- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Agrobacterium-Mediated Transient Gene Expression Optimized for the Bioenergy Crop Camelina sativa
Published: Vol 14, Iss 7, Apr 5, 2024 DOI: 10.21769/BioProtoc.4964 Views: 2112
Reviewed by: Wenrong HeJianyan HuangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Application of Cadaverine to Inhibit Biotin Biosynthesis in Plants
Nicole M. Gibbs [...] Patrick H. Masson
Apr 20, 2022 2305 Views

Identification of S-locus F-box Protein Sequences in Diploid Potato, Solanum okadae, via Degenerate PCR
Amar Hundare [...] Timothy P. Robbins
Jun 5, 2025 1991 Views

Quantitative Analysis of the Arabidopsis Leaf Secretory Proteome via TMT-Based Mass Spectrometry
Sakharam Waghmare [...] Rucha Karnik
Nov 20, 2025 2013 Views
Abstract
Camelina sativa, a Brassicaceae family crop, is used for fodder, human food, and biofuels. Its relatively high resistance to abiotic and biotic stresses, as well as being a climate-resilient oilseed crop, has contributed to its popularity. Camelina's seed yield and oil contents have been improved using various technologies like RNAi and CRISPR/Cas9 genome editing. A stable transformation system for protein localization and other cell autonomous investigations, on the other hand, is tedious and time consuming. This study describes a transient gene expression protocol for Camelina sativa cultivar DH55 leaves using Agrobacterium strain C58C1. The method is suitable for subcellular protein localization and colocalization studies and can be used with both constitutive and chemically induced genes. We report the subcellular localization of the N-terminal ER membrane signal anchor region (1–32 aa) of the At3G28580 gene-encoded protein from Arabidopsis in intact leaves and the expression and localization of other known organelle markers. This method offers a fast and convenient way to study proteins in the commercially important Camelina crop system.
Key features
• This method is based on the approach of Zhang et al. [1] and has been optimized for bioenergy crop Camelina species.
• A constitutive and inducible transient gene expression in the hexaploid species Camelina sativa cultivar DH55.
• Requires only 16–18 days to complete with high efficacy.
Graphical overview

Agrobacterium-mediated transient gene expression optimized for Camelina sativa
Background
Camelina sativa, an important Brassicaceae family crop and crop model, is used to make fodder, human food, industrial chemical raw materials, biofuels, and compost to enhance soil properties [2]. It is planted worldwide, particularly in North America, Europe, and Asia, and is also known as large-seeded False Flax seed due to its high level of polyunsaturated fatty acids [3]. Camelina has lately become noted for its potential to be a high-value, climate-resilient oilseed crop owing to its resistance to a variety of abiotic and biotic stresses [4].
Using different technologies like classical breeding, transgenics, and CRISPR/Cas9 genome editing, various genetic improvements in Camelina have been made to increase its seed yield and oil contents and reduce its anti-nutritional glucosinolate content. Stable transformation systems that employ the floral-dip method and in vitro leaf explants are available in Camelina sativa [5]. Nevertheless, the transformation efficiency is very low (i.e., 0.8%–1%) and the method is laborious [6,7]. While an Agrobacteria-mediated infiltration system for rapid and transient gene expression for studying protein localization, protein–protein interaction, etc., has been well established in Nicotiana benthamiana, this technique has had limited applications for other crop species [8]. Hence, with regards to time and labor efficiency, the development of a transient expression system will provide an alternative and convenient way to study genes in Camelina.
Previously, Zhang et al. [1] developed a transient gene expression protocol using different strains of Agrobacteria in the model plant Arabidopsis thaliana, along with seven other plant species: Brassica oleracea, Capsella rubella, Thellungiella salsuginea, T. halophila, Solanum tuberosum, Capsicum annuum, and N. benthamiana. Herein, we modified this protocol for Camelina sativa cultivar DH55. The major modification is the age of the plants, which is younger than that described by Zhang et al. [1], enabling more rapid acquisition of data. In addition, this optimized protocol functions with constitutive and inducible promoters and is suitable for both subcellular localization and colocalization of proteins in Camelina. Using the leaves of 10–12-days-old plants infected with Agrobacterium strain C58C1, we show the subcellular localization of the organelle markers to chloroplasts, peroxisomes, Golgi bodies, and ER [9]. Leaves of 14-day-old plants showed a significantly decreased transformation efficiency, highlighting the benefit of using younger plants for these experiments. We also report the intracellular localization of the N-terminal ER signal (1–32 aa) region of the protein encoded by the At3G28580 gene in intact leaves. This study demonstrates an efficient transient gene expression protocol for Camelina.
