- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Standardized Protocol for Extraction and Homogenization of Ocular Tissues
Published: Vol 14, Iss 10, May 20, 2024 DOI: 10.21769/BioProtoc.4988 Views: 2903
Reviewed by: Vivien J. Coulson-ThomasAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Expression and Analysis of Flow-regulated Ion Channels in Xenopus Oocytes
Shujie Shi and Marcelo D. Carattino
Apr 20, 2017 10310 Views

Probe-Seq: Method for RNA Sequencing of Specific Cell Types from Animal Tissue
Ryoji Amamoto [...] Constance L. Cepko
Sep 20, 2020 7233 Views
Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1158 Views
Abstract
The eye is a complex organ composed of multiple tissues in anterior and posterior eye segments. Malfunctions of any of these tissues can lead to ocular diseases and loss of vision. A detailed understanding of the ocular anatomy and physiology in animal models and humans contributes to the development of ocular drugs by enabling studies on drug delivery and clearance routes, pharmacokinetics, and toxicity. This protocol provides step-by-step instructions for the extraction and homogenization of ocular tissues for enzymatic and proteomics analyses.
Key features
• Suitable protocol for the extraction and isolation of ocular tissue from humans and laboratory animals (rabbit, pig, rat, mouse) while minimizing cross-contamination.
• Hard or soft tissue homogenates can be prepared efficiently using a Bead Ruptor homogenizer.
• Allows to determine the protein contents in prepared homogenates.
Keywords: EyeGraphical overview
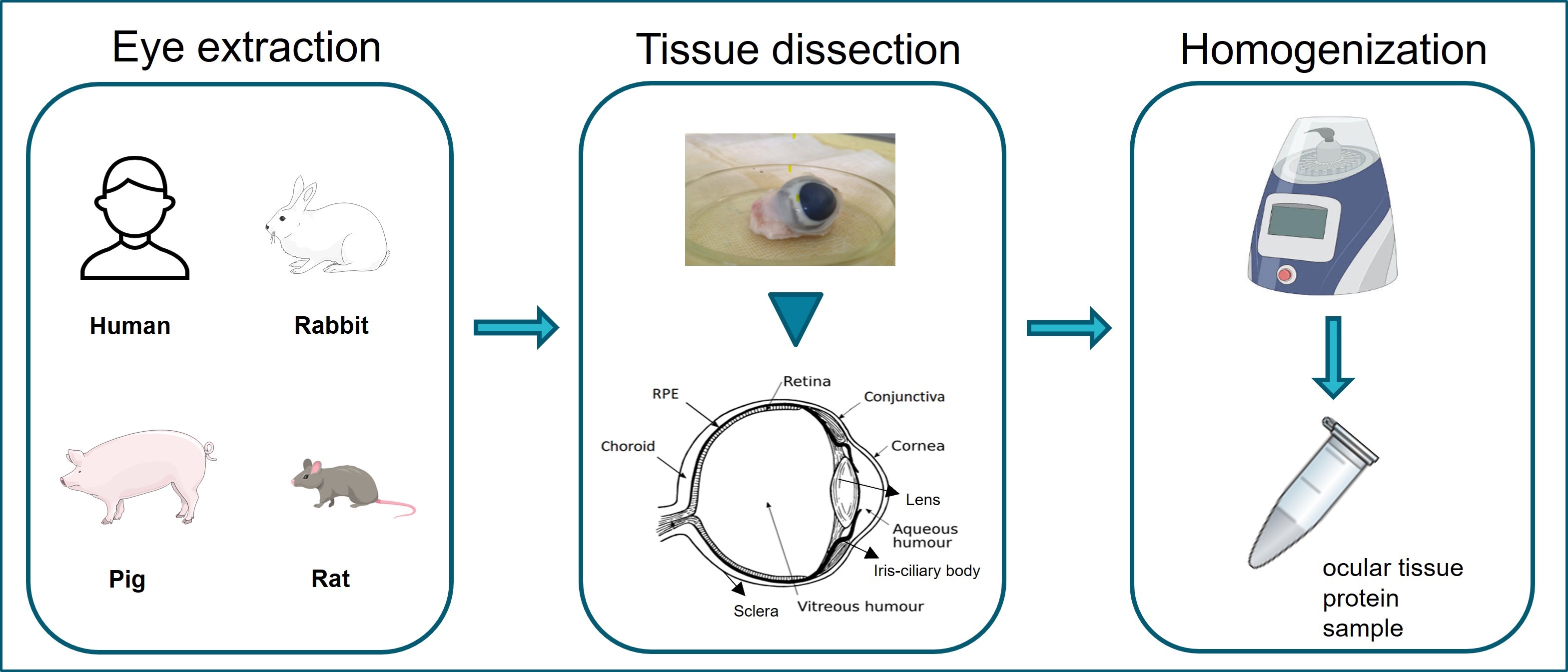
Graphical overview of ocular tissue extraction starting from eye enucleation from human or laboratory animal samples. Extraction and separation of ocular tissues are shown in tissue dissection. Homogenization of ocular tissues collected using the Bead Ruptor Elite homogenizer.
