- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Addressing the Role of Conventional CD8αβ+ T Cells and CD4+ T Cells in Intestinal Immunopathology Using a Bone Marrow–Engrafted Model
Published: Vol 14, Iss 4, Feb 20, 2024 DOI: 10.21769/BioProtoc.4934 Views: 2949
Reviewed by: Andrea GramaticaMarco Di GioiaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Multi-color Flow Cytometry Protocol to Characterize Myeloid Cells in Mouse Retina Research
Wei Xiao [...] Abdelrahman Y. Fouda
Aug 20, 2023 2976 Views
Isolation and Analysis of B-cell Progenitors from Bone Marrow by Flow Cytometry
Hongchang Zhao [...] Jacqueline H. Barlow
Oct 5, 2023 3466 Views
PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3962 Views
Abstract
Inflammatory bowel disease (IBD) is characterized by an aberrant immune response against microbiota. It is well established that T cells play a critical role in mediating the pathology. Assessing the contribution of each subset of T cells in mediating the pathology is crucial in order to design better therapeutic strategies. This protocol presents a method to identify the specific effector T-cell population responsible for intestinal immunopathologies in bone marrow–engrafted mouse models. Here, we used anti-CD4 and anti-CD8β depleting antibodies in bone marrow–engrafted mouse models to identify the effector T-cell population responsible for intestinal damage in a genetic mouse model of chronic intestinal inflammation.
Key features
• This protocol allows addressing the role of CD4+ or CD8αβ+ in an engrafted model of inflammatory bowel disease (IBD).
• This protocol can easily be adapted to address the role of other immune cells or molecules that may play a role in IBD.
Keywords: T cellBackground
Inflammatory bowel disease (IBD) is a chronic and recurring inflammation in the gastrointestinal tract that affects more than 1.3 million people in Europe (Zhao et al., 2021). IBD includes ulcerative colitis and Crohn’s disease. It is common for patients with ulcerative colitis and Crohn's disease to experience diarrhea, rectal bleeding, abdominal pain, fatigue, and weight loss. Furthermore, bearers of this debilitating disease are at high risk of inflammation-associated cancers such as colorectal cancer (Kim and Chang, 2014). Although it is widely accepted that IBD is characterized by an excessive immune response to microbiota, the etiology of IBD remains a mystery, being crucial to create novel models that can unravel the intricate microbial, molecular, and cellular interactions contributing to the onset of IBD.
Several immune-regulatory mechanisms prevent IBD. Particularly, SMAD4 in T cells, a significant downstream component of the cytokine TGF-β pathway, prevents mice from developing chronic intestinal inflammation. Indeed, researchers have demonstrated that ablation of SMAD4 specifically in T cells using a CD4-CRE system leads to severe chronic intestinal inflammation (Hahn et al., 2011; Kim et al., 2006). This inflammation typically emerges around 5–7 months of age. T cells deficient in SMAD4 contribute to intestinal inflammation. However, the model employed to illustrate this is based on the CD4-CRE system. As both CD4+ and CD8αβ+ T cells undergo a stage expressing both CD4 and CD8 markers during their generation in the thymus, the deletion of SMAD4 occurs in both CD4+ and CD8αβ+ T cells. It is crucial to specify which subset of T cells is responsible for mediating the observed pathology in the context of the immunopathology observed in IBD.
At present, no animal model entirely recapitulates Crohn’s disease or ulcerative colitis. Models such as the SMAD4 model present the benefit of not using chemical agents such as dextran sulfate sodium (DSS) and closely mimicking the chronicity of the inflammation without external intervention, in contrast to chemical-induced colitis models. Although useful, colitis models based on the transfer of naïve T cells into mice lacking adaptive immunity (SCID or RAG mice) do not recapitulate the entire onset of IBD. Indeed, those models lack an adaptive immune system. The SMAD4 model provides some interesting insights that closely resemble what happens in humans, such as impairment on TGFβ signaling pathway (Monteleone et al., 2001).
The present protocol offers an approach to address the implication of different populations of T cells in IBD. It utilizes a bone marrow–engrafted mouse model and specific anti-CD4 and anti-CD8β depleting antibodies to eliminate conventional CD8αβ+ and CD4+ T cells, sparing unconventional T cells such as TCRγδ T cells (Figure 1). This protocol can be adapted for the study of other cellular and molecular mechanisms potentially implicated in IBD.