Materials and reagents
Biological materials
Camelina sativa cultivar DH55 seeds
Agrobacterium strain C58C1 containing constitutive or chemically inducible plasmid expression constructs.
Plasmids used in this study harbor constructs encoding chloroplast-GFP (CD3-995), peroxisomes-mCherry (CD3-983), Golgi body-mCherry (CD3-967), ER-mCherry (CD3-959) as described in Nelson et al. [9], and Dex-inducible pBAV150 [10] plasmid with DNA encoding the N-terminal (1–32 aa) region of a protein coded by the At3G28580 gene
Reagents
Plastic domes (Hummert International, catalog number: 11-33480) and trays (Hummert International, catalog
number: 11-33010)
Pots 8 cells; width × length × depth: 12.34 × 12.34 × 5.77 cm (T.O. PLASTICS, catalog number: 715352C)
Soil (Berger 60, catalog number: BM2; BM6, mixed in 1:1 ratio)
Yeast extract (Fisher Scientific, catalog number: BP1422-500)
BactoTM peptone (Thermo Fisher Scientific, catalog number: 211677)
Sucrose (Fisher Scientific, catalog number: S5-500)
MgSO4·7H2O (Fisher Scientific, catalog number: 10034-99-8)
NH4Cl (Sigma, catalog number: 12125-02-9)
KCl (Fisher Scientific, catalog number: P217-500)
CaCl2 (Sigma, catalog number: C2661)
FeSO4·7H2O (Sigma, catalog number: F-7002)
NaH2PO4·H2O (JT Baker, catalog number: JTB-3818-05)
Na2HPO4 (Fisher Scientific, catalog number: 7558-79-4)
Glucose (Sigma, catalog number: G7021)
MES (Sigma, catalog number: M8250)
MgCl2·6H2O (Fisher Scientific, catalog number: BP214-500)
Murashige and Skoog medium (Sigma, catalog number: M5519)
Bleach (Clorox Concentrated Germicidal Bleach)
Ethanol (Decon Labs Inc., catalog number: 2701)
Agarose (GOLDBIO, catalog number: 9012-36-6)
Silwet L-77 (Vac-In-Stuff, catalog number: VIS-30)
Tween® 20 (Hoefer, catalog number: 9005-64-5, GR128-500)
Antibiotics: kanamycin sulfate (Kan) and rifampicin (Rif) (Fisher Scientific, catalog number: BP906-5, 13292-46-1, respectively)
NalgeneTM Rapid-FlowTM sterile disposable filter units (Thermo Fisher Scientific, catalog number: 568-0020)
Dexamethasone (Sigma, catalog number: D1756)
Perfluorodecalin (Strem Chemicals, catalog number: 09-5960)
Dimethyl sulfoxide (DMSO) (Sigma, catalog number: D8418)
Agar (Fisher Scientific, catalog number: BP1423-500)
Acetosyringone (Sigma, catalog number: D134406)
Solutions
Washing solution (see Recipes)
Infiltration solution (see Recipes)
Agrobacterium growth media (see Recipes)
30 μM Dexamethasone (Dex) solution with 0.04% Tween-20 (see Recipes)
70% ethanol (see Recipes)
0.1% agar media (see Recipes)
Phosphate buffer (50 mM, 25×, pH 5.5) (see Recipes)
Recipes
Recipes 1, 2, and 3 are adapted from Zhang et al. [1].
Washing solution (1 L)
Reagent Final Concentration Volume MgCl2 (1 M) 10 mM 10 mL Acetosyringone (0.1 M) 100 μM 1 mL ddH2O n/a 989 mL Total n/a 1 L Infiltration solution (1 L)
Note: Water should be added in increments of 100 mL. Allow the solution to mix completely before adding any more water. Murashige and Skoog medium and sucrose will take a substantial volume when fully dissolved. Adjust pH to 6.0 using 1 M KOH; use freshly prepared.