Background
Ocular diseases lead to vision loss in the aging global population [1,2], decreasing quality of life and increasing costs of health care [3]. Ocular drug development seeks to alleviate eye diseases by developing improved ocular therapeutics [4]. With that intention, a detailed understanding of ocular compartments, tissues in the anterior and posterior segments, and barriers is required [5,6].
The eye encompasses multiple tissues that individually play a significant role in the vision [7]. Anatomically, the eyeball is composed of a smaller anterior and a larger posterior segment [8]. The anterior tissues include the conjunctiva, cornea, aqueous humor, lens, iris, and ciliary body. The posterior segment contains vitreous humor, retinal pigment epithelium (RPE), neural retina, choroid, and sclera [9].
Isolation of contamination-free (devoid of neighboring/cross tissues and foreign-substance contaminants that could interfere with subsequent analysis or experiments) individual ocular tissues is challenging [3] due to the small tissue sizes and complexity. This protocol aims to provide details for (i) the separation and isolation of the ocular tissues, (ii) the preparation of ocular tissue homogenates, and (iii) the quantification of protein contents in prepared homogenates, e.g., enzymatic and proteomic analyses. Minimizing cross-contamination from anatomically adjacent tissues and preserving protein integrity is of fundamental importance.
This optimized protocol allows the preparation of tissue homogenates from fresh or frozen eyeballs. The procedure begins with the aspiration of the aqueous humor. Thereafter, all anterior eye tissues (conjunctiva, cornea, lens, iris, and ciliary body) can be individually separated from each other, leaving the vitreous exposed for collection. Further steps allow the collection of posterior eye tissues (retina, RPE, choroid, and sclera). The differences in tissue hardness influence the homogenization steps, which are described in detail.
Materials and reagents
Biological material
Human or laboratory animal (e.g., rabbit or pig) eyeballs. This protocol is equally suitable for rat and mouse eyes, but dissection and tissue isolation are recommended to be performed under the microscope
Reagents
Dulbecco's phosphate-buffered saline, 10× (DPBS) (Thermo Fisher, catalog number: 14200166)
Potassium phosphate buffer (PBS) (Sigma-Aldrich, catalog number: P3813)
Sterile water (Baxter, catalog number: KKF7113)
Ethanol (Sigma-Aldrich, catalog number: 64-17-5)
Syringe 1 mL (Luer Slip IV Syringes, catalog number: FWC345)
26 G needle 1/2″ (0.45 × 12 mm) (Medoject, catalog number: CH26012)
Scissors: Sharp straight (115 mm 122-010) (Elcon Scissor Tenotomy Stevens Straight, catalog number: 547456), curved pointed scissors (115 mm, curved, pointed St) (Elcon Iris Scissors, catalog number: ELC122011)
Beakers
Scalpel (Swann-Morton sterile disposable) (Sigma-Aldrich, catalog number: S2771-100EA)
Small brushes to scrape RPE; size 4–6 mm (round or flat depending on the size of eye and species; for rats and mice, smaller sizes are more suitable)
Eppendorf tubes, 1.5/2 mL sterile [Reaction tube, 1.5 mL (SARSTEDT, catalog number: 72.690.001); reaction tube, 2 mL (SARSTEDT, catalog number: 72.691)]
50 mL Falcon tubes (Screw cap, 114 × 28 mm) (SARSTEDT, catalog number: 62.547.254)
Pipettes and pipette tips [FinnpipetteTM F2 Good Laboratory Pipetting (GLP) Kit] (Thermo Fisher Scientific, catalog number: 4700880)
Bradford assay kit [10] (Bio-Rad Laboratories, catalog number: 5000205)
2 mL reinforced Polypropylene, 2.8 mm ceramic beads tube (Omni Internationals Kennesaw, catalog number: 19-628D)
Costar® 6-well flat-bottom plate, clear, polystyrene (Costar, catalog number: 38015)
Petri dishes, 92 × 16 mm, transparent (SARSTEDT, catalog number: 82.1472)
Filter papers (Whatman® qualitative filter paper) (Sigma-Aldrich, catalog number: WHA1001929)
Note: Use only sterile and clean equipment during the dissection. Also, tissue isolation should be performed in a sterile environment by cleaning surfaces and equipment thoroughly, followed by disinfection and sterilization.
