- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Bacterial Pathogen-mediated Suppression of Host Trafficking to Lysosomes: Fluorescence Microscopy-based DQ-Red BSA Analysis
Published: Vol 14, Iss 5, Mar 5, 2024 DOI: 10.21769/BioProtoc.4951 Views: 2876

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
HS–GC–MS Method for the Diagnosis of IBD Dynamics in a Model of DSS-Induced Colitis
Olga Yu. Shagaleeva [...] Natalya B. Zakharzhevskaya
Mar 20, 2025 3058 Views
In Vitro Co-culture of Bacterial and Mammalian Cells to Investigate Effects of Potential Probiotics on Intestinal Barrier Function
Ajitpal Purba [...] Dulantha Ulluwishewa
Jun 20, 2025 2411 Views
In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1606 Views
Abstract
Intracellular bacterial pathogens have evolved to be adept at manipulating host cellular function for the benefit of the pathogen, often by means of secreted virulence factors that target host pathways for modulation. The lysosomal pathway is an essential cellular response pathway to intracellular pathogens and, as such, represents a common target for bacterial-mediated evasion. Here, we describe a method to quantitatively assess bacterial pathogen–mediated suppression of host cell trafficking to lysosomes, using Salmonella enterica serovar Typhimurium infection of epithelial cells as a model. This live-cell imaging assay involves the use of a BODIPY TR-X conjugate of BSA (DQ-Red BSA) that traffics to and fluoresces in functional lysosomes. This method can be adapted to study infection with a broad array of pathogens in diverse host cell types. It is capable of being applied to identify secreted virulence factors responsible for a phenotype of interest as well as domains within the bacterial protein that are important for mediating the phenotype. Collectively, these tools can provide invaluable insight into the mechanisms of pathogenesis of a diverse array of pathogenic bacteria, with the potential to uncover virulence factors that may be suitable targets for therapeutic intervention.
Key features
• Infection-based analysis of bacterial-mediated suppression of host trafficking to lysosomes, using Salmonella enterica serovar Typhimurium infection of human epithelial cells as a model.
• Live microscopy–based analysis allows for the visualization of individually infected host cells and is amenable to phenotype quantification.
• Assay can be adapted to a broad array of pathogens and diverse host cell types.
• Assay can identify virulence factors mediating a phenotype and protein domains that mediate a phenotype.
Background
Bacterial pathogens employ diverse strategies to manipulate host cellular function to establish infection [1]. Among the many approaches, modulation of host cells can occur at the level of immune signalling and immune cell function [1,2], cellular immune responses such as lysosomal and autophagy function [3–6], organelle function [7,8], or cytoskeletal dynamics [1,9,10]. The lysosomal pathway represents a fundamental signalling hub and cellular immune response pathway in eukaryotic cells [11,12]. Lysosomes are membrane-bound organelles responsible for the degradation of proteins, lipids, nucleic acids, and carbohydrates [11,12]. These vesicles exhibit a characteristically low pH that promotes the activation of over 60 different hydrolases, ultimately contributing to cellular homeostasis and clearance of intracellular pathogens [11,12]. Defects in lysosome function can lead to one of more than 50 different disorders, collectively defined as lysosome storage diseases, with implications in neurodegeneration and aging [12–15].
Given that lysosomes function as a primary organelle responsible for the clearance of intracellular bacterial pathogens, they represent an important target for pathogen manipulation. Intracellular bacteria have evolved diverse mechanisms to manipulate host function to evade lysosomal degradation [2,10]. Lysosomal evasion can occur through either direct or indirect means. An example of an indirect mechanism of evasion is the manipulation of host trafficking to acquire membrane from other organelles. For example, the pathogen Legionella pneumophila uses a cooperative approach involving a series of secreted virulence proteins to acquire membrane from the endoplasmic reticulum [16]. This acquisition of membrane disguises the pathogen-containing vacuole as the host compartment, ultimately evading detection by the lysosomal pathway [17]. Many pathogens have evolved more direct means of targeting lysosomal function [10,18]. For example, the foodborne pathogen Vibrio parahaemolyticus uses the secreted virulence protein VepA to target the lysosomal V-ATPase and induce lysosome rupture [5].
Effective characterization of pathogen-mediated suppression of host lysosome function involves identifying the mechanism of host evasion and ultimately establishing the mechanism of action of the virulence factor responsible. This requires the development of robust cell biological assays for the study of these phenotypes. Among the most commonly proposed direct mechanisms of evasion is the suppression of trafficking of host lysosomes [10, 18]. Here, we describe a host–pathogen infection assay that can be used to investigate this phenotype [19]. This fluorescence-based microscopy assay involves the use of a BODIPY TR-X conjugate of BSA (DQ-Red BSA). This highly labelled BSA derivative exists as a self-quenched, non-fluorescent form (Figure 1A) and, upon treatment of host cells, is internalised by endocytosis. Upon trafficking to functional lysosomes, the acquisition of lysosomal proteases results in BSA cleavage, resulting in unquenching and bright-red fluorescence [20]. DQ-Red BSA is a substrate for a broad array of lysosome hydrolases and, therefore, is capable of detecting global lysosome function [21].