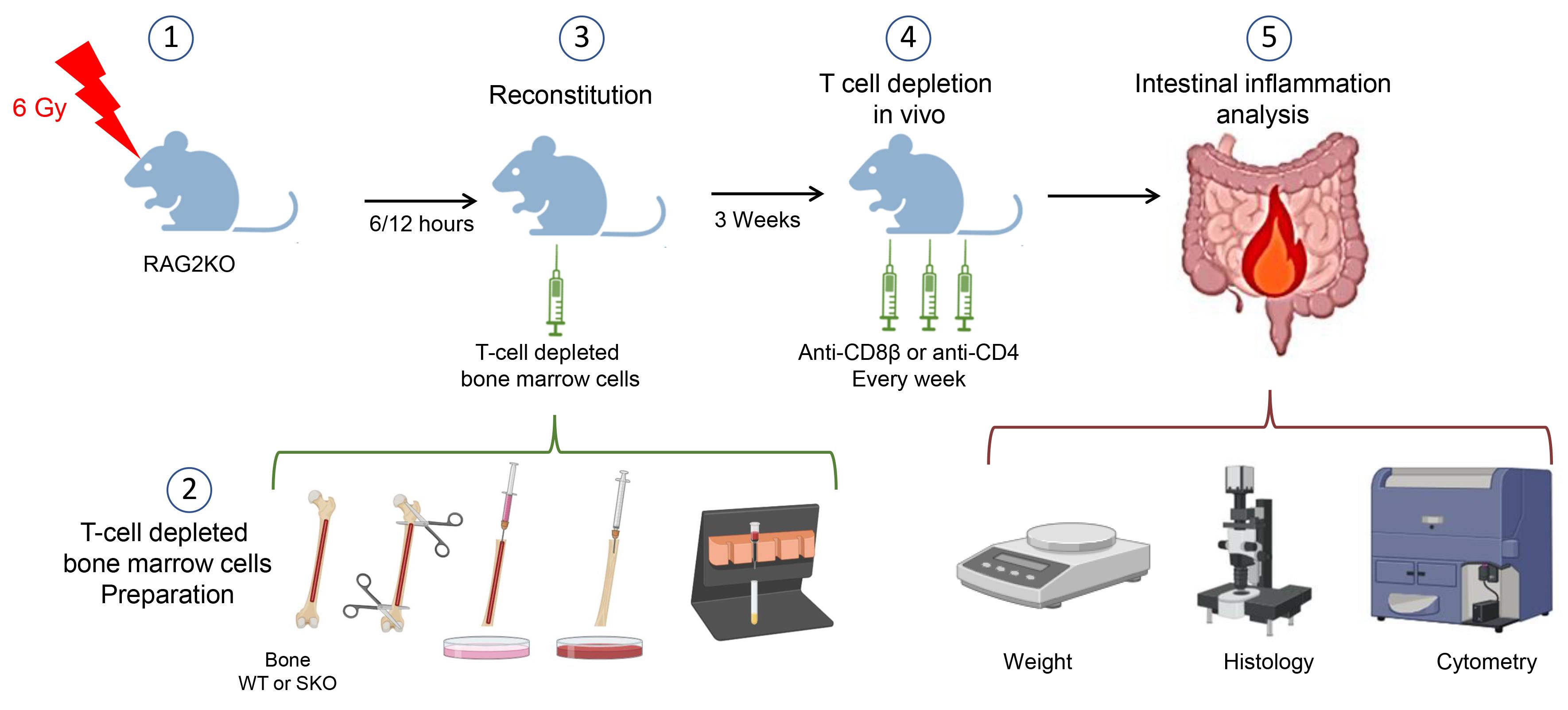
Figure 1. Schematic model of the procedures. This figure was created with BioRender.com.
