- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunofluorescent Staining Assay of 3D Cell Culture of Colonoids Isolated from Mice Colon
Published: Vol 14, Iss 5, Mar 5, 2024 DOI: 10.21769/BioProtoc.4950 Views: 2480
Reviewed by: Samantha HallerAbhijnya KanugoviAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3752 Views
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1249 Views

Intraepidermal Nerve Fiber Quantification of the Mouse Hind Paw Footpads: A Detailed and Simplified Protocol
Anastasia Yerushkin [...] Amir Dori
Dec 5, 2025 1219 Views
Abstract
Here, we describe immunofluorescent (IF) staining assay of 3D cell culture colonoids isolated from mice colon as described previously. Primary cultures developed from isolated colonic stem cells are called colonoids. Immunofluorescence can be used to analyze the distribution of proteins, glycans, and small molecules—both biological and non-biological ones. Four-day-old colonoid cell cultures grown on Lab-Tek 8-well plate are fixed by paraformaldehyde. Fixed colonoids are then subjected to antigen retrieval and blocking followed by incubation with primary antibody. A corresponding secondary antibody tagged with desired fluorescence is used to visualize primary antibody–marked protein. Counter staining to stain actin filaments and nucleus to assess cell structure and DNA in nucleus is performed by choosing the other two contrasting fluorescences. IF staining of colonoids can be utilized to visualize molecular markers of cell behavior. This technique can be used for translation research by isolating colonoids from colitis patients’ colons, monitoring the biomarkers, and customizing their treatments.
Key features
• Analysis of molecular markers of cell behavior.
Protocol to visualize proteins in 3D cell culture.
• This protocol requires colonoids isolated from mice colon grown on matrigel support.
• Protocol requires at least eight days to complete.
Graphical overview
Background
Inflammatory bowel disease (IBD) represents a chronic intestinal inflammation with unknown etiology, which has been linked to genetic and environmental factors. Dysregulation of epithelial-mucosal homeostasis is a key feature of IBD pathogenesis. Epithelial-mucosal lining acts as a defensive barrier against harmful luminal microenvironment. In the past, to understand the progression of IBD, immunofluorescent staining was used to assess the key markers of cell differentiation and proliferation. Staining has been used mostly either for fixed intestinal tissues (as an in vivo model) or for traditional 2D cultures of immortal cell lines (as an in vitro model), which lack the spatial 3D orientation of the cells. There are other staining techniques such as in situ hybridization, immunohistochemistry, and Alcian blue staining utilizing colonoids [1]. Here, we describe the use of immunofluorescent staining of colonoids to mimic the intestinal physiological and pathophysiological relevance. This is an assay that shows the expression of a protein as well as its subcellular location in a realistic way, providing significant benefits to translational research by screening potential therapeutic drugs and their effects on cell differentiation and proliferation for IBD patients.
Materials and reagents
Biological materials
4–6-weeks-old C57BL/6Jmice (Jackson Laboratories, strain number: 000664)
Reagents
Pipette tip: 1,000 µL, 20–200 µL, 1–10 µL (Thermo Fisher Scientific, catalog numbers: P1126, P1179, and P1060, respectively)
Matrigel phenol-red free (Fisher Scientific, catalog number: CB-40234C)