Equipment
Olympus CK2 inverted phase contrast microscope (Microscope Central, model: ULWCD 0.3 N.A.)
Microcentrifuge (Thermo Fisher Scientific, model: Heraeus Biofuge 24 Place)
Bead Ruptor Elite homogenizer Omni Internationals (Kennesaw, GA, USA)
2 mL reinforced tubes filled with 2.8 mm diameter ceramic beads (Omni Internationals. Kennesaw, GA, USA)
Microplate reader (PerkinElmer Wallac, model: Victor2)
Procedure
Extraction
Obtain the eyeballs from a slaughterhouse (pig) or extract eyeballs by sacrificing the rabbit in an animal lab facility, usually by injecting the lethal dose of 60 mg/mL pentobarbital (Mebunat; Orion Pharma, Finland) into the marginal ear veins. The eyeball can be removed via enucleation, which is a surgical process to remove and detach the eye from the orbit. The outer part of the eyelid is usually cut with the help of sharp scissors. All tissue connections should be separated between the eyeball and the surrounding orbit with the help of sharp scissors. Detach the eyeball and place it on ice in prelabeled plastic bags, named with left/right eye, animal numbers, and further experimental details.
Keep the eyeballs immersed in ice-cold 1× DPBS, pH 7.4, on ice after extraction and during transportation.
Set up the required equipment for dissection, including sharp straight and curved scissors, beakers, Petri dishes, scalpel, filter papers, brushes to scrape RPE, and sterile Eppendorf tubes. In addition, buffer (approximately 5–6 mL of PBS/eye), sterile water, and ethanol for washing purposes should be placed in Falcon tubes (Figure 1).
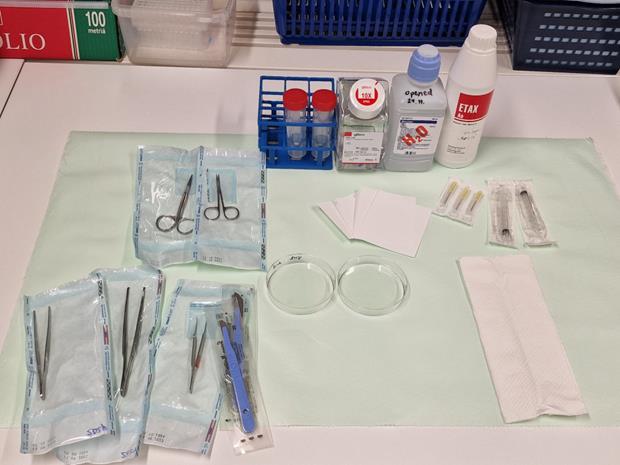
Figure 1. Equipment and reagents for ocular tissue dissectionLabel the collection tubes (1.5–2 mL Eppendorf tubes), weigh each empty tube (conjunctiva, lens, iris-ciliary body, cornea, aqueous humor, vitreous, neural retina, RPE, choroid, sclera), and keep them on ice.
Note the weights of the empty tubes and record them on the provided sheet. This data will be used later to calculate the weight of the tissues. To obtain the tissue weight, subtract the weight of the tube with tissues from the weight of the empty tube (Table 1).
Table 1. Sheet to record the weight of isolated ocular tissues
Date: Eye no: Organism: Tissue Empty tube [w] g/mg Tube + tissue [w] g/mg Tissue [w] g/mg Notes (1) Conjunctiva (2) Cornea (3) Aqueous humor (4) Lens (5) Iris-ciliary body (6) Vitreous (7) Neural retina (8) RPE (9) Choroid (10) Sclera Start the dissection by removing the eye from the buffer and placing it on filter paper in the Petri dish or on a cooled pad (for thermosensitive samples, to preserve the protein/sample integrity) on filter paper.
First, remove all extraocular parts, as shown in Figures 2 and 3 (A and B).