Materials and reagents
Biological materials
Animals
All mice were on the C57BL/6J background and were maintained in PPAC, a specific pathogen-free animal facility of the Cancer Research Center of Lyon (CRCL), France. RAG2-KO mice were obtained from Charles River Laboratories and bred and maintained in the animal facility (specific pathogen-free). Mice were older than 8 weeks when used for the experiment. CD4-Cre+ Smad4floxed/floxed (SKO) mice and littermate CD4-Cre-Smad4floxed/floxed (WT) mice were the donors of bone marrow cells. In terms of gut inflammation development, no discernible difference was observed between males and females. However, the mice used should be of the same age and sex in the same experiment.
Materials
Pipette tips 10 μL, 20 μL, 200 μL, and 1 mL (Starlab)
5 mL sterile syringe (BD, catalog number: 3095971)
50 mL centrifuge tube (Falcon, catalog number: 352070)
15 mL conical centrifuge tube (Falcon, catalog number: 352097)
Petri dishes (100 × 20 mm) (FALCON, catalog number 353003)
10 mL serological pipettes (SARTSTEDT, catalog number: 86.1254.001)
25 G needle (BD, catalog number: 300600)
BD U-100 insulin syringe with a 29 G needle (BD, catalog number: 324891)
Neubauer chamber (KOVA international, catalog number: 87144 F)
Tubes 1.5 mL, autoclaved (STARLAB, catalog number: S1615-5500)
Nylon meshes with a mesh size of 70 μm (SEFAR NITEX, catalog number: 03-80/29), sterilized by autoclaving
Reagents
Surface disinfectants (Anios, SURFA’SAFE)
LD columns (Miltenyi Biotec, catalog number: 130-042-901)
Anti-CD8α BV605 (clone 53-6.7) (BioLegend, catalog number: 100748)
Anti-CD8b Alexa 700 (clone YTS156.7.7) (BioLegend, catalog number: 126618)
Anti-CD4 Brilliant Violet 711 (clone RM4.5) (BD, catalog number: 563726)
Anti-CD3e Brillant Violet 650 (clone 17A2) (BioLegend, catalog number: 100229)
Anti-CD45 APC-Cy7 (clone 30-F11) (BioLegend, catalog number: 103116)
Anti-CD103 e450 (clone 2E7) (eBioscience, catalog number: 48-1031-82)
Anti-Ly6g PE/Dazzle594 (clone 1A8) (BioLegend, catalog number: 127648)
Anti-CD11b APC (clone M1/70) (BioLegend, catalog number: 553312)
Anti-TCRgd FITC (clone GL3) (BD, catalog number: 553177)
LIVE/DEAD™ Fixable Yellow Dead Cell Stain kit (Life technologies, catalog number: L34968)
InVivoMAb anti-mouse CD8β (Lyt 3.2) (Clone 53-5.8) (BioXcell, catalog number: BE0223)
InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase (Clone HRPN) (BioXcell, catalog number: BE0088)
InVivoMAb anti-mouse CD4 (Clone GK1.5) (BioXcell, catalog number: BE0003-1)
InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin (Clone LTF-2) (BioXcell, catalog number: BE0090)
Fetal bovine serum (FBS) (Gibco®, catalog number: A5256701)
RPMI-1640 medium, GlutaMAX™ supplement (Life Technologies, Gibco, catalog number: 61870036)
HEPES buffer solution, 1 M (Gibco, catalog number: 15630-080)
Hank’s buffered salt solution (HBSS) (Gibco, catalog number: 14175-053)
Phosphate buffered saline (PBS 10×) (Life Technologies, Gibco, catalog number: 14200-059)
Ethanol 70% (Alcool modifié COOPER®) used as an antiseptic
Ethanol absolute (VWR Chemicals, catalog number: 20821.365)
CD3ϵ microbead kit, mouse (Miltenyi Biotec, catalog number: 130-094-973)
Trypan blue solution, 0.4% (Thermo Fisher, catalog number: 15250061)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906)
EDTA, pH 8.0 (EUROMEDEX, catalog number: EU0084)
Isoflurane
Gentamicin (50 mg/mL) (Gibco, catalog number: 15750-037)
Penicillin-Streptomycin (Life Technologies, catalog number: 15140-122)
Solutions
RPMI complete media (see Recipes)
Intra-epithelial lymphocyte enrichment buffer (see Recipes)
MACS buffer (see Recipes)
Recipes
RPMI complete media
RPMI-1640 enriched with 10% heat-inactivated FBS, 10 mM HEPES, and penicillin-streptomycin (1,000 U/mL).
