 2 × 104 cells/mL to working spheroid medium (see Recipe 2) supplemented with 0.5 µM FAD.
Seed 100 µL of medium (2 × 103 cells) into 96-well suspension U-bottom plates.
Centrifuge the plates at 200× g for 3 min at room temperature for the cells to collect at the centre of the well.
Incubate the cells at 37 °C with 5% CO2 under blue light (20.4 μW/cm2) or in dark conditions up to three days.
Acquire images of every well with a brightfield microscope at 10× magnification (see Figure 3).
Determine the volume of the spheroids with the MATLAB code SpheroidSizer1_0 [28].
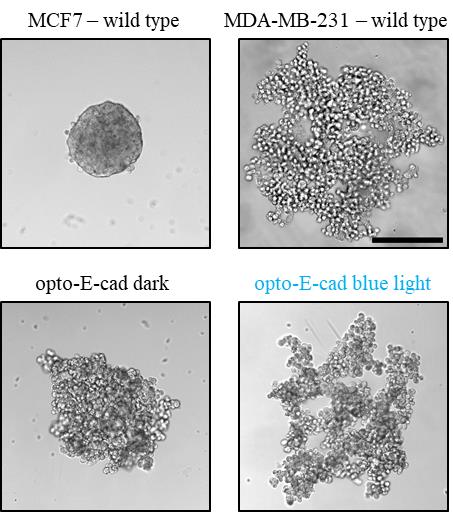
Figure 3. Representative images of cells in 3D culture. MCF7 cells and MDA opto-E-cad cells in the dark, form of compact spheroids overnight. MCF7 is present here to depict a spheroid formed from cells with high levels of native E-cadherin. MDA-MB-231 wild type and MDA opto-E-cad cells under blue light, form loose, ramified structures. Scale bar is 250 µm.
Data analysis
After image acquisition of 2D or 3D opto-E-cad cell clusters, ImageJ is a useful tool to quickly analyse differences in cell aggregation between dark and blue light conditions and reversibility. Below is a useful list of commands in IJ1 Macro language, which can be directly run as a macro for image analysis.
run("Sharpen");
run("8-bit");
run("Gaussian Blur...", "sigma=2");
run("Find Edges");
setAutoThreshold("Default dark");
waitForUser("adjust Threshold and press OK.");
//adjusting the threshold manually may be necessary to make sure that cluster edges are accurately distinguished from the background.
setOption("BlackBackground", true);
run("Convert to Mask");
run("Fill Holes");
//double-check image quality and manually remove stitching artefacts or dirt, which may be falsely recognised as a cell cluster.
run("Set Scale...", "distance=xxxx known=xxxx unit=um");
//set the scale to um if images are saved in a different unit from the microscope
waitForUser("Check Cluster accuracy and press OK.");
run("Analyze Particles...", "size=10000-Infinity display clear include add");
//the same commands can be used when analysing 3D clusters by changing the particle size detection to ≥ 5,000.
The experiments are run with two technical replicates and three biological replicates per condition. All comparisons are performed using Fisher’s one-way ANOVA test, and p < 0.05 was treated as the significance threshold.
The analysis method for the spheroid size in MATLAB has been described in detail [28]. The spheroids experiments are run with 60 technical replicates and three biological replicates per condition. All comparisons are performed using a two-tailed Student’s t-test, and p < 0.05 was treated as the significance threshold.
Validation of protocol
This protocol or parts of it has been used and validated in the following research articles:
Mueller et al. [19]. The Importance of Cell-Cell Interaction Dynamics in Bottom-Up Tissue Engineering: Concepts of Colloidal Self-Assembly in the Fabrication of Multicellular Architectures. Nano Letters (Figures 1–3).
Rasoulinejad et al. [20]. Orthogonal Blue and Red Light Controlled Cell-Cell Adhesions Enable Sorting-out in Multicellular Structures. ACS Synthetic Biology (Figures 1–5).
Nzigou Mombo et al. [21]. Spatiotemporal Control Over Multicellular Migration Using Green Light Reversible Cell-Cell Interactions. Advanced Biology (Figures 1–3).
Nzigou Mombo et al. [22]. Reversible photoregulation of cell-cell adhesions with opto-E-cadherin. Nature Communications (Figures 1–3, 6). Source data can be found here [22].
General notes and troubleshooting
General notes
In the context of tissue formation, sorting, or cell invasion, it is important that cells express homogenous levels of cell adhesion molecules for reproducible results. The methods described here were primarily used to find and characterise opto-E-cad clones with good switching behaviour, i.e., large differences in cell clustering in the dark and under blue light. However, the experiments can be expanded to provide further valuable information.
2D clustering: Immunostaining of proteins involved in cell adhesion (e.g., p120 catenin) gives insights on how opto-E-cad influences signal transduction and is connected to the cytoskeleton in a spatio-temporal manner. This can be further elucidated with subsequent western blot and RNA expression analysis.