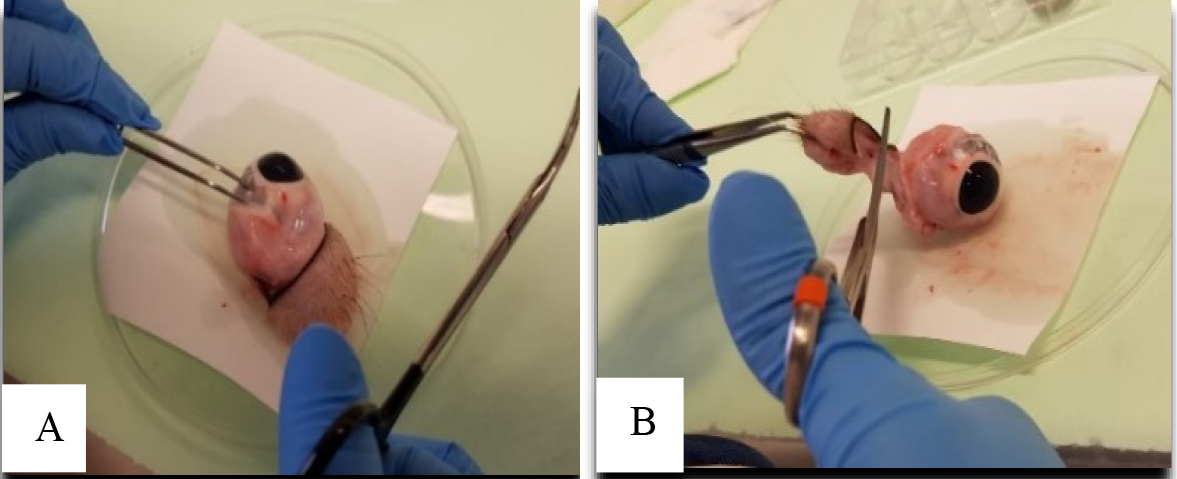
Figure 2. Removing extraocular muscles and parts during ocular tissue extraction. A. Holding the eyeball with the help of tweezers. B. Cutting extra ocular parts with the help of scissors.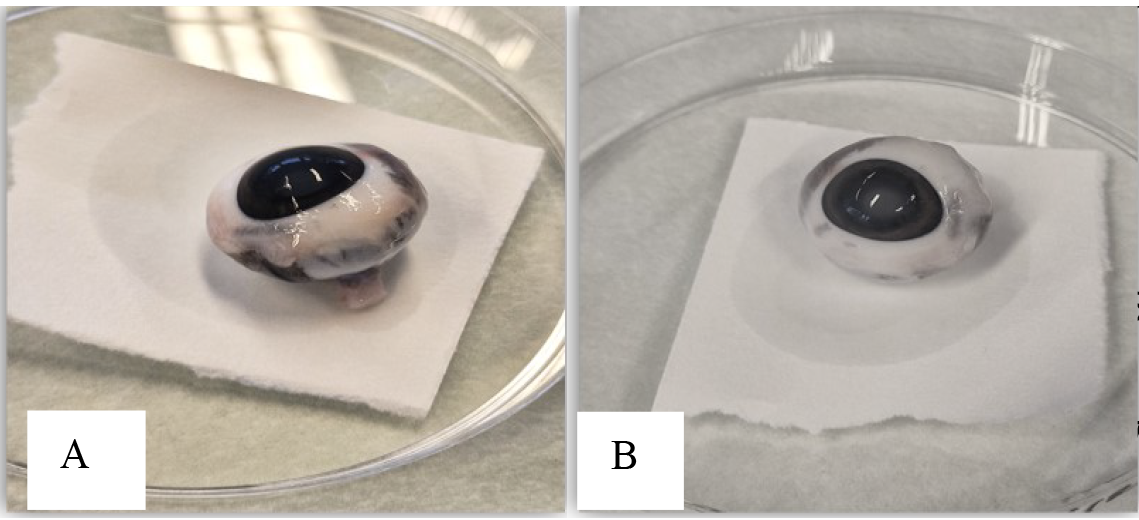
Figure 3. Clean eyeball after removing all extraocular muscles and parts. A. Side of the eyeball. B. Front of the eyeball.Aspirate the aqueous humor through the limbus using a 1 mL syringe and 26 G needle (Figure 4A) before cutting the eyeball, as it will collapse during the extraction of intraocular tissues. Hold the syringe in one hand and, with the other hand, hold the eyeball to keep it in a stable and fixed position. Insert the syringe near the peripheral cornea from the limbus into the anterior chamber, which is the space between the cornea (the clear surface) and the iris (the colored part). Avoid inserting directly into the center to avoid damage to the lens (Figure 4). The amount of aqueous humor collected can vary depending on species and handling, ranging between 20 and 200 µL.