MACS buffer
PBS 1× with 2 mM EDTA and 2% BSA
Equipment
Irradiator (Elekta Synergy® or any alternate irradiator)
Sterile cell culture hood (Thermo, model: HERAsafe KS 12 1.2m/41489710/2013)
QuadroMACS magnetic cell separator (Miltenyi Biotec, catalog number: 130-090-976)
Microscope (Zeiss, model: Primovert)
Serological pipettes [SARSTEDT, catalog number: 86.1253.001 (5 mL), 86.1254.001 (10 mL), and 86.1685.001 (25 mL)]
Pipette controller (INTEGRA, model: pipetboy)
Surgical scissors and forceps (Dutscher, catalog number: 005064, 005065, 711198, 005072)
Ice bucket (Dutscher, catalog number: 830116)
Anesthesia chamber (Tem Sega® or any alternate instrument)
Weigh balance (OHAUS®, model: CS200)
Pie-shape chambers (model equivalent of pie cage for X-ray irradiation from Bioseb Lab instrument®)
Animal transfer box [InnoVive, model: M-BTM (bottom) and MSX4 (lid)]
LSRII flow cytometer (BD Biosciences, model: LSR Fortessa HTS)
Centrifuge (Thermo Fisher, model: Heraeus Megafuge)
Software
FlowJoTM software (BD Biosciences) (v. 10.9, 2023)
GraphPad Prism (v. 10.1.2, 2023)
Procedure
Isolation of bone marrow cells for engraftment into recipient mice
Euthanize the donor mouse using an approved method; here, we used CO2 inhalation. Generally, 8–10 mice can be reconstituted from a single donor.
Disinfect the surgical area with 70% ethanol.
With scissors, make a small incision in the skin overlying the femur and tibia. Collect and place the femurs and tibias in RPMI complete media (see Recipes).
Under the sterile culture hood, initiate the process by immersing the bone within a Petri dish filled with 70% ethanol in order to eliminate potential contaminations (30 s). Try to meticulously remove a substantial portion of muscle and connective tissues from the bone. Leaving bones in ethanol for an extended period of time while flushing each bone is possible, but caution is advised because ethanol may penetrate the bone, potentially compromising viable cells. Subsequently, relocate the bone to a fresh Petri dish containing approximately 15 mL of RPMI complete media.