Reagent Final Concentration Quantity or Volume Murashige and Skoog medium 1/4 × (w/v) 1.1 g Sucrose 1% (w/v) 10 g Acetosyringone (0.1 M) 100 µM 1 mL Silwet L-77 0.01% (v/v) 100 μL ddH2O n/a see note Total n/a 1 L Agrobacterium growth media (1 L)
Note: Adjust the pH to 5.5 using 1 M KOH and autoclave the media. After autoclaving, add the filtered induce buffer components. Acetosyringone is prepared in DMSO.
Reagent Final Concentration Quantity or Volume Yeast extract 0.1% (w/v) 1.0 g BactoTM peptone 0.5% (w/v) 5.0 g Sucrose 0.5% (w/v) 5.0 g MgSO4·7H2O 2.03 mM 0.5 g Agarose 1% (w/v) 10.0 g NH4Cl 18.70 mM 1.0 g KCl 2.01 mM 0.15 g CaCl2 90.10 μM 0.01 g FeSO4·7H2O 8.99 μM 0.0025 g ddH2O n/a 889 mL (see note) Induce buffer composition: Phosphate buffer (50 mM, 25×, pH 5.5) 1× 40 mL Glucose (20%, 20×) 1× 50 mL MES (1 M, 50×, pH 5.5) 1× 20 mL Acetosyringone (200 mM, 1,000×) 1× 1 mL Total n/a 1 L 30 μM Dexamethasone (Dex) solution with 0.04% Tween-20 (100 mL)
Note: Dex is prepared in DMSO.
Reagent Final Concentration Volume Dex (30 mM) 30 μM 100 μL (see note) Tween-20 0.04% 40 μL ddH2O n/a 99.86 mL Total n/a 100 mL 70% ethanol (100 mL)
Reagent Final Concentration Volume Ethanol (95%) 70% 73.7 mL ddH2O n/a 26.3 mL Total n/a 100 mL 0.1% agar media (100 mL)
Note: Autoclave the media and stir before it cools.
Reagent Final Concentration Volume Agar 0.1% 100 mg ddH2O n/a 100 mL Total n/a 100 mL (see note) Phosphate buffer (50 mM, 25×, pH 5.5; 100 mL)
Note: Adjust the pH to 5.5 using 1 M KOH.
Reagent Final Concentration Volume NaHPO.HO 48.02 mM 662.7 mg NaHPO 1.98 mM 28.05 mg ddH2O n/a 100 mL Total n/a 100 mL (see note)
Laboratory supplies
Laboratory glassware
Pipette tips: 10 μL, 200 μL, and 1,000 μL (USA Scientific, catalog number: 1111-3700, 1111-1700, 1112-1720)
Black marker (Sharpie Permanent Markers, catalog number: 33861PP)
Kimwipes (Fisher Scientific, catalog number: 06-666A)
15 mL and 50 mL centrifuge tubes (Fisher Scientific, catalog numbers: 14-959-53A, 14-432-22)
9 cm round Petri dishes (Fisher Scientific, catalog number: FB0875712)
1.5 mL microcentrifuge tubes (Fisher Scientific, catalog number: 01-549-746)
1 mL tuberculin syringes without needle (BD Biosciences, catalog number: 309659)
Damp paper towel (Georgia-Pacific, catalog number: P200)
Sterile double-distilled water
Micropipettes 20 μL, 200 μL, and 1 mL (Gilson, catalog number: F144056M, F144058M, F144059M, respectively)
1 L beaker (Pyrex, catalog number: 1000)
Equipment
Cork borer 4 mm diameter
Autoclave (Primus Sterilizer Co. Inc. 1317, catalog number: 09415.1256)
Fisher vortex Genie 2 (Fisher Scientific, catalog number: 12-812)
Plant growth chamber or walk-in growth room with humidity maintained at ≥ 50%. Light intensity should be 135–145 μmols-1·m-2 at soil level
Freezer (-80 °C) (Panasonic VIP Plus, model: MDF-V76VC-PA)
Balance (Mettler Toledo, model: PB1501)
Spectrophotometer (Bio-Mini, model: SHIMADZU)
Laminar flow hood (SterilGARD, model: 3 Advance)
Incubator at 28 °C (VWR, model: 3020)
Analytical balance for weighing chemicals
pH meter (SevenCompact, serial number: B408309625)
Confocal microscope (Zeiss, model: LSM 800)
Software and datasets
Fiji (ImageJ, version 2.15.0, 10/12/2023)
Photoshop (Adobe PS, 22.4.3, 07/19/2021)
Prism v10.1 (GraphPad, 07/01/2023)
BioRender.com online tool
Procedure
Plant growth and maintenance
Soak non-sterilized, dry Camelina seeds (~100–150) in 4–6 mL of 0.1% sterile agar in 15 mL falcon tubes and keep at 4 °C in the dark for 3–7 days. The Camelina seeds utilized in this study were multiplied and bulked inside the greenhouse chamber.