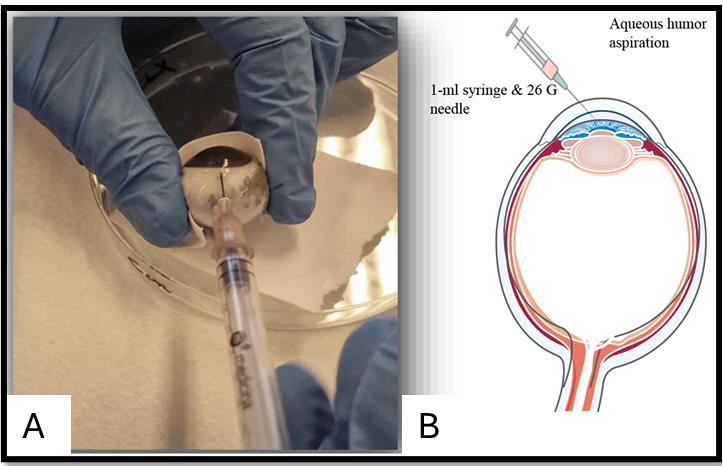
Figure 4. Aqueous humor aspiration. A. Aspiration aided by a syringe and needle. B. Graphical representation of the aspiration of aqueous humor.After aqueous humor collection, the conjunctiva can be collected from the outer interior part with the help of a scalpel or scissors (Figure 5A–C). Place the eyeball on the Petri dish and pull up the conjunctival layer 3–5 mm away from the limbus with the help of a small tweezer; make a hole in the layer between the conjunctiva and the connective tissues beneath. Start cutting and moving forward to get a layer of conjunctiva while avoiding the adjacent muscles. Cut and collect pieces of bulbar conjunctiva in a tube and place the tube on ice after collection.
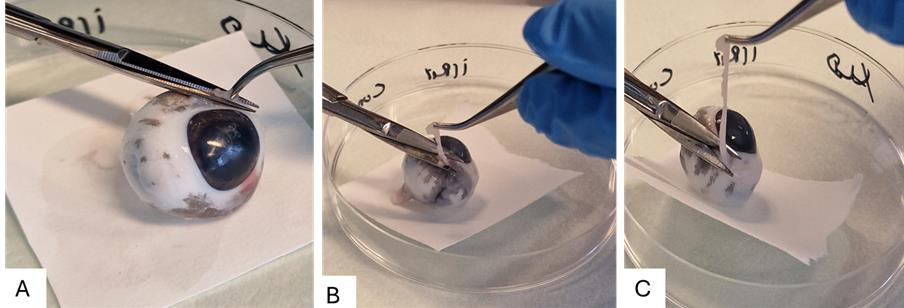
Figure 5. Steps for collecting the bulbar conjunctiva. A. Placing the eyeball on the Petri dish, holding the conjunctival layer. B. Cutting with the help of tweezers and scissors. C. Collection of the bulbar conjunctival layer.Next, grab the eyeball tightly by holding it with the help of filter paper and mark a cut on the limbus (2 mm from the edge of the cornea). Carefully cut the eye with the help of curved scissors around the iris (Figure 6). The eyecup is now cut into two parts: the anterior and the posterior eye cup. The anterior eye cup contains tissues such as the lens, iris-ciliary body, and cornea, while the posterior contains vitreous, retina, retinal pigment epithelium, choroid, and sclera.

Figure 6. Cutting the eye with the help of scissors to open the eye cupOpen the eye cup and collect all the anterior eye tissues [lens, iris-ciliary body, and cornea (Figure 7)] into labeled tubes on ice. The lens is a crystalline, transparent, gelatinous, and round structure (Figure 7A). The iris and ciliary body are ring-shaped and dark-colored structures next to the lens (Figure 7B), while the cornea is a transparent top layer of the eye cup (Figure 7C). From the anterior eye cup, the lens can be easily pulled out with the help of forceps; then, the iris-ciliary body can be detached by holding it with the help of tweezers while peeling and separating gently away from the back of the cornea. In the end, the cornea is visible, isolated from the rest of the anterior ocular tissues.