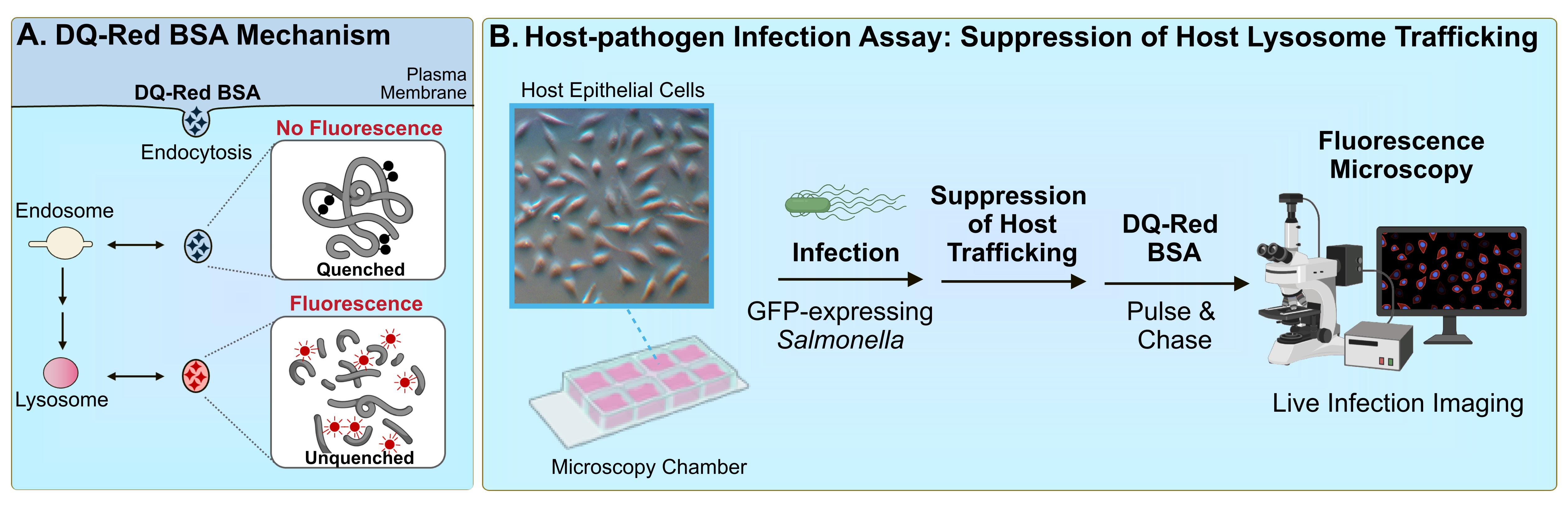
Figure 1. DQ-Red BSA assay for bacterial pathogen–mediated suppression of host trafficking. A. DQ-Red BSA dye mechanism. The fluorophores within the DQ-Red BSA dye exist in a quenched form and therefore do not generate intrinsic fluorescence. When host cells are treated with the dye, the compound is internalised by general endocytic mechanisms, and is trafficked to intermediates of the endocytic pathway. When dye-containing vesicles are trafficked to lysosomes, active lysosomal proteases cleave the BSA backbone, resulting in unquenching of the fluorophores and the generation of bright-red fluorescence. B. Host–pathogen infection assay to assess suppression of host lysosomal trafficking. Host cells are seeded in a microscopy chamber slide and infected with the bacterial pathogen of interest expressing green fluorescent protein (GFP). At the timepoint of interest, cells are subjected to a pulse-chase with DQ-Red BSA. Infected host cells are imaged by fluorescence microscopy, and suppression of host lysosome trafficking is quantified by imaging software.
The following summarises a method to quantitatively assess bacterial pathogen–mediated suppression of host cell trafficking to lysosomes, using Salmonella enterica serovar Typhimurium infection of epithelial cells as a model (Figure 1B). S. enterica is a Gram-negative pathogen that is among the leading causes of foodborne gastroenteritis worldwide [22]. As an intracellular vacuolar pathogen, S. Typhimurium evades targeting for death by lysosomes to establish a replicative niche within host cells [10]. S. Typhimurium was shown to suppress host trafficking to lysosomes (Figure 2) using this fluorescence microscopy–based imaging assay [19], which allows the visualization of trafficking to lysosomes in individual live-infected host cells alongside neighbouring non-infected host cell counterparts. This method was also used to identify the secreted virulence factor responsible for the phenotype, as well as domains within the bacterial protein that contribute to the phenotype [19]. The methods outlined highlight important considerations for adapting this method for the study of other host cell types and other bacterial pathogens, as well as for modifying the assay setup to tailor to the experimental question of interest.
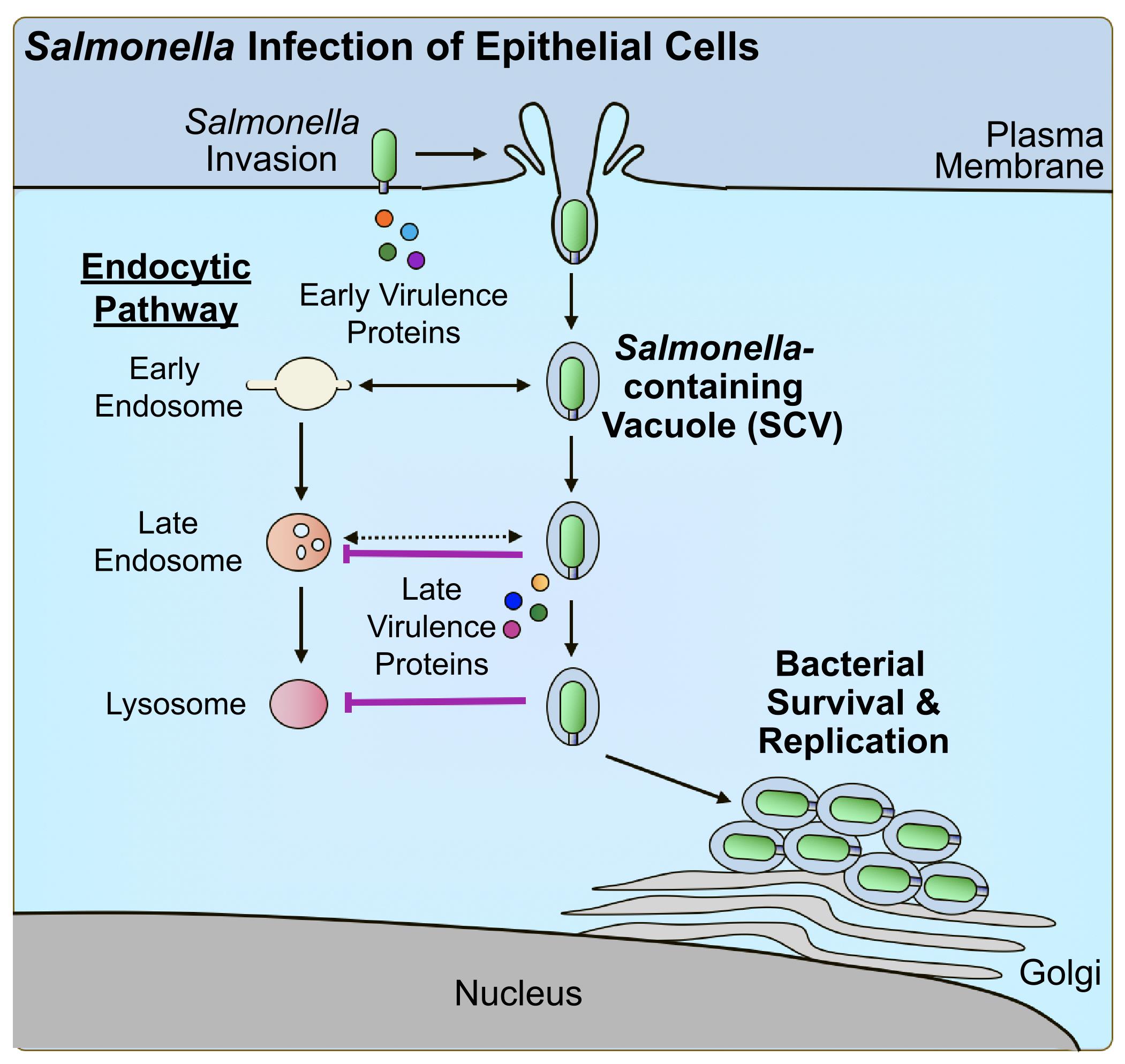
Figure 2. Salmonella virulence factor–mediated suppression of host trafficking to lysosomes. Salmonella Typhimurium manipulates the host cytoskeleton to direct its uptake into host epithelial cells using early-secreted virulence proteins. Upon uptake, Salmonella-containing vacuoles (SCVs) begin to undergo interactions with early intermediates of the endocytic pathway. As a mechanism of evasion, Salmonella produces late-secreted virulence proteins to suppress trafficking to lysosomes and promote intracellular survival and replication.