Gibco DMEM/F-12 (Thermo Fisher Scientific, catalog number: 11320033)
Penicillin-Streptomycin (Thermo Fisher Scientific, catalog number: 15140122)
L-Glutamine solution (Sigma-Aldrich, catalog number: G7513-100ML)
Fetal bovine serum (FBS), heat inactivated (Corning, catalog number: 35-011-CV)
GlutaMAX supplement (Thermo Fisher Scientific, catalog number: 35050079)
HEPES buffer solution (Sigma-Aldrich, catalog number: 83264-100ML-F)
N-2 supplement (100×) (Thermo Fisher Scientific, catalog number: 17502048)
B27 supplement (50×), serum free (Thermo Fisher Scientific, catalog number: 17504044)
Epidermal growth factor (EGF) (Sigma-Aldrich, catalog number: E1257-0.1MG)
ROCK inhibitor (Sigma-Aldrich, catalog number: 688001-500UG)
1× phosphate buffered saline (PBS) (without calcium and magnesium) (Fisher Scientific, catalog number: MT21040CM)
Paraformaldehyde 16% (Thermo Fisher Scientific, catalog number: 043368-9M)
FocusClear solution (CelExplorer, catalog number: FC-101)
Triton X-100 solution (Fisher Scientific, catalog number: P185111)
Tween 20 solution (Fisher Scientific, catalog number: P185113)
Sodium azide (Sigma-Aldrich, catalog number: S2002-5G)
Vector Laboratories goat serum (Cole-Parmer, catalog number: S-1000)
ProLong Gold Antifade Mountant with DNA blue fluorescence stain DAPI (Thermo Fisher Scientific, catalog number: P36931)
Ted Pella Inc clear nail polish (Fisher Scientific, catalog number: NC 1849418)
Solutions
Minigut medium (see Recipes)
4% paraformaldehyde solution (see Recipes)
Immunofluorescence (IF) buffer (see Recipes)
Permeabilization solution (see Recipes)
Blocking buffer (see Recipes)
Dilution buffer (see Recipes)
Recipes
Minigut medium
Conditioned medium (1 volume of L-cell medium and 1 volume of DMEM F12 containing 1:100 vol/vol penicillin-streptomycin, 1:100 vol/vol glutamine, and 20% vol/vol FBS) [2]
GlutaMAX 1:100 vol/vol
10 mM HEPES buffer
Penicillin-streptomycin 1:100 vol/vol
N-2 supplement 1:100 vol/vol
B27 supplement 1:50 vol/vol
EGF 50 ng/mL (final concentration)
ROCK inhibitor 10 µM (final concentration)
4% paraformaldehyde solution
1:4 vol/vol 16% paraformaldehyde and 1× PBS
Immunofluorescence (IF) buffer
Triton X-100 0.2% (vol/vol), Tween 20 0.05% (vol/vol), 1× PBS 99.75% (vol/vol)
Permeabilization solution
Triton X-100 2% (vol/vol) and 1× PBS 98% (vol/vol)
Blocking buffer
Goat serum 10% (vol/vol), Triton X-100 2% (vol/vol), sodium azide 0.02% (wt/vol), 1× PBS 88% (vol/vol)
Dilution buffer
Triton X-100 0.25% (vol/vol), goat serum 1% (vol/vol), sodium azide 0.02% (wt/vol), 1× PBS 98.75% (vol/vol)
Equipment
Costar 24-well clear TC-treated multiple well plates individually wrapped, sterile (Corning, catalog number: 3524)
Pipetman—PK1000, PK200, PK20 (Gilson, catalog numbers: F144059M, F144058M, and F144056M, respectively)
Lab-Tek chamber slide 8-well glass slide (Nunc, catalog number: 154941)
Laminar flow hood (LabGard Class II type A2 Biological Safety cabinet) (Nuaire, model: NU-540)
3110 CO2 water jacketed cell incubator (Thermo Fischer Scientific, model: 4120)
Digital water bath (VWR, catalog number: 89501-460)
Sorvall ST 16R centrifuge (Fisher Scientific, catalog number: 75-004-240)
All-in-One fluorescence microscope (Keyence BZ-X series, model: BZ-X810) (Figure 1)
Rectangular ice pan (ice tray/bucket) (Fisher Scientific, catalog number: 07-210-107)
Micro cover glass (VWR, catalog number: 48393252)
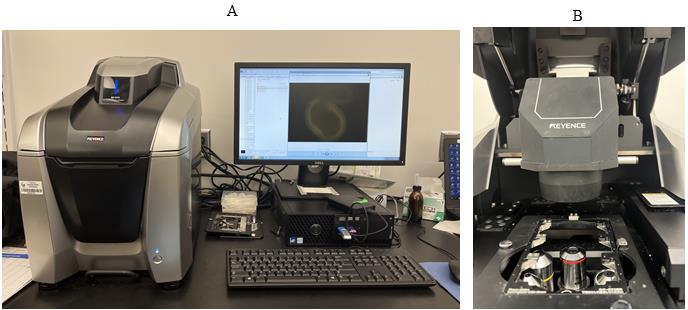
Figure 1. Keyance microscope. A. Keyence microscope with software showing the immunofluorescent staining of a colonoid. B. Keyance microscope showing the holder to be used for 8-well Lab-Tek chamber slide.