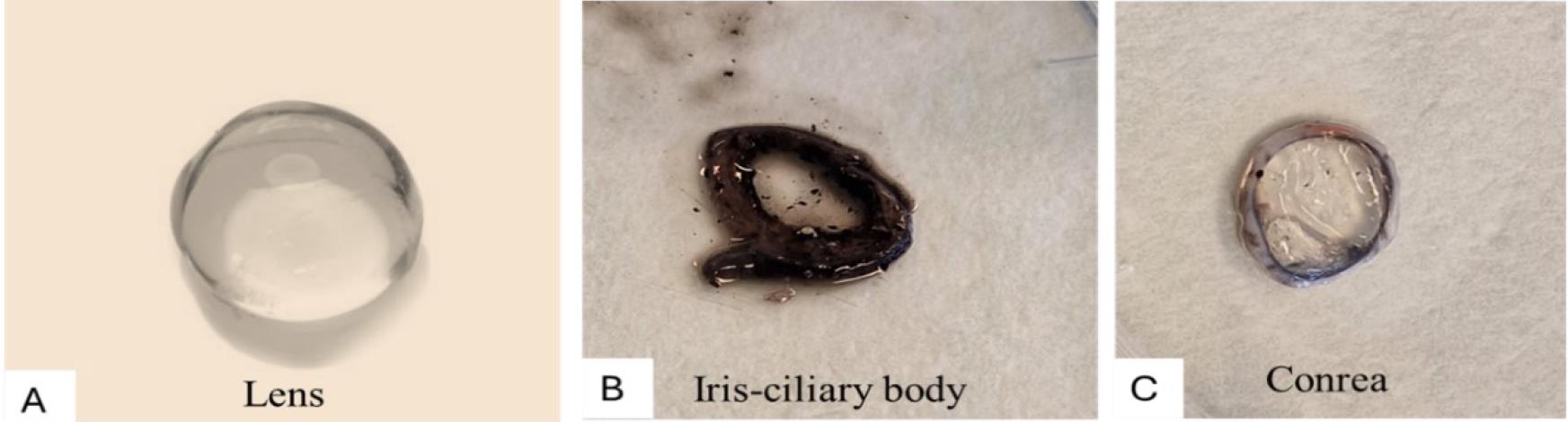
Figure 7. Anterior eye tissues. A. Lens B. Iris-ciliary body. C. Cornea.From the posterior eye cup, collect the vitreous and neural retina first and then the retinal pigment epithelium cells; finally, separate the choroid from the sclera. The vitreous can be aspirated via pipetting and carefully separating from the neural retina. Collection will be smoother when using a broader pipette tip (e.g., 1 mL or bigger) compared with a narrow and small tip because of the gel-like structure of the vitreous, as shown in Figure 8. Collect only the clear and transparent vitreous to avoid contamination by retinal tissues.
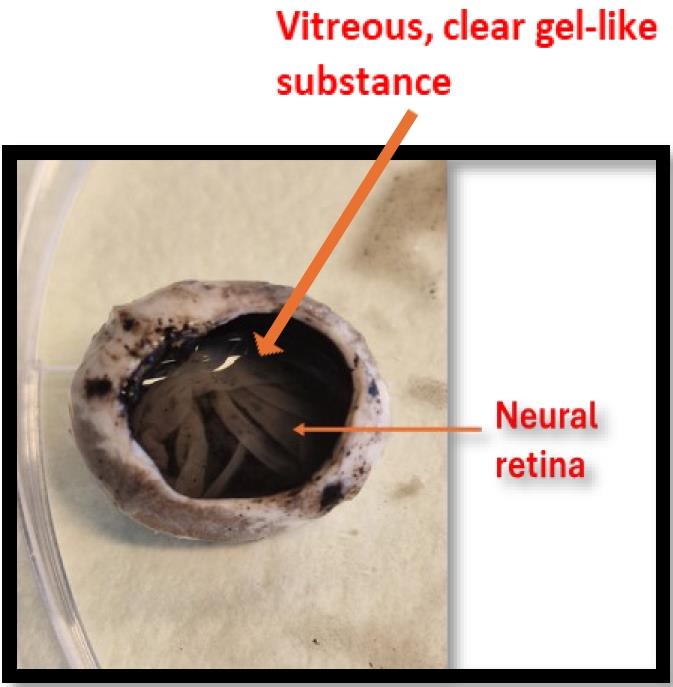
Figure 8. Neural retina and vitreous visible on top of the posterior eye cupAfter removing the vitreous, the neural retina can be pulled out gently and detached easily from the RPE layer (shown in Figure 8) with the help of forceps or broad-tip tweezers. The isolation of the neural retina from RPE is easier when using a fresh eye.
Now, the eye cup has RPE on the top. The dark-colored pigmented RPE can be easily visually distinguished from the retina (Figure 9). To place the posterior eye cup in a multi-well plate, use sterile forceps or tweezers to grip its edges. Carefully lower the eye cup into the selected well and ensure it is placed in any required well. Adjust the position by moving it to locate it exactly in the middle (Figure 9B). Pipette ~1 mL of 1× PBS on the eye cup while placing it in a multi-well plate. Wait at least 5 min to help RPE cells detach and immerse in the buffer. After 5 min, scrape the RPE layer with the help of a small brush to recover the RPE cells. Repeat twice until all RPE is collected (as shown in Figure 9A–E).
Centrifuge the RPE suspensions at 6,000× g for 5 min at 4 °C to pellet the RPE. The supernatant can be discarded; the pellet contains the desired RPE cells that are to be stored (Figure 9E).