Materials and reagents
Biological materials
HeLa (ATCC, catalog number: CCL-2)
Salmonella enterica serovar Typhimurium SL1344 harbouring pFPV25.1 [23]
Note: The pathogen of interest must be expressing a fluorescent protein that is not associated with red wavelengths (e.g., GFP) for detection purposes. This may be in the form of a shuttle plasmid such as pFPV25.1 (expressing GFP) or a strain harbouring a chromosomal insertion. Should this not be previously established for the pathogen of interest, broad host spectrum shuttle plasmids exist that might be worthy of investigation [24].
Reagents
Dulbecco modified Eagle medium (DMEM), 4.5 g/L glucose, with L-glutamine, sodium pyruvate, and phenol red) (Wisent, catalog number: 319-005-CL)
Fetal bovine serum (FBS) (Wisent, catalog number: 080-450)
LB Miller broth (BioShop, catalog number: LBL407)
Agar, bacteriological grade (BioShop, catalog number: AGR001)
Streptomycin sulfate (BioShop, catalog number: STP101)
Carbenicillin sodium salt (BioShop, catalog number: CAR544)
Phosphate-buffered saline with calcium and magnesium (PBS+/+) (Wisent, catalog number: 311-011-CL)
Gentamicin sulfate (BioShop, catalog number: GTA401)
DQ-Red BSA (Thermo Fisher, catalog number: D12051)
Phosphate-buffered saline without calcium and magnesium (PBS-/-) (Wisent, catalog number: 311-425-CL)
Roswell Park Memorial Institute 1640 (RPMI), L-glutamine & HEPES, no sodium bicarbonate (Wisent, catalog number: 350-025-CL)
Solutions
Streptomycin stock solution (see Recipes)
Carbenicillin stock solution (see Recipes)
LB-agar solution (for LB-agar plates) (see Recipes)
DQ-Red BSA solution (see Recipes)
Recipes
Streptomycin stock solution (1 mL)
Note: Dissolve powder in water and vortex well until powder dissolves. In a sterile area such as a Bunsen burner or Class II biosafety cabinet, filter sterilise the stock into a sterile tube.
Reagent Final concentration Quantity or Volume Streptomycin sulfate 50 mg/mL 50 mg Double-distilled water (ddH2O) n/a to 1 mL Carbenicillin stock solution (1 mL)
Note: Dissolve powder in water and vortex well until powder dissolves. In a sterile area such as a Bunsen burner or Class II biosafety cabinet, filter sterilise stock into a sterile tube.
Reagent Final concentration Quantity or Volume Carbenicillin sodium salt 100 mg/mL 100 mg Double-distilled water (ddH2O) n/a to 1 mL LB-agar solution (500 mL)
Note: Dissolve powder in water and mix well using a stir bar until powder dissolves as much as possible. Autoclave using standard settings for liquid laboratory media. Where supplementation with antibiotics is applicable, when the autoclaved media is cooled such that it is warm to the touch, add the appropriate concentration of antibiotic stock in a sterile area. After stirring on a stir plate, use a sterile area to pour a layer of agar-supplemented media in each Petri dish. Allow to solidify at room temperature and store solidified plates at 4 °C.
