- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Apolipoprotein B Secretion Assay from Primary Hepatocytes
Published: Vol 14, Iss 9, May 5, 2024 DOI: 10.21769/BioProtoc.4982 Views: 1629
Reviewed by: Ralph Thomas BoettcherKrishna NakuluriAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Enhancement of Mucus Production in Eukaryotic Cells and Quantification of Adherent Mucus by ELISA
Christian Reuter and Tobias A. Oelschlaeger
Jun 20, 2018 11771 Views
In-vitro GLP-1 Release Assay Using STC-1 Cells
Liu Qi [...] Sifuentes-Dominguez Luis
Aug 20, 2020 6285 Views
Muscle Biopsy Sample Preparation and Proteomics Analysis Based on UHPLC-MS/MS
Jiawei Du [...] Yafeng Song
Dec 20, 2024 1912 Views
Abstract
Apolipoprotein B (APOB) is the primary structural protein of atherogenic lipoproteins, which drive atherogenesis and thereby lead to deadly cardiovascular diseases (CVDs). Plasma levels of APOB-containing lipoproteins are tightly modulated by LDL receptor–mediated endocytic trafficking and cargo receptor–initiated exocytic route; the latter is much less well understood. This protocol aims to present an uncomplicated yet effective method for detecting APOB/lipoprotein secretion. We perform primary mouse hepatocyte isolation and culture coupled with well-established techniques such as immunoblotting for highly sensitive, specific, and semi-quantitative analysis of the lipoprotein secretion process. Its inherent simplicity facilitates ease of operation, rendering it a valuable tool widely utilized to explore the intricate landscape of cellular lipid metabolism and unravel the mechanistic complexities underlying lipoprotein-related diseases.
Key features
• A pipeline for the isolation and subsequent culture of mouse primary hepatocytes.
• A procedure for tracking the secretion of APOB-containing lipoproteins.
• A rapid and sensitive assay for detecting the APOB level based on immunoblotting.
Keywords: Cardiovascular diseasesBackground
Cardiovascular diseases (CVDs) continue to be the leading cause of death worldwide, accounting for over 20 million deaths each year. Hyperlipidemia, characterized by elevated plasma levels of cholesterol and triglycerides, is the most common risk factor for CVDs [1–3]. Due to their hydrophobic nature, lipids are loaded onto specialized carriers named lipoproteins before entering the circulation to meet the lipid demands of various body parts. Excess plasma lipids, especially those carried by apolipoprotein B (APOB)-containing lipoproteins, are the major cause of plaque buildup on the arteries, which leads to narrowed blood vessels, obstruction of blood flow, and ultimately CVDs. The APOB is the primary and unexchangeable structural protein of atherogenic lipoproteins, including low-density lipoproteins (LDL) and chylomicrons (CM), originating from hepatocytes and enterocytes, respectively [4–6]. Stoichiometrically, each APOB-containing lipoprotein contains one APOB molecule, which renders the plasma APOB levels a more reliable risk indicator for CVDs than plasma lipid levels per se.
Understanding the secretion process of lipoproteins is crucial for unraveling their roles in hyperlipidemia and CVDs. Abnormalities in lipoprotein secretion may result in elevated blood lipoprotein levels, further contributing to the progression of atherosclerosis. Therefore, developing an efficient and accurate method for detecting lipoprotein secretion is essential for a profound understanding of their biological functions and roles in disease development [7–11]. Here, we report a straightforward approach including the isolation and culture of primary mouse hepatocytes, followed by detecting the secreted APOB in the medium using established techniques like immunoblotting. Enzyme-linked immunosorbent assay (ELISA) can also be used for APOB secretion measurement, and the above methods are compatible. Our method provides a reliable, sensitive, specific, and semi-quantitative strategy for lipoprotein secretion detection, which can be feasibly applied to explore cellular lipid metabolism. This method may contribute to advancing the understanding of the regulatory mechanisms of lipoprotein export itinerary and holds promise for providing new insights into the prevention and treatment of CVDs.