Spheroid formation: Spheroids formed from opto-E-cad cells can be used to study tumour invasion in collagen or by chorioallantoic membrane assay in vivo.
Troubleshooting
Problem 1: Cells do not cluster in 2D or 3D.
Possible causes: The expression level of opto-E-cad in the clone is not sufficient. / Cells were lifted with trypsin or incubated with Accutase for too long.
Solution: Prior to performing experiments, determine which clones have relative high expression levels of opto-E-cad by flow cytometry or fluorescent microscopy. If low levels of opto-E-cad expression are desired, reducing the rotation speed of the orbital shaker during 3D clustering allows weaker cell–cell adhesions to be sufficient for clustering. / Lift cells with EDTA or Accutase and avoid too-long incubation with proteases.
Problem 2: Cells cluster in 2D or 3D regardless of light condition.
Possible cause: The expression level of opto-E-cad in the clone is too high. Even though the binding affinity under blue light is greatly reduced, high expression levels may lead to sufficient cell–cell adhesions, resulting in cell clustering. Another possible cause can be that light intensities are too low for effective photoswitching.
Solution: Perform the experiments with opto-E-cad clones that have lower expression levels. Increase the light intensity but check for light toxicity with the untransfected cell line under the same conditions. Use a power meter to determine the absolute intensity values.
Problem 3: Opto-E-cad cells do not form compact spheroids, and rather loose and ramified structures persist.
Possible cause: FBS and insoluble components influence spheroid structure.
Solution: Filter FBS with a 0.2 µm filter before adding it to the spheroid medium. Decrease the concentration of FBS in the spheroid medium.
Acknowledgments
This work was funded by the European Research Council ERC Starting Grant ARTIST (# 757593, S.V.W) and published as Nzigou Mombo et al. [22].
Competing interests
The authors declare no financial or non-financial competing interests.
References
- Gumbiner, B. M. (1996). Cell Adhesion: The Molecular Basis of Tissue Architecture and Morphogenesis. Cell. 84(3): 345–357.
- Szekanecz, Z. and Koch, A. E. (2000). Cell-cell interactions in synovitis. Endothelial cells and immune cell migration. Arthritis Res. 2(5): 368–373.
- Grace, E. A. and Busciglio, J. (2003). Aberrant Activation of Focal Adhesion Proteins Mediates Fibrillar Amyloid β-Induced Neuronal Dystrophy. J Neurosci. 23(2): 493–502.
- Okegawa, T., Pong, R. C., Li, Y. and Hsieh, J. T. (2004). The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 51(2): 445–457.
- Perrais, M., Chen, X., Perez-Moreno, M. and Gumbiner, B. M. (2007). E-Cadherin Homophilic Ligation Inhibits Cell Growth and Epidermal Growth Factor Receptor Signaling Independently of Other Cell Interactions. Mol Biol Cell. 18(6): 2013–2025.
- Bruner, H. C. and Derksen, P. W. (2017). Loss of E-Cadherin-Dependent Cell–Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harbor Perspect Biol. 10(3): a029330.
- Petrova, Y. I., Schecterson, L. and Gumbiner, B. M. (2016). Roles for E-cadherin cell surface regulation in cancer. Mol Biol Cell. 27(21): 3233–3244.
- Thiery, J. P., Acloque, H., Huang, R. Y. and Nieto, M. A. (2009). Epithelial-Mesenchymal Transitions in Development and Disease. Cell. 139(5): 871–890.
- Kremer, M., Quintanilla-Martinez, L., Fuchs, M., Gamboa-Dominguez, A., Haye, S., Kalthoff, H., Rosivatz, E., Hermannstädter, C., Busch, R., Höfler, H. and Luber, B. (2003). Influence of tumor-associated E-cadherin mutations on tumorigenicity and metastasis. Carcinogenesis. 24(12): 1879–1886.
- Medina Rangel, P. X., Moroni, E., Merlier, F., Gheber, L. A., Vago, R., Tse Sum Bui, B. and Haupt, K. (2019). Chemical Antibody Mimics Inhibit Cadherin‐Mediated Cell–Cell Adhesion: A Promising Strategy for Cancer Therapy. Angew Chem Int Ed. 59(7): 2816–2822.
- Das, T., Safferling, K., Rausch, S., Grabe, N., Boehm, H. and Spatz, J. P. (2015). A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol. 17(3): 276–287.
- Selden, N. S., Todhunter, M. E., Jee, N. Y., Liu, J. S., Broaders, K. E. and Gartner, Z. J. (2011). Chemically Programmed Cell Adhesion with Membrane-Anchored Oligonucleotides. J Am Chem Soc. 134(2): 765–768.