Reagent Final concentration Quantity or Volume LB Miller broth (powder) 25 g/L 12.5 g Agar 15 g/L 7.5 g Double-distilled water (ddH2O) n/a to 500 mL DQ-Red BSA stock solution (1 mL)
Note: Dissolve powder in PBS-/- and mix well by vortexing. If needed, sonicate to assist with solubilizing the reagent. Once solubilised, the reagent can be stored at 4 °C for several weeks. Note that DQ-Red BSA is light-sensitive. Store in a sample tube that is either covered in foil or opaque and, wherever possible, handle in the dark.
Reagent Final concentration Quantity or Volume DQ-Red BSA 2 mg/mL 1 mg PBS-/- n/a to 500 μL
Laboratory supplies
Glass-bottom microscopy chambers (ibidi, catalog number: 80827-90)
Centrifuge tubes, polypropylene, sterile (Corning, catalog number: C352098)
Serological pipettes, sterile (Sarstedt, catalog number: 86.1253.001)
Petri dishes for bacteriology, sterile (Sarstedt, catalog number: 82.1473.001)
Round-bottom culture tubes, sterile (Corning, catalog number: C352059)
Syringe, sterile (BD, catalog number: B309659)
Syringe filter, sterile (Millipore Sigma, catalog number: SLGP033RS)
Magnetic stir bar (Fisherbrand, catalog number: 800371113)
Equipment
CO2 incubator (Nuaire, model: NU-425-400)
Bacterial incubator (New Brunswick, model: Innova 42)
Quorum spinning disk fluorescence microscope (Leica, model: DMIRE2, Hamamatsu, model: CMOS FL-400)
Bunsen burner (EISCO, model: CH0095B) or Class II biosafety cabinet (Nuaire, model: NU-602-600)
Analytical balance (Mettler Toledo, model: ML304T)
Stir plate (Thermo Scientific, model: SP131525)
Autoclave
Vortex (VWR, model: G-560)
Microcentrifuge (Eppendorf, model: 5425)
Software and datasets
Fiji (ImageJ, v1.54f 29, June 2023) (free analysis software)
Procedure
Bacterial pathogen infection
The following method is used for the study of S. Typhimurium infection of host epithelial cells (Figure 1B). The protocol is adaptable for infection with diverse intracellular pathogens and a broad array of cell types. Highlighted within the protocol are important considerations for tailoring this assay to study different host cell types, pathogens, and applications. Information is also provided regarding commonly encountered challenges and suggested troubleshooting strategies.
Seed host cells in glass-bottom microscopy chambers. For comparison studies, such as identifying virulence factor(s) associated with the phenotype or domains within the virulence factor responsible for function (additional information in Table 1), one well is needed per strain. For analysis of S. Typhimurium infection of HeLa cells, seed μ-Slide 8-well glass-bottom chambers with HeLa cells at 6 × 104 cells/well using DMEM supplemented with 10% FBS as a culture medium.
Note: For comparison studies with isogenic knockout strains, the use of a complementation strain is required to rule out the possibility of polar effects. Complementation plasmids for these purposes have been established for diverse intracellular pathogens.
Table 1. Applications of DQ-Red BSA assay for the study of bacterial pathogenesis
Assay purpose Bacterial strains required Additional information and considerations Validation of suppression of host lysosomal trafficking Wildtype • Kinetics of trafficking suppression may vary by strain and host cell line; if the timepoint of interest has not been established, a time-course analysis may be needed
• Seeding: one well per timepoint of interest
Virulence factor identification Wildtype, isogenic knockout(s), complementation strain(s) • If needed, assay can be scaled down to a smaller format to be amenable for larger scale screen of knockouts
• Once a candidate virulence factor is identified, the experiment must be repeated in comparison to a complementation strain
• Complementation: all strains must harbour the same complementation base plasmid, either with or without the virulence factor gene of interest
Virulence factor domain mapping Wildtype, isogenic knockout, complementation strains harbouring wildtype and truncation mutants • All strains must harbour the same complementation base plasmid, either with or without the virulence factor gene of interest
• For virulence factors with limited information, in silico tools such as Phyre2 [26] may provide valuable insight to guide hypotheses
• If the virulence factor has known or predicted catalytic sites or functional residues, point mutations can also be assessed in parallel
Grow host cells in a CO2 incubator until the optimal confluency for infection of the pathogen of interest. For S. Typhimurium infection of epithelial cells, incubate overnight until host cells are at a confluency of 70%–80%.
Concurrently, prepare the pathogen inoculum so that it is ready for infection when the host cells are at the optimal level of confluency for infection. For S. Typhimurium SL1344 harbouring pFPV25.1, this requires setting up an overnight culture on the day of seeding host cells. Inoculate a sterile bacterial culture tube with 2 mL of LB supplemented with 50 μg/mL streptomycin (for selection of the strain) and 100 μg/mL carbenicillin (for selection of pFPV25.1) with several single colonies grown on LB-agar supplemented with 50 μg/mL streptomycin and 100 μg/mL carbenicillin. Incubate for 16 h at 37 °C and 250 rpm.
On the day of infection, prepare the pathogen inoculum as required prior to infection. For S. Typhimurium SL1344 infection of epithelial cells, in a sterile 125 mL flask containing 10 mL of LB, subculture from the overnight culture (1:33) [25] and incubate for 3 h at 37 °C and 250 rpm. Prepare inocula by pelleting 1 mL of the culture at 10,000× g for 2 min, resuspending the pellet in PBS+/+ to 1 mL, and diluting in PBS+/+ to the appropriate multiplicity of infection. For S. Typhimurium SL1344, 1:100 is suggested.