With sterile scissors, cut both ends of the femur and tibia to expose the bone marrow cavity.
After exposing the bone marrow cavity, carefully grasp the bone with forceps. Load a 5 mL syringe with complete media, using a 25 G needle, for the extraction process. Gently insert the needle into the bone marrow cavity and push the syringe plunger to flush out bone marrow cells into the Petri dish. Repeat this process iteratively until the bone transitions from its initial red hue to a paler, whitish appearance, indicating that the bone marrow cells have been extracted (Figure 2).
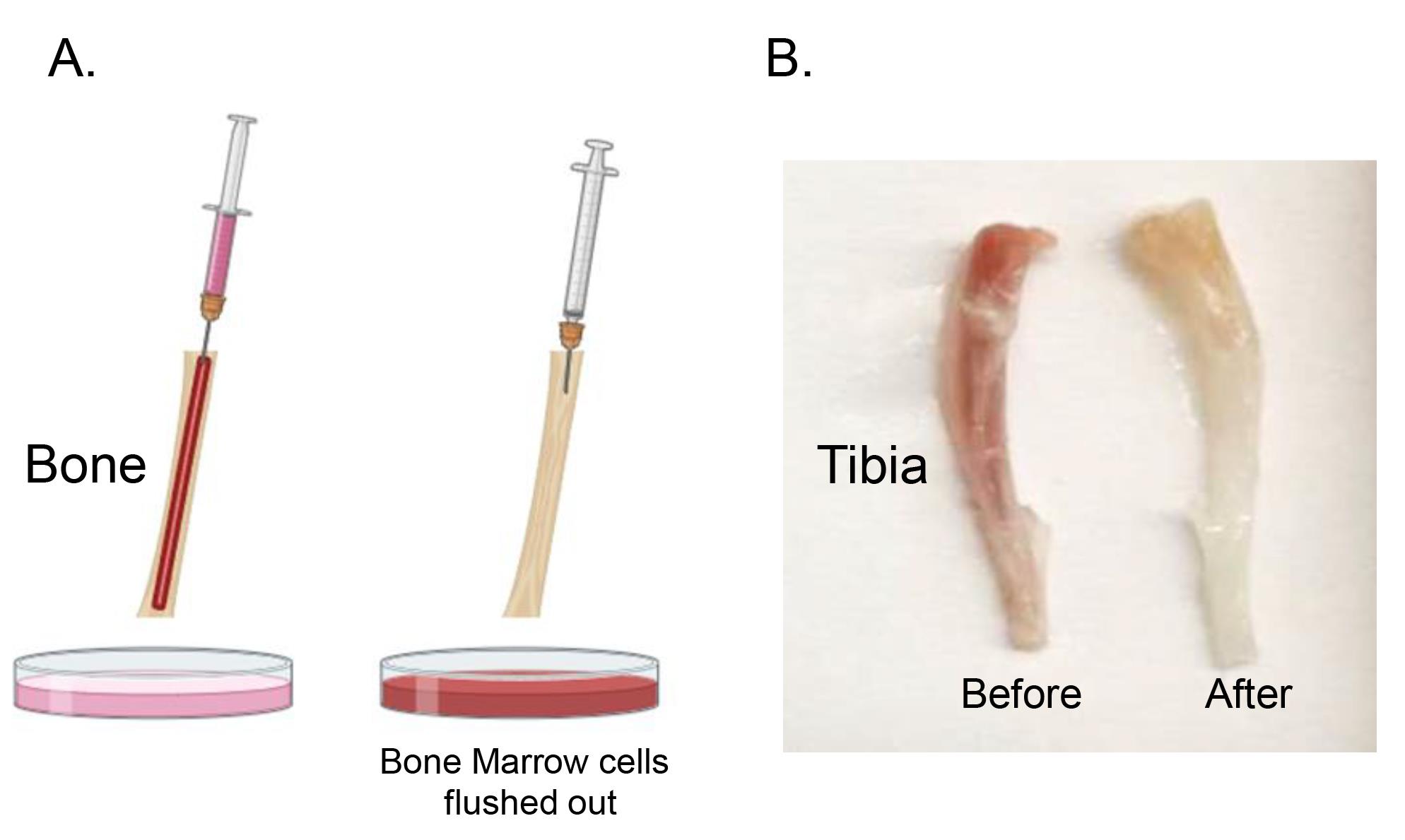
Figure 2. Bone marrow cell extraction. Utilize a 5 mL syringe with a 25 G needle filled with RPMI complete media for extraction of bone marrow cells (A). Insert the needle into the bone marrow cavity, push the syringe plunger to flush out cells into the Petri dish, and repeat until the bone shifts from red to a whitish appearance. Photo of mouse tibias (B). Figure 2A was created with BioRender.com.Thoroughly resuspend the bone marrow cells using a p1000 pipette. This step is crucial to ensure the bone marrow cells do not form any aggregate.
Using a 70 μm sterile nylon mesh, filter the solution into a 50 mL tube to eliminate any possible clumps. Then, centrifuge the cells at 600× g for 5 min at 4 °C.
Discard the supernatant, resuspend the pellet into 500 μL of MACS buffer (see Recipes), and add 50 μL of CD3ϵ-biotin antibody. Incubate for 15 min at 4 °C. CD3e biotin antibodies will bind to T cells, permitting their specific elimination in the subsequent step of the protocol. After the incubation period, retrieve the tube and add 5 mL of MACS buffer, and spin down the cells by centrifugation at 600× g for 5 min at 4 °C.
Resuspend the pellet with 500 μL of MACS buffer and add 100 μL of anti-biotin microbeads. Mix well and incubate at 4 °C for 15 min.
After incubation, add 5 mL of MACS buffer and load the cell suspension to a LD column placed on the QuadroMACS magnetic cell separator. Using a 15 mL Falcon tube, collect the unlabeled cells that pass through.