Figure 9. Steps for collecting retinal pigment epithelium (RPE) from the eyecup. A. Take the posterior eye cup. B. Pipette 1× PBS to the eye cup (e.g., 1 mL, 2×) while placing it in a multi-well plate and wait for 5 min to let RPE cells detach. C. Scrape with the help of a small brush to detach RPE cells. D. Remove the eye cup and take the buffer containing RPE cells with the help of a pipette in the Eppendorf tube. E. Centrifuge the RPE suspensions at 6000× g for 5 min at 4 °C to pellet the RPE. Discard the supernatant and keep the pellet containing RPE cells.The choroid is a sticky tissue that can be scraped with a scalpel to separate it from the sclera. The pigmented choroid will be visually distinguishable from the sclera. Peel off the choroid, starting from one edge from the top of the sclera to separate both layers. The detailed steps are shown in Figure 10.

Figure 10. Steps showing the peeling off of the choroid from the top of the scleraWash the sclera with PBS to eliminate traces of choroid. Take a clean beaker and fill it with 1× PBS. Make sure there is enough PBS to completely immerse the sclera and repeat this three times to ensure complete removal of choroid traces.
Weigh the individual tubes containing tissues and subtract the empty tube weight to get the actual tissue weight. Keep a record of tissue weights while placing tissues on ice during and after weighing.
Homogenization using Bead Ruptor Elite homogenizer
Homogenization of ocular tissues can be done using a Bead Ruptor Elite homogenizer and 2.8 mm diameter ceramic beads.
Label 2 mL hard tissue homogenizing reinforced tubes prefilled with four beads per tube (2.8 mm diameter).
Precool the Cryo cooling unit of the homogenizer using liquid nitrogen or dry ice with ethanol according to the manufacturer's instructions to maintain a constant temperature at 4 °C before loading the heat-sensitive samples.
Add tissue pieces (cut the tough tissues into smaller pieces using a scalpel to aid in homogenization) to the tubes and 1× DPBS buffer (3:1, volume/tissue weight or depending on working dilution required).
Unlock the finger plate on top of the tube carriage and remove it by rotating it. Load the 2 mL sample tubes on a precooled (~4 °C) 24-bead raptor tube carriage. Samples must be spaced evenly (it is recommended to load at least four tubes simultaneously with approximately equal weight; for that, weigh the tubes beforehand). Lock the carriage before the run.
For hard tissues such as the cornea and conjunctiva, use a 1-min cycle 4× at a speed of 6 m/s as described earlier [11]. The cycle speed (m/s), run time (s), and dwell time between runs can be adjusted according to the tissue. The vitreous and aqueous humor can be homogenized without adding buffer (or depending upon the dilution required) at a low cycle speed and using less run time, e.g., a 1-min cycle one time at a speed of 6 m/s).
Note: Both aqueous humor and vitreous are homogenized to ensure uniformity during protein quantification, enhance the accuracy of analyses, and achieve consistency in results and subsequent analysis.
Here, the starting temperature of each cycle is very critical for temperature-sensitive samples. If the start temperature of the cycle is at -2 °C, for a 1-min cycle it will not exceed the limit of 4 °C. Before the next cycle, turn on the cooling chamber and bring the temperature of the sample chamber down to -2 °C.
In addition, the sample loading carriage can be placed in a fridge at 4 °C to keep it cold.
Once the sample carriage chamber is cool, load the samples and press Run to start the cycle and Stop to stop the run on the screen.
Check with the pipette tip if the sample is completely homogenized; otherwise, repeat the cycle to get a completely homogenized sample.
Centrifuge homogenates at 10,000× g for 5 min at 4 °C to collect the supernatant. Aliquot and store at -80 °C.
Protein quantification
Note: Not all labs have Bead Ruptor Elite homogenizers. In that case, a traditional manual or electric homogenizer or grinder can be used, such as a Dounce homogenizer or Potter-Elvehjem homogenizer.
We routinely use the Bradford assay [10] using bovine serum albumin (0.25–2 mg/mL) as the standard for protein quantification.
Validation of protocol
This protocol has been validated in the following research article:
Hammid et al. [12]. Carboxylesterase Activities and Protein Expression in Rabbit and Pig Ocular Tissues. Molecular Pharmaceutics (Figure 1, panels A, B, and C).
General notes and troubleshooting
Following this protocol, the extraction of tissues from fresh eye samples is relatively easy. Separation of the ocular tissues from frozen eyeballs, especially posterior tissues, is more variable and challenging: clean isolation of RPE and choroid from frozen human eyes may be impossible, and we have often combined RPE and choroid into a single tissue homogenate to avoid variability.