Sow approximately 18–20 seeds in individual soil mix–containing pots (12.34 × 12.34 × 5.77 cm) with the help of a Pasteur pipette and cover with a transparent plastic lid to maintain moisture. Keep pots in a plant growth chamber or growth room chamber under 12 h light and 12 h dark conditions at 22 ± 2 °C day/night temperature regime and light intensity of ~135–145 μmols-1·m-2. We use a mix of 50/50 sodium and metal halide light (but other lights might be suitable) at 50%–70% relative humidity.
After 4–5 days, thin seedlings to nine plants per pot and remove the lid. Select the seedlings that look visibly similar and discard the rest (Figure S1).
Allow seedlings to establish for the next 5–7 days until the first true leaves are completely opened (Figure 1).

Figure 1. Representative image depicting Agrobacterium-infiltrated plants of Camelina sativa DH55 cultivar. Ten-to-twelve-day-old plants with opened true leaves were infiltrated with Agrobacterium strain C58C1 carrying desired constructs. White arrows indicate representative leaves imaged immediately after infiltration. The abaxial part of the leaves will have a wet appearance until the infused liquid containing Agrobacteria evaporates. Scale bar is in centimeters.
Agrobacterium strain suspension preparation for infiltration
Agrobacterium suspension preparation
Streak an Agrobacterium strain C58C1 stock with the desired constructs on a sterile plate of Agrobacterium growth media containing appropriate antibiotics and 200 μM acetosyringone inside a laminar airflow cabinet.
Keep plates with bacteria at 28 °C for 24–36 h. In this work, marker plasmids of plastid-GFP (CD3-995), peroxisomes-mCherry (CD3-983), Golgi body-mCherry (CD3-967), ER-mCherry (CD3-959), and the N-terminal ER signal (1–32 aa) region of the At3G28580-encoded protein were used, and plates contained Rif (34 mg/mL) + Kan (50 mg/mL).
Scrape a thin layer of Agrobacterial cells from each plate using a 200 μL pipette tip and resuspend bacteria in 1 mL of washing solution (see Recipes) in a 1.5 mL microcentrifuge tube by vortexing.
Dilute an aliquot of 100 μL of resuspended Agrobacteria 10× in order to measure the OD600. A value equal to or greater than 0.6 is desired in the final infiltration solution. Multiply this OD value by 10 to determine the OD600 of the original undiluted resuspended Agrobacteria. This is done to obtain enough inoculum, sufficient to infiltrate all the true leaves per pot for each construct. After that, adjust the original suspension of Agrobacteria to an OD600 of 0.6 using the infiltration solution (see Recipes). For colocalization studies, mix strains in a 1:1 ratio using an OD600 of 0.6 for each strain and immediately infiltrate the leaves.
Agrobacterium infiltration in Camelina
Infiltrate the Agrobacterium in the infiltration solution into the abaxial surface of Camelina leaves (2 true leaves per plant) with the help of a 1 mL needleless plastic syringe. Use four or more plants per construct for infiltrations. Keep plants in the light for 1 h to allow the water-soaked leaves to dry before placing plants in the dark for 24 h at room temperature using plastic trays.
Return the Agrobacterium-infiltrated plants to a growth chamber or growth room for another 3–5 days before observing leaves using confocal microscopy. If using an inducible promoter, spray the inducer over leaves 21 h before examination, as described in Banday et al. [11], using a confocal microscope (Figure 2A and 2B). In the example in Figure 2A, 30 μM Dex with 0.04% Tween-20 was applied.