Note: The goal of this experimental setup should be to have host cells infected with the intracellular pathogen such that, at the time of microscopy analysis, primary infected cell(s) are in the same field of view with neighbouring non-infected host cells. The multiplicity of infection and host cell confluency can be adjusted during the optimization process to achieve this.
Aspirate culture medium from host cells and wash with 200 μL of PBS+/+. Apply bacterial inoculum as previously established for the pathogen of interest. A volume of 200 μL is suggested. For S. Typhimurium infection of epithelial cells, incubate infected host cells in CO2 incubator at 37 °C for 10 min.
After the appropriate amount of time previously established for the pathogen, aspirate the bacterial inoculum from the host cells and wash three times with PBS+/+. Apply growth medium appropriate for the host cell type of interest and incubate in CO2 incubator.
Note: Prewarm all culture media used in this experiment to 37 °C. This step is important to allow completion of bacterial uptake such that the pathogen-containing vacuole has completed fission from the host plasma membrane. Proceeding to Step A7 too early could result in bacterial killing and therefore little or no infected host cells.
At the appropriate timepoint for the pathogen, change the culture media to growth medium supplemented with antibiotic for the selection of intracellular bacteria. For S. Typhimurium SL1344 infection of epithelial cells, change the media at 30 min post-infection (p.i.) to DMEM supplemented with 10% FBS and 100 μg/mL gentamicin [19]. At 2 h p.i., change the media to DMEM supplemented with 10% FBS and 10 μg/mL gentamicin for maintenance purposes.
Note: Selection for intracellular bacteria is essential to ensure that the phenotype of interest is not due to factors produced by extracellular bacteria. The antibiotic of interest is chosen based on previous knowledge about antibiotic internalization into host cells. For example, gentamicin is commonly used because of its limited penetration into a wide range of host cells, allowing selection for intracellular bacteria [27]. The sensitivity profile of the pathogen strain of interest, as well as previously established acceptable antibiotic concentration ranges for host cell treatment, will affect the concentration selection. With respect to pathogen sensitivity, this information has been previously established in the literature for many intracellular pathogens. If unknown for the pathogen of interest, a minimum inhibitory concentration assay can be performed.
At the timepoint of infection where the suppression of host trafficking to lysosomes occurs, change media to the culture medium used at the end of Step A7 supplemented with DQ-Red BSA. For S. Typhimurium infection of HeLa cells, perform this step at 8 h p.i. using DMEM supplemented with 10% FBS, 10 μg/mL gentamicin, and 0.25 mg/mL DQ-Red BSA [19].
Note: The infection timepoint associated with the phenotype will most often be determined based on previous literature for the pathogen. Should the pathogen be poorly understood in this context, a series of timepoints can be investigated to determine this. The concentration of DQ-Red BSA for treatment will vary depending on the cell line used and its ability to uptake dye. For example, for phagocytic RAW 264.7 cells, a concentration of 10 μg/mL may be used [19]. This is often previously established in the literature but may be optimised experimentally if needed.
Incubate in CO2 incubator for 1 h. This treatment, with culture medium supplemented with dye, is called the pulse step.
Note: The pulse period can be adjusted if 1 h proves to be ineffective for the cell type of interest.
Wash infected host cells with PBS+/+ and change media to the culture medium used at the end of Step A7. Incubate in CO2 incubator for 4 h. This incubation in the absence of dye to allow for trafficking to lysosomes is called the chase step.
Note: This chase period will allow the DQ-Red BSA to traffic to lysosomes for detection. The time period associated with this step may vary depending on the cell line. Should the pathogen of interest function to block trafficking to lysosomes, DQ-Red BSA trafficking should occur at a reduced level.
Wash infected host cells with PBS+/+ and change medium to HEPES-buffered RPMI 1640 or an equivalent culture medium with HEPES buffer, supplemented with 10% FBS and the antibiotic used for intracellular selection (10 μg/mL gentamicin for S. Typhimurium). The infected sample is ready for live imaging by fluorescence microscopy.
Note: The HEPES-buffered media is used to allow live-cell imaging on a routine microscope. Should the microscope of interest be outfitted with a CO2-infused system, a culture medium with sodium bicarbonate may be used.
Fluorescence microscopy imaging and image quantification
On a fluorescence microscope with a 40× or 63× objective, examine each chamber for primary infected cells in the channel associated with the fluorescent protein expressed. For S. Typhimurium SL1344 expressing pFPV25.1, use the green channel. For each infected host cell identified, examine the field of view for an adjacent non-infected cell. Image each field of view, capturing the channel to image the bacterially expressed fluorescent protein and DQ-Red BSA (Figure 3). Host cells can also be captured in the differential interference contrast (DIC) channel to assist with identifying cell boundaries.