Centrifuge the collected cells at 600× g for 5 min at 4 °C. Resuspend the pellet in 1 mL of PBS 1× buffer. Count the viable cells using 0.4% Trypan blue solution, a Neubauer chamber, and an optical microscope.
Adjust the cell concentration such that 2 million cells in 200 μL of PBS 1× can be engrafted into each mouse. Alternatively, if there are insufficient cells, 1 million cells may be engrafted. Optionally, to prevent potential contamination, gentamicin (50 μg/mL) can be added to the preparation. Then, keep cells at 4 °C until they are ready to be used.
Irradiation and T cell–depleted bone marrow transplantation
The day before the irradiation, weigh the recipient mice to obtain the starting weight. Tag the mice (earmark) prior to the irradiation to identify them and try, as much as possible, to place the control and future treated mice in the same cage, in order to minimize cage effects.
On the day of irradiation, place mice in a pie cage and transfer to the irradiator. This process must be conducted under the hood and all materials must be sterilized. In order to comply with the facility's specific pathogen-free conditions, mice should be transferred in a sterile transfer box to the irradiator.
To ablate the hematopoietic system of recipient mice, administer a single dose of sub-lethal irradiation. This is accomplished by irradiating 8–12-week-old RAG2KO mice with a total body irradiation dose of 6 Gy (600 rads).
After irradiation, replace the recipient mice in their cage with caution to maintain the specific pathogen-free conditions of the animal facility (e.g., the outside of the box is disinfected with Anios for at least 10 min).
After irradiation, reconstitute mice within 6–12 h. For the reconstitution of irradiated mice, inject 2 million T cell–depleted bone marrow cells from WT or SKO mice in 200 μL of PBS intravenously via the retro-orbital vein with a BD U-100 insulin syringe. To minimize mouse stress and discomfort, anesthetize mice with isoflurane in an anesthesia chamber.
Post-irradiation, as the mice undergo substantial immunosuppression and are consequently susceptible to infections, these animals should be housed within a sterile environment. For reconstituted mice, regular observation is particularly crucial during the first 14 days following irradiation to identify any indicators of pain or distress. In cases where animals exhibit signs of suffering for any reason (loose feces, rectal bleeding, or constant hunched posture), the ethical decision is to euthanize them to alleviate pain and distress. It is important to highlight that the reconstituted mice are intentionally not administered antibiotics, as this can adversely influence the development of IBD in SMAD4-deficient reconstituted mice (Igalouzene et al., 2022).
Wait 21 days after bone marrow reconstitution to allow the donor hematopoietic cells to establish within the recipient and then proceed to the T-cell depletion.
Weigh the mice twice a week to determine whether they are losing weight relative to their initial weight. This constitutes an objective indication that the mice are suffering from chronic intestinal inflammation.
CD8αβ+ and CD4+ T-cell depletion in vivo
On day 21 after bone marrow reconstitution, intraperitoneally inject each mouse with 150 μg of one of the following antibodies in 200 μL of PBS 1×: anti-CD8β antibody (clone 53-5.8), anti-CD4 antibody (clone GK1.5), rat IgG1 isotype control antibody (clone HRPN), or rat IgG2b isotype control antibody (clone LTF-2) (Figure 3).