Software and datasets
BZ-X analyzer (https://www.keyence.com/bz-x810)
Procedure
As described by O’Rourke et al. [2], use 5–6-week-old wild-type mice to isolate colonoids. Briefly, the colon of the mice was cut into small pieces and strained to remove tissues. Filtrate was shaken vigorously with 1× PBS-EDTA and was continuously checked for crypts enrichment under a basic microscope. Once optimum crypts are observed, the supernatant is centrifuged at 112× g (rcf) at 10 °C. The pellet is mixed with matrigel. Mix isolated colonoids with matrigel (10 µM) in a laminar flow hood. Mix the suspension by pipetting using a PK200 without creating bubbles.
Add 50 µL of matrigel and colonoid mix (~10 colonoids/50 µL matrigel) in each well of a prewarmed (at 37 °C) 24-wells plate in the laminar flow hood and keep it in a cell incubator set at 37 °C with 5% CO2 and 90% humidity for 5 min for polymerization.
After polymerization, overlay each well with 1 mL of prewarmed Minigut medium using a PK100 in the laminar flow hood. Dispense the medium along the wall of the well.
Replace the medium of the wells after 18–24 h and on day 3 with 1 mL of prewarmed Minigut medium using a PK1000 in the laminar flow hood. Use PK1000 to aspirate the medium along the wall of the well without disturbing the matrigel drop.
On day 5, aspirate the medium using a PK1000 in the laminar flow hood and use prewarmed 1× PBS (without calcium and magnesium) as one-time wash to remove the medium.
Place the 24-well plate on an ice pan in the laminar flow hood and add 1 mL of ice-cold 1× PBS (without calcium and magnesium) using a PK1000. Gently break the matrigel drop by repeated pipetting using a PK200 avoiding bubbling.
Collect the 1× PBS containing the dissolved matrigel with colonoids in a 50 mL centrifuge tube and centrifuge at 28× g (rcf) for 5 min at 4 °C.
Aspirate out 1× PBS with dissolved matrigel using a PK1000 as quick as possible, keeping the centrifuge tube in ice in the ice pan.
Resuspend the colonoid pellet in 400 µL of matrigel and mix it by pipetting with 200 µL Pipetman. Add 50 µL of the suspension to prewarmed 8-well Lab-Tek chamber slide and place it straight (not upside down) in the cell incubator for 5 min. After polymerization, add 500 µL of prewarmed Minigut medium to each well of the Lab-Tek chamber slide and place it in the cell incubator (Figure 2).
Figure 2. 8-well Lab Tek chamber slide. A. 50 μL of matrigel drop with colonoid suspension. B. Minigut medium overlaying the polymerized matrigel drop with colonoid in cell incubator.Replace the medium of the wells on days 1 and 3 with 1 mL of prewarmed Minigut medium using a PK1000 in the laminar flow hood. Use a 1 mL Pipetman to aspirate the medium along the wall of the well without disturbing the matrigel drop.
On day 4 or day 5, colonoids will show branching (Figure 3), which is the optimum state to start immunofluorescent staining. Take out the Lab-Tek chamber slide from the cell incubator. Use a PK1000 to aspirate the medium along the wall of the well without disturbing the matrigel drop. Wash each well (once only) with 500 µL of prewarmed 1× PBS using a PK1000.
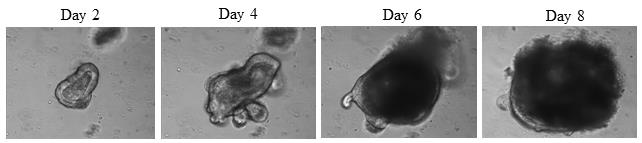
Figure 3. Day 2 (no branching), Day 4 (branching starts appearing), Day 6 (branching is not very clear in multilayer colonoid), and Day 8 (branching is not visible in very dense colonoid) of wild-type mice culture on matrigel in 8-well Lab-Tek chamber plate. Magnification is 20×.After aspirating 1× PBS, fix the colonoids using 4% paraformaldehyde solution at room temperature for 2 h.