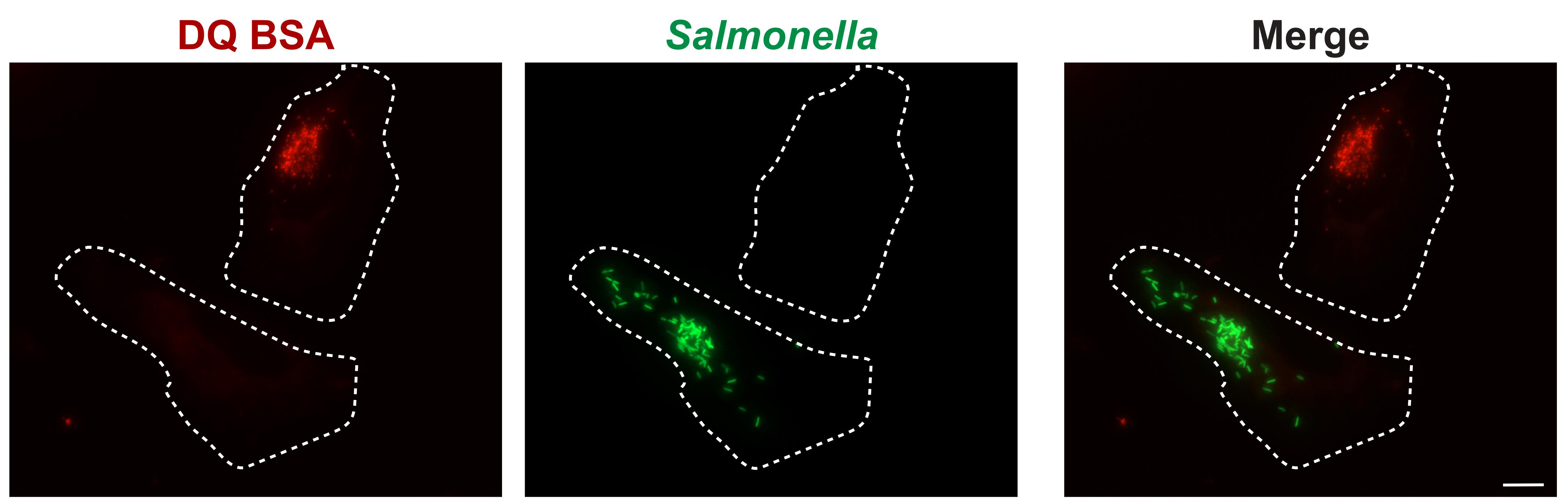
Figure 3. DQ-Red BSA assay: Salmonella-infected epithelial cells. HeLa cells were infected with wildtype Salmonella enterica serovar Typhimurium SL1344 harbouring the GFP-expressing plasmid pFPV25.1 (green) for 8 h. Cells were subjected to a pulse-chase with DQ-Red BSA (red) and subsequently imaged live. The scale bar represents 10 μm.Continue imaging as described in Step B1. For each strain of interest, it is ideal to capture images of at least 100 infected cells with adjacent non-infected counterparts.
Note: If the microscope is outfitted with a platform capable of automated imaging, capture as many fields of view as required to fulfil the criteria described above.
Analysis can be performed using the commonly available software Fiji (ImageJ). Open imaging file of interest in the software (Figure 4A).
Note: An equivalent analysis can be performed using other microscopy analysis software, such as Volocity.
For each primary infected cell, assess fluorescence intensity per area in the DQ-Red BSA channel:
Open the image of interest. To assist with assessment, use the Composite setting to allow all channels to be visualised as an overlay.
Open the Channels Tool (Image → Color → Channels Tool) to allow for the turning on and off of individual channels.
To select which measurements will be performed, go to Analyze → Set Measurements. Select Area and Mean Value.
Open ROI Manager by selecting Analyze → Tools → ROI Manager.
As the DQ-Red BSA channel will be measured, use the slider at the bottom of the image to select the corresponding channel.
Select the Freehand Tool (Figure 4B).
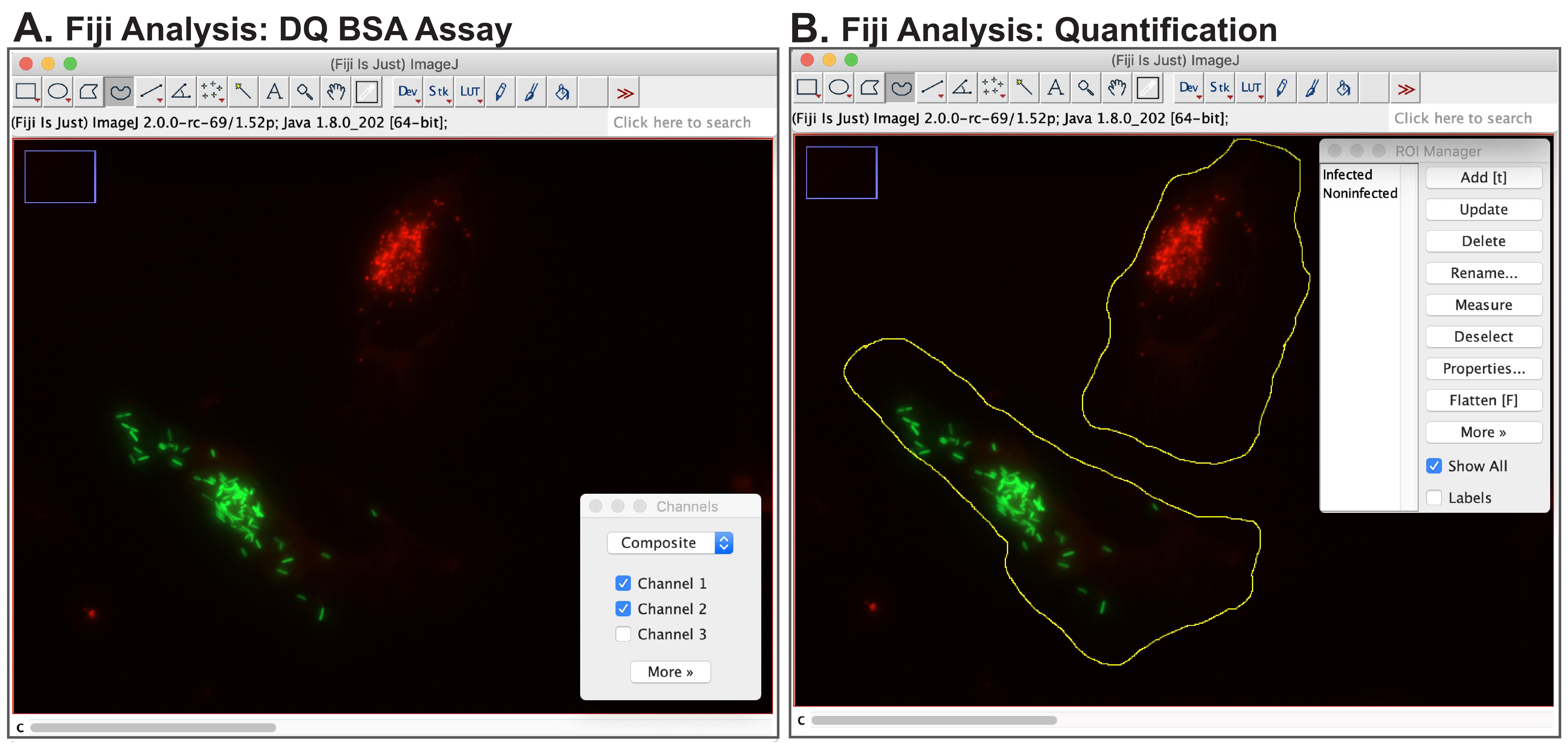
Figure 4. Fiji analysis of DQ-Red BSA assay: Salmonella-infected epithelial cells. (A) Cells should be imaged such that each field of view contains an infected cell of interest and a neighbouring non-infected cell. Visualization in the Composite mode allows for the viewing of both bacterial (green) and DQ-Red BSA (red) channels simultaneously. (B) DQ-Red BSA quantification is performed in Fiji. The Freehand Tool is used to outline both infected and non-infected cells as regions of interest (ROIs) prior to measurement using ROI Manager.Draw a region of interest corresponding to the cell boundary of the infected host cell.