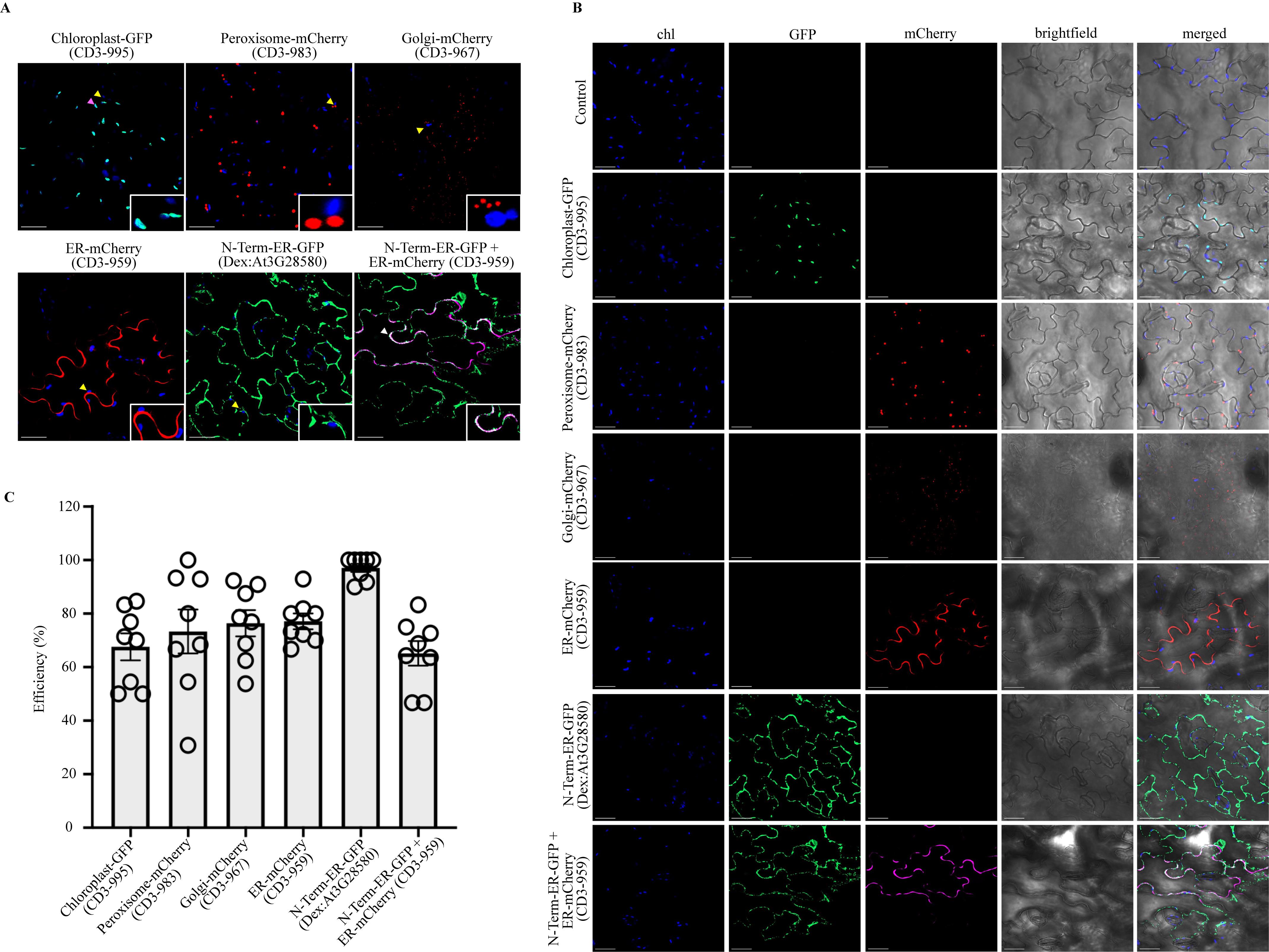
Figure 2. Agrobacterium-mediated transient subcellular localization in Camelina sativa DH55 cultivar. A. Upper panel: Confocal micrographs showing localization of Chloroplast-GFP (CD3-995), Peroxisome-mCherry (CD3-983), and Golgi-mCherry (CD3-967). Lower panel: Confocal micrographs of ER-mCherry (CD3-959), N-Term-ER-GFP (Dex: At3G28580; 1-32 aa region), and colocalization of N-Term-ER-GFP (Dex: At3G28580) with ER-mCherry (CD3-959; shown in magenta color). For localization and colocalization studies, images were taken 3 and 5 days after transfer to the light conditions, respectively. Chloroplast autofluorescence is shown in blue. Yellow arrowheads indicate chloroplast autofluorescence (shown in insets), magenta arrowheads indicate chloroplast-GFP overlap with chloroplast autofluorescence, and white arrows indicate colocalization of N-Term-ER-GFP (Dex: At3G28580) and ER-mCherry (CD3-959). Scale bars, 100 μm. B. Details of the separate channels of individual confocal micrographs: chloroplast autofluorescence (Blue), GFP signal (Green), and mCherry signal (Red). For colocalization, the mCherry signal is shown in magenta color. Scale bars, 100 μm. C. Efficiency of each transiently expressed construct. Data represent the average ± SE (n = 8) in percentage (%) obtained from experiments performed on two different days. The four images from each independent experiment were used for the calculations. See Table S1 for details.