Materials and reagents
25 mg/mL IV collagenase (Sigma-Aldrich, catalog number: C5138)
DMEM culture medium (Gibco, catalog number: 8123306)
Penicillin-Streptomycin solution (P/S), 100× (Caisson, catalog number: PSL01)
Phosphate buffered saline (PBS) (Meilunbio, catalog number: MA0015)
Fetal bovine serum (FBS) (Vistech, catalog number: SE100-011)
Acetone (TGREAG, catalog number: 105003)
APOB polyclonal antibody (Proteintech, catalog number: 20578-1-AP)
ALBUMIN monoclonal antibody (Proteintech, catalog number: 66051-1-Ig)
Glucose (HARVEYBIO, catalog number: SR1742)
NaHCO3 (TGREAG, catalog number: 114005)
NaCl (TGREAG, catalog number: 112008)
HEPES (Sigma, catalog number: V900477)
KCl (Sinopharm Chemical Reagent Co., Ltd, catalog number: 10016328)
KH2PO4 (Sinopharm Chemical Reagent Co., Ltd, catalog number: 10017628)
MgSO4·7H2O (Sinopharm Chemical Reagent Co., Ltd, catalog number: 10013018)
Tris-HCl (Sigma, catalog number: V900483)
Nonidet P-40 (Sigma, catalog number: 56741)
Glycerol (Sigma, catalog number: G7893)
EGTA (Sigma, catalog number: 03777)
CaCl2 (TGREAG, catalog number: 104034)
Solutions
Krebs Ringer with glucose (KRG) buffer (see Recipes)
Solution C (see Recipes)
Buffer 1 (see Recipes)
Buffer 2 (see Recipes)
Lysis buffer (see Recipes)
Recipes
KRG buffer (1 L)
Note: KRG buffer (pH = 7.4) should be prepared immediately before use. Filter through a 0.22 μm membrane before use.
Reagent Final concentration Quantity or Volume Glucose 20 mM 3.6 g NaHCO3 25 mM 2.0 g NaCl 120 mM 7.0 g HEPES 1 M (pH 7.45) 5 mL Solution C (Recipe 2) N/A 10 mL Total N/A Set volume to 1 L with ddH2O Solution C (1 L)
Reagent Final concentration Quantity or Volume KCl 480 mM 35.79 g KH2PO4 120 mM 16.33 g MgSO4·7H2O 120 mM 29.58 g Total N/A Set volume to 1 L with ddH2O Buffer 1
Add 100 μL of 50 mM EGTA to 50 mL of KRG buffer.
Buffer 2
Add 100 μL of 1 M CaCl2 and 25 mg of IV collagenase to 50 mL of KRG buffer.
Lysis buffer
Note: Store at 4 °C and add protease inhibitor before use.
Reagent Final concentration Tris-HCl (pH 7.4) 50 mM Nonidet P-40 1% Glycerol 10% NaCl 150 mM
Laboratory supplies
Cell strainer (Falcon, catalog number: 352350)
Millex-GP Filter, 0.22 μm, PES 33 mm, non-sterile (Millex, catalog number: SLGP033NS)
Axygen MaxyClear Snaplock microtubes (Axygen, catalog number: MCT-150-C)
10 cm cell culture dish (JET, catalog number: TCD010100)
6-well cell culture dish (JET, catalog number: TCP010006)
50 mL centrifuge tube (JET, catalog number: CFT011500)
10 mL pipettes (JET, catalog number: GSP010010)
Perfusion set (BD Intima II, catalog number: 383405; TNTC-1066)
Eye scissors (Beijing Hongbai Technology Co., Ltd., catalog number: 2101157)
Forceps (Beijing Hongbai Technology Co., Ltd., catalog number: HC10704)
Nitrocellulose membrane (DiNing, catalog number: 66485)
Equipment
Centrifuge (Eppendorf, model: 5424R)
Centrifuge (Eppendorf, model: 5810)
GE Amersham Imager600 (GE Healthcare, catalog number: 29083463)
Western blot equipment (Bio-Rad, catalog number: 1658033)
Procedure
Isolation and culture of primary mouse hepatocytes
Sterilize eye scissors and forceps.
Preheat two 50 mL solutions of KRG buffer in a 37 °C water bath for 30 min.
Using preheated KRG buffer, prepare buffer 1 and buffer 2 (see Recipes).