In ROI Manager, add the newly selected ROI by clicking Add.
Draw a region of interest corresponding to the cell boundary of the neighbouring non-infected host cell.
In ROI Manager, add the newly selected ROI by clicking Add.
To take measurements, in ROI Manager, select Measure. A Results window will appear with the corresponding measurements for the two ROIs. These values can be copied and pasted into Excel for subsequent analysis.
Repeat steps B4g–k for all images of interest.
Note: Should the host cell type demonstrate abnormal cells that are characteristically smaller and/or larger than normal cells, a size threshold range can be predetermined such that abnormal cells are excluded from analysis. Also, if needed, a background correction can be performed.
Data analysis
For each primary infected cell and its neighbouring non-infected host cell counterpart, individually calculate the associated intensity/µm2 of the DQ-Red BSA channel by dividing the total intensity measured for the ROI (mean value) by the area of the ROI (area).
For each primary infected cell and its neighbouring non-infected host cell counterpart, calculate the intensity/µm2 of the infected cell as a percentage of the non-infected comparison. This can be accomplished by dividing the intensity/µm2 of the infected cell by the intensity/µm2 of the non-infected cell and multiplying by a factor of 100.
Repeat steps 1 and 2 for each primary infected cell and neighbouring non-infected cell set. A total of 100 data points is ideal.
For each strain or condition of interest, calculate the mean of the 100 data points. For comparison studies of wild type with isogenic knockout strains and complementation strain(s), mean DQ-Red BSA signal intensity/µm2, presented as a percentage of control (non-infected cells), can be compared by bar graph.
Note: Should the data indicate a statistically significant difference with a new pathogen, or should a virulence factor be implicated, a supplementary analysis is needed to rule out the possibility of a defect in dye internalization. This can be performed as a separate independent assay or in combination with DQ-Red BSA using a secondary dye (e.g., dextran Alexa Fluor 647) [19]. Also, it would be valuable to consider validating that DQ-Red BSA dye uptake in non-infected cells is equivalent to that of uninfected cells. To assess this, a comparison of uninfected conditions can be performed for subsequent analysis.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article:
D’Costa et al. [19]. Salmonella Disrupts Host Endocytic Trafficking by SopD2-Mediated Inhibition of Rab7. Cell Reports (Figure 1, panels A–D; Figure 3, panels C–D; Figure 5, panel B; Supplemental Figure 1, panels A–C; Supplemental Figure 2).
General notes and troubleshooting
Troubleshooting
Problem 1: Poor infection efficiency.
Possible cause: Suboptimal host cell conditions.
Solution: Host cell confluency can affect bacterial uptake. If this is suspected, a confluency assessment can be performed by seeding at several host cell densities.
Problem 2: Poor infection efficiency.
Possible cause: Suboptimal bacterial inoculum.
Solution: A colony-forming unit analysis of the inoculum can be performed to confirm that the bacterial growth conditions resulted in viable bacteria. If applicable, it may also be possible that virulence factor production to induce uptake was suboptimal. As virulence factor production in pathogens such as Salmonella can be growth phase–dependent, a growth curve analysis can be performed to assess this.
Problem 3: No intracellular bacteria.
Possible cause: Ineffective intracellular protection conditions.
Solution: If intracellular protection is performed before pathogen uptake into intact pathogen-containing vacuoles is complete, the antibiotic is accessible to the bacteria, which will subsequently be targeted for death. The time period prior to antibiotic treatment can be increased if needed.
Problem 4: Extracellular bacteria observed.
Possible cause: Ineffective intracellular protection conditions.
Solution: Perform a minimum inhibitory concentration assay with the strain of interest to confirm its sensitivity to the antibiotic of interest. This may indicate that a slight adjustment in the drug concentrations used in the assay may be needed. If the strain is resistant to the antibiotic of preference (e.g., gentamicin), an alternate drug class may be considered (e.g., polymyxins).
Problem 5: DQ-Red BSA signal is too low.
Possible cause: Low sensitivity microscopy system.
Solution: Increase light source intensity or exposure length.
Problem 6: DQ-Red BSA signal is too low.
Possible cause: Suboptimal dye uptake.
Solution: The pulse treatment conditions may need optimization, depending on the intrinsic uptake properties of the host cell line. If needed, consider increasing the dye concentration or uptake period.