Wash two times with 500 µL (per well) of prewarmed 1× PBS using a PK1000 (5 min each wash, no shaking).
Add 200 µL of FocusClear solution to each well using a PK1000 and incubate for two nights at room temperature on a working laboratory bench.
Wash two times with 200 µL (per well) of IF buffer using a PK200 (5 min each wash, no shaking).
Incubate with 200 µL of permeabilization solution in each well for three nights at room temperature.
Aspirate out the permeabilization solution using a P200 and wash three times with IF buffer (200 µL per well) at room temperature for 5 min (each wash) without shaking.
Add 200 µL of blocking buffer to each well and incubate for 4 h at room temperature.
Aspirate out the blocking buffer using a P200.
Use a P200 to add 150 µL (per well) of primary antibody diluted in dilution buffer and incubate overnight at room temperature.
Aspirate out the primary antibody using a P200 and wash three times with IF buffer (200 µL per well) at room temperature for 5 min (each wash) without shaking.
Add 150 µL (per well) of secondary antibody (against the primary antibody, as per the manufacturer’s instruction) conjugated with, for example, green fluorescence (GFP). Dilute the secondary antibody (as per the manufacturer’s instruction) in dilution buffer and incubate for two nights at room temperature in the dark.
Aspirate out the secondary antibody using a P200 and wash three times with IF buffer (200 µL per well) at room temperature for 5 min (each wash) without shaking.
Add 150 µL (per well) of fluorescence-counterstaining antibody (for example, if secondary antibody is conjugated with green fluorescence, then red fluorescence conjugated Phalloidin, which stains actin filaments, should be used) diluted in dilution buffer for one night at room temperature.
Aspirate out the counterstaining antibody using a P200 and wash three times with IF buffer at room temperature (200 µL per well) for 5 min (each wash) without shaking.
Add 100 µL of ready-to-use ProLong Gold Antifade Mountant with DNA blue fluorescence stain DAPI to each well for one night at room temperature.
As per manufacturer’s instructions, a tool is provided which separates and removes the upper chamber from the silicone gasket of the Lab-Tek slide.
Coverslip the Lab-Tek slide without upper chamber with a micro cover glass and seal with white-colored nail polish.
Store in the dark at room temperature.
The mounted and cover-slipped slide should be used for taking images at the earliest (within 2–3 days of staining) and can be stored at room temperature for 4–5 days only under dark conditions.
Representative data: For example, we demonstrate in Figure 4 that 4-day-old colonoids isolated from wild-type mice were stained with anti-PCNA (proliferating cell nuclear antigen, a proliferation marker) conjugated with green fluorescence. TRITC-conjugated phalloidin and DAPI (blue fluorescence) were used to counterstain actin filaments with red fluorescence and nuclei with blue fluorescence, respectively, using 40× magnification. We also captured the image in brightfield using 40× magnification.
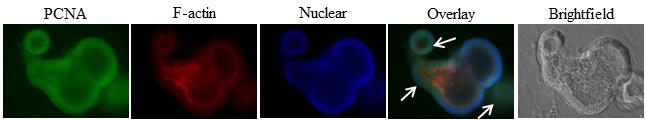
Figure 4. Representative result of immunofluorescence staining of 4-day-old organoid of wild-type mice, cultured and fixed on matrigel in 8-well Lab-Tek chamber plate and probed with anti-PCNA (proliferation marker). FITC as green fluorescence was used as secondary antibody. Images are the Z-sections at 40× magnification. DAPI was used to counterstain nuclei with blue fluorescence and red fluorescence is for actin-phalloidin. Branching of colonoid shows proliferating cells as indicated by white arrows.
Data analysis
We used a Keyence microscope (BZ-X810 model) and the BZ-X series software to capture images of the immunofluorescent-stained colonoid in brightfield and under green, red, and blue channel. With the help of software, an overlay image of all three channels can be created, which will depict the expression of the protein of interest as well as its location due to actin and nuclear counterstaining.