- Koo, H., Choi, M., Kim, E., Hahn, S. K., Weissleder, R. and Yun, S. H. (2015). Bioorthogonal Click Chemistry-Based Synthetic Cell Glue. Small. 11(48): 6458–6466.
- Ollech, D., Pflästerer, T., Shellard, A., Zambarda, C., Spatz, J. P., Marcq, P., Mayor, R., Wombacher, R. and Cavalcanti-Adam, E. A. (2020). An optochemical tool for light-induced dissociation of adherens junctions to control mechanical coupling between cells. Nat Commun. 11(1): 472.
- Bian, Q., Wang, W., Han, G., Chen, Y., Wang, S. and Wang, G. (2016). Photoswitched Cell Adhesion on Azobenzene‐Containing Self‐Assembled Films. ChemPhysChem. 17(16): 2503–2508.
- Endo, M., Iwawaki, T., Yoshimura, H. and Ozawa, T. (2019). Photocleavable Cadherin Inhibits Cell-to-Cell Mechanotransduction by Light. ACS Chem Biol. 14(10): 2206–2214.
- Yüz, S. G., Rasoulinejad, S., Mueller, M., Wegner, A. E. and Wegner, S. V. (2019). Blue Light Switchable Cell–Cell Interactions Provide Reversible and Spatiotemporal Control Towards Bottom‐Up Tissue Engineering. Adv Biosyst. 3(4): e201800310.
- Yüz, S. G., Ricken, J. and Wegner, S. V. (2018). Independent Control over Multiple Cell Types in Space and Time Using Orthogonal Blue and Red Light Switchable Cell Interactions. Adv Sci. 5(8): e1800446.
- Mueller, M., Rasoulinejad, S., Garg, S. and Wegner, S. V. (2020). The Importance of Cell–Cell Interaction Dynamics in Bottom-Up Tissue Engineering: Concepts of Colloidal Self-Assembly in the Fabrication of Multicellular Architectures. Nano Lett. 20(4): 2257–2263.
- Rasoulinejad, S., Mueller, M., Nzigou Mombo, B. and Wegner, S. V. (2020). Orthogonal Blue and Red Light Controlled Cell–Cell Adhesions Enable Sorting-out in Multicellular Structures. ACS Synth Biol. 9(8): 2076–2086.
- Nzigou Mombo, B., Bijonowski, B. M., Rasoulinejad, S., Mueller, M. and Wegner, S. V. (2021). Spatiotemporal Control Over Multicellular Migration Using Green Light Reversible Cell–Cell Interactions. Adv Biol. 5(5): e202000199.
- Nzigou Mombo, B., Bijonowski, B. M., Raab, C. A., Niland, S., Brockhaus, K., Müller, M., Eble, J. A. and Wegner, S. V. (2023). Reversible photoregulation of cell-cell adhesions with opto-E-cadherin. Nat Commun. 14(1): 6292.
- Di Iorio, D., Bergmann, J., Higashi, S. L., Hoffmann, A. and Wegner, S. V. (2023). A disordered tether to iLID improves photoswitchable protein patterning on model membranes. Chem Commun. 59(29): 4380–4383.
- St Croix, B., Sheehan, C., Rak, J. W., Flørenes, V. A., Slingerland, J. M. and Kerbel, R. S. (1998). E-Cadherin–dependent Growth Suppression is Mediated by the Cyclin-dependent Kinase Inhibitor p27KIP1. J Cell Biol. 142(2): 557–571.
- Ramirez Moreno, M., Stempor, P. A. and Bulgakova, N. A. (2021). Interactions and Feedbacks in E-Cadherin Transcriptional Regulation. Front Cell Dev Biol. 9: e701175.
- Lewis, J. E., Wahl, J. K., Sass, K. M., Jensen, P. J., Johnson, K. R. and Wheelock, M. J. (1997). Cross-Talk between Adherens Junctions and Desmosomes Depends on Plakoglobin. J Cell Biol. 136(4): 919–934.
- Höhener, T. C., Landolt, A. E., Dessauges, C., Hinderling, L., Gagliardi, P. A. and Pertz, O. (2022). LITOS: a versatile LED illumination tool for optogenetic stimulation. Sci Rep. 12: 13139.
- Chen, W., Wong, C., Vosburgh, E., Levine, A. J., Foran, D. J. and Xu, E. Y. (2014). High-throughput Image Analysis of Tumor Spheroids: A User-friendly Software Application to Measure the Size of Spheroids Automatically and Accurately. J Visualized Exp: e3791/51639.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Raab, C. A. and Wegner, S. V. (2024). Reversible Photoregulation of Cell–Cell Adhesions With Opto-E-cadherin. Bio-protocol 14(10): e4995. DOI: 10.21769/BioProtoc.4995.
Category
Cell Biology > Cell-based analysis > Cell adhesion
Molecular Biology > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