The extraction protocol does not guarantee 100% purity of each individual tissue, although precautions against cross-contamination of adjacent tissues can be made by washing and visual inspection. It is advised to estimate this by immunoblotting with tissue-specific antibodies against marker proteins [11].
In these preparations, we omitted protease and phosphatase inhibitors to avoid interference with the activity of specific enzymes (particularly esterases) under study. However, protease and phosphatase inhibitor cocktails are essential for cell lysis and protein extraction. They protect proteins from degradation and preserve their phosphorylation status by blocking or neutralizing enzymes released during cell lysis, which could otherwise break down proteins and affect their activation states.
Acknowledgments
This protocol was adapted from the publication of rabbit and pig [12] and human eyes [11]. The author acknowledges Professor Paavo Honkakoski at the University of Eastern Finland for kindly reviewing the manuscript. This work was supported by EU-ITN project OCUTHER (H2020-MSCA-ITN-2016, grant number 722717) and the Doctoral Programme in Drug Research (University of Eastern Finland). The author would like to acknowledge The Maud Kuistila Memorial Foundation, The Finnish Cultural Foundation, and Oskar Öflunds Foundation for financial support.
Competing interests
The author declares no conflict of interest.
Ethical considerations
The study used human and animal eye samples. Human eye samples were provided by a biobank [11] at the University Hospital in Santiago de Compostela, Spain. The use of laboratory animals and human tissue [11,12] was approved by the local animal welfare committee (license # ESAVI/8621/04.10.07/2017).
References
- Steinmetz, J. D., Bourne, R. R. A., Briant, P. S., Flaxman, S. R., Taylor, H. R. B., Jonas, J. B., Abdoli, A. A., Abrha, W. A., Abualhasan, A., Abu-Gharbieh, E. G., et al. (2021). Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob. Health 9(2): e144–e160.
- Bourne, R., Steinmetz, J. D., Flaxman, S., Briant, P. S., Taylor, H. R., Resnikoff, S., Casson, R. J., Abdoli, A., Abu-Gharbieh, E., Afshin, A., et al. (2021). Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob. Health 9(2): e130–e143.
- Dumouchel, J. L., Chemuturi, N., Milton, M. N., Camenisch, G., Chastain, J., Walles, M., Sasseville, V., Gunduz, M., Iyer, G. R., Argikar, U. A., et al. (2018). Models and Approaches Describing the Metabolism, Transport, and Toxicity of Drugs Administered by the Ocular Route. Drug Metab. Dispos. 46(11): 1670–1683.
- Gote, V., Sikder, S., Sicotte, J. and Pal, D. (2019). Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 370(3): 602–624.
- Maurice, D. M. and Mishima, S. (1984). Ocular pharmacokinetics. In: Pharmacology of the Eye (pp. 19–116). Berlin, Heidelberg: Springer Berlin Heidelberg.
- Gower, N. J. D., Barry, R. J., Edmunds, M. R., Titcomb, L. C. and Denniston, A. K. (2016). Drug discovery in ophthalmology: past success, present challenges, and future opportunities. BMC Ophthalmol. 16: 11.
- Sebastian, E. T. (2010). The complexity and origins of the human eye: A brief study on the anatomy, physiology, and origin of the eye. Senior Honors Theses. 116.
- Mafee, M. F. (1996). Eye and orbit. In: Head and Neck Imaging. Sore, P. M. (Ed.), St. Louis; Mosby, 1109-I 114.
- Malhotra, A., Minja, F. J., Crum, A. and Burrowes, D. (2011). Ocular Anatomy and Cross-Sectional Imaging of the Eye. Semin. Ultrasound CT MRI 32(1): 2–13.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
- Hammid, A., Fallon, J. K., Lassila, T., Vieiro, P., Balla, A., Gonzalez, F., Urtti, A., Smith, P. C., Tolonen, A., Honkakoski, P., et al. (2022). Activity and Expression of Carboxylesterases and Arylacetamide Deacetylase in Human Ocular Tissues. Drug Metab. Dispos. 50(12): 1483–1492.
- Hammid, A., Fallon, J. K., Lassila, T., Salluce, G., Smith, P. C., Tolonen, A., Sauer, A., Urtti, A. and Honkakoski, P. (2021). Carboxylesterase Activities and Protein Expression in Rabbit and Pig Ocular Tissues. Mol. Pharmaceutics 18(3): 1305–1316.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Hammid, A. (2024). A Standardized Protocol for Extraction and Homogenization of Ocular Tissues. Bio-protocol 14(10): e4988. DOI: 10.21769/BioProtoc.4988.
Category
Cell Biology > Cell isolation and culture > Cell isolation
Biochemistry > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