Data analysis
The confocal microscope method was adapted from Banday et al. [11]. Zeiss LSM 800 was used to visualize GFP and mCherry fluorescence (Argon laser/excitation: 488 nm; emission collection: 505–530 nm for GFP; Argon laser/excitation: 594 nm; emission collection: 610–630 nm for mCherry) and chlorophyll autofluorescence (He-Ne laser/excitation: 633nm; emission collection: 650–750 nm or 645–700 nm). Images of abaxial leaf surface were captured using EC Plan-Neofluar 40/1.3 Oil DIC M27 objectives, pinhole at 1 AU for each channel, and photomultipliers master gain between 450 and 700. The images were optical sections captured at 1,024 × 1,024 pixels scanning resolution in maximum speed mode. Fluorescence of GFP, mCherry, and chlorophyll was acquired in sequential acquisition mode. Plant leaves were mounted in perfluorodecalin for optical enhancement [12]. ImageJ (Fiji) and Adobe PS were used to process the images. Each C. sativa imaging experiment included four independent samples per construct, and the experiments were done twice. Four images from different plant leaves from each experiment were used to calculate the efficiency of the transformation of cells per image. Using this protocol, all infiltrated leaves showed expression of the respective constructs. However, the efficiency of cells transformed/image was lower in the leaves of older plants (Table S1).
For calculation of efficiency analysis, the formula was adapted from Zhang et al. [1]. The graph was plotted using Prism software (Figure 2C and Table S1).
Efficiency (%) = Number of transiently transformed cells *100/Total number of cells
Validation of protocol
This protocol was adapted from Zhang et al. [1] and optimized for the Camelina crop. The protocol was validated using five different constitutive or chemically inducible plasmid expression constructs.
Each Camelina imaging experiment included four independent samples per construct, and the experiments were done twice. Four images from different plant leaves from each experiment were used to calculate the efficiency of the transformation of cells per image.
The student’s t-test shows that the efficiency of cells transformed/image was significantly lower in leaves of older plants (Table S1).
General notes and troubleshooting
Plants' age is very important. Leaves of older Camelina plants require needle punctures for successful infiltration and the efficiency of transformation declines (Table S1).
Pots must be well watered during the experiment to maintain humidity.
Keep only nine plants in one pot; plant density affects the growth of plants. A higher number of plants results in slower growth. Conversely, a lower number of plants per pot hastens the growth of plants.
Do not keep bacterial cultures for more than 36 h.
For better infiltration, the infiltration solution should be introduced into the abaxial surfaces of leaves.
Take confocal images of the abaxial surface to obtain a greater number of cells in the same focal plane.
Use perfluorodecalin to prevent the leaves from drying and for better imaging.
Clean the workbench before making an Agrobacterial suspension with the help of 70% ethanol. Later on, after completion of infiltration in plants, the remaining bacterial suspension must be treated with bleach for 24 h before being discarded into the sink.
Acknowledgments
This work was supported by a Dropkin Postdoctoral Fellowship awarded to PK and an Undergraduate fellowship from Arnold and Mabel Beckman Foundation to JLR. This protocol was adapted from Zhang et al. [1] and modified for the Camelina crop. We thank Dr. Isobel Parkin (Agriculture and Agri-Food Canada) for providing Camelina seeds. We thank Dr. Joanna Jelenska for valuable suggestions.
Competing interests
The authors declare that they have no conflicts of interest.