Validation of protocol
We have demonstrated in Walter et al. [3] that 4-day-old colonoids were stained with anti-SEPP1 conjugated with green fluorescence (Figure 5C-ii). TRITC-conjugated phalloidin and DAPI were used to counterstain actin filaments with red fluorescence and nuclei with blue fluorescence, respectively, using 40× magnification.
General notes and troubleshooting
The protocol was used on freshly isolated colonoids from mice colon. However, it should work with any colonoid culture as long as they are grown on matrigel and are healthy and less branched. See in Figure 4 the difference in branching of colonoids on Day 4 and Day 6.
The 50 µL of colonoid and matrigel suspension in prewarmed 8-well Lab-Tek chamber slide should not be incubated in the cell for more than 5 min. This is the optimum duration for the polymerization of matrigel as well as avoiding nutrient deprivation of colonoids.
While breaking the matrigel-containing colonoid, temperature should be maintained icy cold. Furthermore, frothing and bubbling due to repeated pipetting should be avoided as much as possible.
The entire procedure takes approximately eight days to finish. Therefore, there is a higher chance of losing the polymerized matrigel-containing colonoid during the procedure. Therefore, one slide (meaning all 8 wells) should be used for one condition. This means that, if we are comparing wild-type mice with transgenic mice, one slide should be seeded with wild-type mice colonoids and another slide should be seeded with transgenic mice colonoids. This will give a sufficient number of ‘ns’ for data analysis.
Mounted and cover-slipped slides should be processed for data analysis as soon as possible. Due to matrigel, slides cannot be stored at 4 °C, and fluorescence intensity fades out slowly at room temperature.
Handling of paraformaldehyde should be done in the fume hood while wearing PPE and safety goggles.
Troubleshooting
Matrigel polymerizes at 37 °C and becomes liquid at 4 °C. Therefore, while passaging the colonoids, icy conditions are important to be maintained; during the staining procedure, prewarmed solutions should be used at room temperature.
After seeding colonoids on 8-well Lab-Tek chamber slides, they should be observed daily. Branching of colonoids can start as early as on Day 3 and as late as Day 5 or 6 depending on mice strain or if it is a transgenic mouse. Multilayered colonoids with several branching should be avoided for data analysis purposes, as it will not result into sharp pictures.
If primary antibody staining is not visible, then consider decreasing its dilution.
Acknowledgments
This protocol was adapted from Walter et al. [3].
Competing interests
The authors declare no conflicts of interest within this work.
Ethical considerations
Georgia State University’s Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC) have approved the experiments described in the protocol.
References
- Wilson, S. S., Mayo, M., Melim, T., Knight, H., Patnaude, L., Wu, X., Phillips, L., Westmoreland, S., Dunstan, R., Fiebiger, E., et al. (2021). Optimized Culture Conditions for Improved Growth and Functional Differentiation of Mouse and Human Colon Organoids. Front. Immunol. 11: e547102. https://doi.org/10.3389/fimmu.2020.547102
- O’Rourke, K., Ackerman, S., Dow, L. and Lowe, S. (2016). Isolation, Culture, and Maintenance of Mouse Intestinal Stem Cells. Bio Protoc 6(4): e1733. https://doi.org/10.21769/bioprotoc.1733
- Walter, L., Canup, B., Pujada, A., Bui, T. A., Arbasi, B., Laroui, H., Merlin, D. and Garg, P. (2020). Matrix metalloproteinase 9 (MMP9) limits reactive oxygen species (ROS) accumulation and DNA damage in colitis-associated cancer. Cell Death Dis. 11(9): 767. https://doi.org/10.1038/s41419-020-02959-z
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Mehrotra, T., Shi, X. and Merlin, D. (2024). Immunofluorescent Staining Assay of 3D Cell Culture of Colonoids Isolated from Mice Colon. Bio-protocol 14(5): e4950. DOI: 10.21769/BioProtoc.4950.
Category
Stem Cell > Organoid culture
Cell Biology > Cell isolation and culture > 3D cell culture
Cell Biology > Cell staining > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link