Weigh and anesthetize the mice used for experiments by intraperitoneal injection of tribromoethanol according to the local animal handling protocols.
After complete anesthesia, fix the mouse on a clean foam board, spray with 75% alcohol, and wipe with sterile gauze.
Cut the abdominal wall, separate the subcutaneous tissue, and detach the abdominal skin on both sides with a needle. Open the abdominal cavity; the liver, portal vein, hepatic artery, and hepatic vein are exposed.
Prepare an infusion set. Insert the venous indwelling needle into the vena cava, open the infusion valve, cut the portal vein, and perfuse the liver at a rate of 2 mL/min using Buffer 1 to remove residual blood.
Add 25 mg of IV collagenase to buffer 2 close to the time of use.
After the blood is thoroughly washed out, switch to buffer 2 for liver digestion. The collagenase perfusion process lasts approximately 1–2 min.
When the liver becomes enlarged, white, and loose (such that pressing lightly leaves a small dent), place the liver into a 10 cm dish with the remaining buffer 2 (approximately 15 mL) and transfer to a biosafety cabinet for further processing.
After collagenase perfusion, the liver becomes loose and soft and can be easily cut up with a pair of 10 cm eye scissors. Cut the liver into small pieces in a culture dish. Pipette the digested liver tissue until dispersed with a 10 mL pipette. Filter the mixture through a 70 μm cell strainer into a 50 mL centrifuge tube to remove tissue debris.
Add pre-chilled DMEM to the tube for cell resuspension
Centrifuge at 50× g for 4 min at 4 °C, discard the supernatant.
Repeat steps A12–A13 three times until the supernatant is clear. Perform all washing steps with DMEM.
Discard the supernatant, add prewarmed complete medium (DMEM with 10% FBS and 1% P/S), and seed the cells in a culture dish according to the desired cell density for subsequent experiments. Recommended cell density: 4 × 105 cells per well of a 6-well dish.
It usually takes 3–4 h for cell adhesion and before the cells can be used for follow-up experiments.
Lipoprotein secretion detection in primary mouse hepatocytes
Change the medium to FBS-free DMEM preheated at 37 °C (other treatments can be performed simultaneously according to experimental requirements): 10 mL for a 10 cm dish and 2 mL per well of a 6-well dish.
Collect the medium after 4–8 h depending on the specific experimental conditions.
Centrifuge at 15,000× g for 15 min at 4 °C to remove cell debris.
Transfer the supernatant to a new centrifuge tube, add four times the sample volume of pre-chilled acetone, mix well, and precipitate at -20 °C overnight or at -80 °C for 2 h.
After centrifugation at 15,000× g for 10 min at 4 °C, resuspend the pellet using lysis buffer and perform western blotting.
Note: The conditions for western blot can be as follows: Run the gel on a 3%–8% tris-acetate gel at 100 V. When the 250 kDa marker reaches the middle, typically after 75 min, transfer to a nitrocellulose membrane overnight at 200 mA by using fresh transfer buffer. The primary antibodies used for overnight incubation were APOB polyclonal antibody and ALBUMIN monoclonal antibody.
The results of analyzing APOB and albumin in the culture medium by immunoblotting are shown in Figure 1.
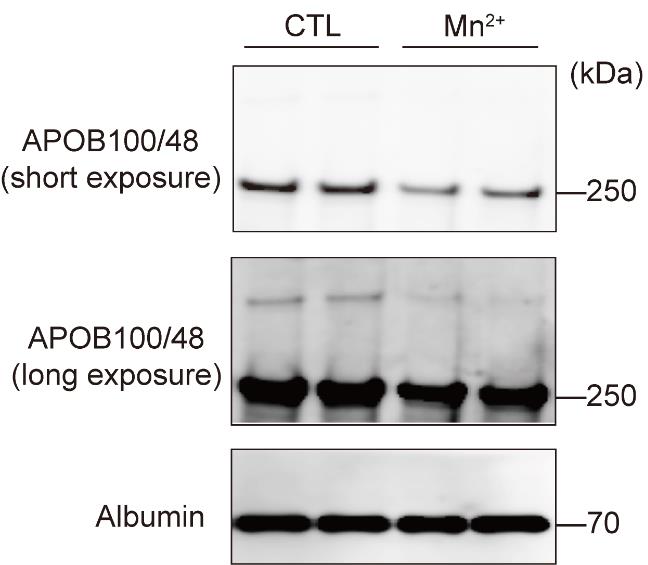
Figure 1. Decreased apolipoprotein B (APOB) secretion after Mn2+ treatment. Primary hepatocytes from wild-type mice were treated with 125 μM MnCl2 for 8 h. APOB and albumin in the medium were analyzed by immunoblotting.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article: Wang et al. [12]. Manganese regulation of COPII condensation controls circulating lipid homeostasis. Nature Cell Biology (Figure 1, panel d; Figure 5, panel a).