References
- Zhang, Y., Chen, M., Siemiatkowska, B., Toleco, M. R., Jing, Y., Strotmann, V., Zhang, J., Stahl, Y. and Fernie, A. R. (2020). A Highly Efficient Agrobacterium-Mediated Method for Transient Gene Expression and Functional Studies in Multiple Plant Species. Plant Commun. 1(5): 100028. https://doi.org/10.1016/j.xplc.2020.100028
- Sydor, M., Kurasiak-Popowska, D., Stuper-Szablewska, K. and Rogoziński, T. (2022). Camelina sativa. Status quo and future perspectives. Ind. Crops Prod. 187: 115531. https://doi.org/10.1016/j.indcrop.2022.115531
- Waraich, E. A., Ahmed, Z. I., Ahmad, R., Ashraf, M. Y., Saifullah, Naeem, M. and Rengel, Z. (2013). Camelina sativa, a climate proof crop, has high nutritive value and multiple uses: A review. Aust. J. Crop Sci. 7(10):1551–1559. https://www.cropj.com/waraich_7_10_2013_1551_1559.pdf
- Neupane, D., Lohaus, R. H., Solomon, J. K. Q. and Cushman, J. C. (2022). Realizing the Potential of Camelina sativa as a Bioenergy Crop for a Changing Global Climate. Plants 11(6): 772. https://doi.org/10.3390/plants11060772
- Ghidoli, M., Ponzoni, E., Araniti, F., Miglio, D. and Pilu, R. (2023). Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Plants 12(3): 570. https://doi.org/10.3390/plants12030570
- Lu, C. and Kang, J. (2007). Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 27(2): 273–278. https://doi.org/10.1007/s00299-007-0454-0
- Liu, X., Brost, J., Hutcheon, C., Guilfoil, R., Wilson, A. K., Leung, S., Shewmaker, C. K., Rooke, S., Nguyen, T., Kiser, J., et al. (2012). Transformation of the oilseed crop Camelina sativa by Agrobacterium-mediated floral dip and simple large-scale screening of transformants. In Vitro Cell. Dev. Biol. Plant48(5): 462–468. https://doi.org/10.1007/s11627-012-9459-7
- Krenek, P., Samajova, O., Luptovciak, I., Doskocilova, A., Komis, G. and Samaj, J. (2015). Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 33(6): 1024–1042. https://doi.org/10.1016/j.biotechadv.2015.03.012
- Nelson, B. K., Cai, X. and Nebenführ, A. (2007). A multicolored set of in vivo organelle markers for co‐localization studies in Arabidopsis and other plants. Plant J. 51(6): 1126–1136. https://doi.org/10.1111/j.1365-313x.2007.03212.x
- Vinatzer, B. A., Teitzel, G. M., Lee, M., Jelenska, J., Hotton, S., Fairfax, K., Jenrette, J. and Greenberg, J. T. (2006). The type III effector repertoire of Pseudomonas syringae pv. syringae B728a and its role in survival and disease on host and non‐host plants. Mol. Microbiol. 62(1): 26–44. https://doi.org/10.1111/j.1365-2958.2006.05350.x
- Banday, Z. Z., Cecchini, N. M., Speed, D. J., Scott, A. T., Parent, C., Hu, C. T., Filzen, R. C., Agbo, E. and Greenberg, J. T. (2022). Friend or foe: Hybrid proline-rich proteins determine how plants respond to beneficial and pathogenic microbes. Plant Physiol. 190(1): 860–881. https://doi.org/10.1093/plphys/kiac263
- Littlejohn, G. R., Gouveia, J. D., Edner, C., Smirnoff, N. and Love, J. (2010). Perfluorodecalin enhances in vivo confocal microscopy resolution of Arabidopsis thaliana mesophyll. New Phytol. 186(4): 1018–1025. https://doi.org/10.1111/j.1469-8137.2010.03244.x
Supplementary information
The following supporting information can be downloaded here:
- Figure S1: Visible appearance of 5-days-old seedling.
- Table S1: Details of efficiency analysis in percentage.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Kumar, P., Banday, Z. Z., Riley, J. L. and Greenberg, J. T. (2024). Agrobacterium-Mediated Transient Gene Expression Optimized for the Bioenergy Crop Camelina sativa. Bio-protocol 14(7): e4964. DOI: 10.21769/BioProtoc.4964.
Category
Plant Science > Plant molecular biology > Protein
Molecular Biology > Protein > Expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