Acknowledgments
This protocol was adapted from a previously published study [19]. We thank members of the D’Costa Lab for helpful discussions. This work was funded by Operating Grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) (PJ4-175369 and PJT-178191) to V.M.D.
Competing interests
The authors have no conflicts of interest or competing interests to declare.
Ethical considerations
There are no ethical considerations relevant to this paper.
References
- Bhavsar, A. P., Guttman, J. A. and Finlay, B. B. (2007). Manipulation of host-cell pathways by bacterial pathogens. Nature 449(7164): 827–834.
- Diacovich, L. and Gorvel, J. P. (2010). Bacterial manipulation of innate immunity to promote infection. Nat. Rev. Microbiol. 8(2): 117–128.
- Jiao, Y. and Sun, J. (2019). Bacterial Manipulation of Autophagic Responses in Infection and Inflammation. Front. Immunol. 10: e02821.
- Tuli, A. and Sharma, M. (2019). How to do business with lysosomes: Salmonella leads the way. Curr. Opin. Microbiol. 47: 1–7.
- Matsuda, S., Okada, N., Kodama, T., Honda, T. and Iida, T. (2012). A Cytotoxic Type III Secretion Effector of Vibrio parahaemolyticus Targets Vacuolar H+-ATPase Subunit c and Ruptures Host Cell Lysosomes. PLoS Pathog. 8(7): e1002803.
- Newton, H. J. and Roy, C. R. (2011). The Coxiella burnetii Dot/Icm System Creates a Comfortable Home through Lysosomal Renovation. mBio 2(5): e00226–11.
- Kellermann, M., Scharte, F. and Hensel, M. (2021). Manipulation of Host Cell Organelles by Intracellular Pathogens. Int. J. Mol. Sci. 22(12): 6484.
- Lobet, E., Letesson, J. J. and Arnould, T. (2015). Mitochondria: A target for bacteria. Biochem. Pharmacol. 94(3): 173–185.
- Stevens, J. M., Galyov, E. E. and Stevens, M. P. (2006). Actin-dependent movement of bacterial pathogens. Nat. Rev. Microbiol. 4(2): 91–101.
- Brumell, J. H. and Scidmore, M. A. (2007). Manipulation of Rab GTPase Function by Intracellular Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 71(4): 636–652.
- Yang, C. and Wang, X. (2021). Lysosome biogenesis: Regulation and functions. J. Cell Biol. 220(6): e202102001.
- Xu, H. and Ren, D. (2015). Lysosomal Physiology. Annu. Rev. Physiol. 77(1): 57–80.
- Platt, F. M., Boland, B. and van der Spoel, A. C. (2012). Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 199(5): 723–734.
- He, L. Q., Lu, J. H. and Yue, Z. Y. (2013). Autophagy in ageing and ageing-associated diseases. Acta Pharmacol. Sin. 34(5): 605–611.
- Carmona-Gutierrez, D., Hughes, A. L., Madeo, F. and Ruckenstuhl, C. (2016). The crucial impact of lysosomes in aging and longevity. Ageing. Res. Rev. 32: 2–12.
- Liu, X. and Shin, S. (2019). Viewing Legionella pneumophila Pathogenesis through an Immunological Lens. J. Mol. Biol. 431(21): 4321–4344.
- Hilbi, H. and Haas, A. (2012). Secretive Bacterial Pathogens and the Secretory Pathway. Traffic 13(9): 1187–1197.
- Ham, H., Sreelatha, A. and Orth, K. (2011). Manipulation of host membranes by bacterial effectors. Nat. Rev. Microbiol. 9(9): 635–646.
- D’Costa, V. M., Braun, V., Landekic, M., Shi, R., Proteau, A., McDonald, L., Cygler, M., Grinstein, S. and Brumell, J. H. (2015). Salmonella Disrupts Host Endocytic Trafficking by SopD2-Mediated Inhibition of Rab7. Cell Rep. 12(9): 1508–1518.
- Reis, R. C., Sorgine, M. H. and Coelho-Sampaio, T. (1998). A novel methodology for the investigation of intracellular proteolytic processing in intact cells. Eur. J. Cell Biol. 75(2): 192–197.
- Marwaha, R. and Sharma, M. (2017). DQ-Red BSA Trafficking Assay in Cultured Cells to Assess Cargo Delivery to Lysosomes. Bio Protoc 7(19): e2571.
- Mead, P. S., Slutsker, L., Dietz, V., McCaig, L. F., Bresee, J. S., Shapiro, C., Griffin, P. M. and Tauxe, R. V. (1999). Food-Related Illness and Death in the United States. Emerg. Infect. Dis. 5(5): 607–625.
- Valdivia, R. H., Hromockyj, A. E., Monack, D., Ramakrishnan, L. and Falkow, S. (1996). Applications for green fluorescent protein (GFP) in the study of hostpathogen interactions. Gene 173(1): 47–52.
- Barbier, M. and Damron, F. H. (2016). Rainbow Vectors for Broad-Range Bacterial Fluorescence Labeling. PLoS One 11(3): e0146827.
- Steele-Mortimer, O., Meresse, S., Gorvel, J. P., Toh, B. H. and Finlay, B. B. (1999). Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell. Microbiol. 1(1): 33–49.
- Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. and Sternberg, M. J. E. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10(6): 845–858.
- Elsinghorst, E. A. (1994). Measurement of invasion by gentamicin resistance. Meth. Enzymol: 405–420.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Mocăniță, M., Martz, K. and D'Costa, V. M. (2024). Bacterial Pathogen-mediated Suppression of Host Trafficking to Lysosomes: Fluorescence Microscopy-based DQ-Red BSA Analysis. Bio-protocol 14(5): e4951. DOI: 10.21769/BioProtoc.4951.
Category
Microbiology > Microbe-host interactions > Bacterium
Cell Biology > Cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link