Acknowledgments
The work is supported by the National Natural Science Foundation of China (NSFC: 32100947 to X.W., 32125021, 92254308, 91957119, 91954001, 31571213 to X.W.C) and the National Key R&D Program (2021YFA0804802). This protocol has been used in Nature Cell Biology [12].
Competing interests
All authors declare no conflict of interest.
Ethical considerations
All animal housing and use were approved by the Institutional Animal Care and Use Committees of Peking University, an AAALAC-accredited laboratory animal.
References
- Roth, G. A., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., Abbastabar, H., Abd-Allah, F., Abdela, J., Abdelalim, A., et al. (2018). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159): 1736–1788.
- Goldstein, J. L. and Brown, M. S. (2015). A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell 161(1): 161–172.
- Bentzon, J. F., Otsuka, F., Virmani, R. and Falk, E. (2014). Mechanisms of Plaque Formation and Rupture. Circ. Res. 114(12): 1852–1866.
- Fisher, E. A. and Ginsberg, H. N. (2002). Complexity in the Secretory Pathway: The Assembly and Secretion of Apolipoprotein B-containing Lipoproteins. J. Biol. Chem. 277(20): 17377–17380.
- Ference, B. A., Kastelein, J. J. P. and Catapano, A. L. (2020). Lipids and Lipoproteins in 2020. JAMA 324(6): 595.
- Mehta, A. and Shapiro, M. D. (2021). Apolipoproteins in vascular biology and atherosclerotic disease. Nat. Rev. Cardiol. 19(3): 168–179.
- Ginsberg, H. N., Le, N. A., Goldberg, I. J., Gibson, J. C., Rubinstein, A., Wang-Iverson, P., Norum, R. and Brown, W. V. (1986). Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J. Clin. Invest. 78(5): 1287–1295.
- Björkegren, J. L. and Lusis, A. J. (2022). Atherosclerosis: Recent developments. Cell 185(10): 1630–1645.
- Bhatia, H. S., Becker, R. C., Leibundgut, G., Patel, M., Lacaze, P., Tonkin, A., Narula, J. and Tsimikas, S. (2023). Lipoprotein(a), platelet function and cardiovascular disease. Nat. Rev. Cardiol. e1038/s41569-023-00947-2.
- Tsimikas, S. and Witztum, J. L. (2023). Oxidized phospholipids in cardiovascular disease. Nat. Rev. Cardiol. 21(3): 170–191.
- Willeit, P., Ridker, P. M., Nestel, P. J., Simes, J., Tonkin, A. M., Pedersen, T. R., Schwartz, G. G., Olsson, A. G., Colhoun, H. M., Kronenberg, F., et al. (2018). Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet 392(10155): 1311–1320.
- Wang, X., Huang, R., Wang, Y., Zhou, W., Hu, Y., Yao, Y., Cheng, K., Li, X., Xu, B., Zhang, J., et al. (2023). Manganese regulation of COPII condensation controls circulating lipid homeostasis. Nat. Cell Biol. 25(11): 1650–1663.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Wang, Y., Li, X., Huang, R., Chen, X. W. and Wang, X. (2024). Apolipoprotein B Secretion Assay from Primary Hepatocytes. Bio-protocol 14(9): e4982. DOI: 10.21769/BioProtoc.4982.
Category
Cell Biology > Cell-based analysis > Protein secretion
Medicine > Cardiovascular system
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