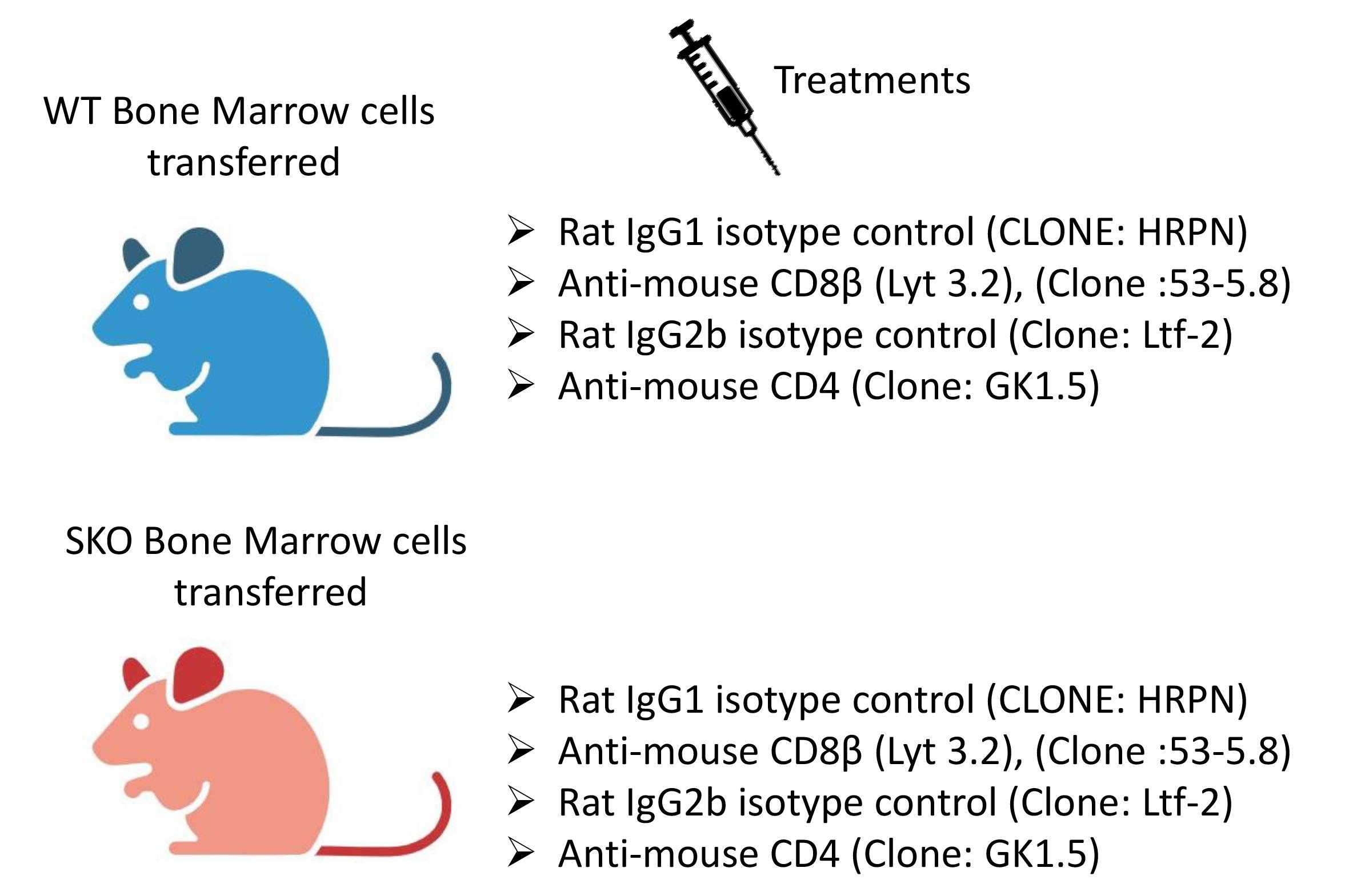
Figure 3. Experimental groups. This figure was created with BioRender.com.Repeat the injection once per week until the SMAD4-deficient mice lose approximately 15%–20% of their original weight and must be euthanized and analyzed. After irradiation and reconstitution, the SKO-reconstituted mice generally become ill 5–7 weeks later, exhibiting loose stools, diarrhea, and rectal bleeding, as well as a hunched-over appearance and a weight loss of 15%–20%. The colon, small intestine, and mesenteric lymph nodes can be harvested for histology or flow cytometry analysis.
As well as monitoring the body weight two times per week, monitor the health status of the mice at least three times per week to intervene promptly and ethically. Carefully check mice for signs of discomfort such as social isolation, hunched posture, immobility, blood in feces, loose feces, or ruffled or unkempt appearance.
Data analysis
Weight loss is indicative of the severity of gut inflammation. Throughout the experiment, mice are weighed twice a week, and the percentage of weight loss is calculated in comparison to the initial weight. Readers are directed to Igalouzene et al. (2022) and Figure 2 of the present manuscript to see how the elimination of CD8αβ+ T cells prevent bone marrow–engrafted SKO mice from developing severe intestinal inflammation. It is important to remind that, for ethical reasons, mice experiencing a 20% loss of their initial body weight are euthanized within 24 h with the corresponding controls. Following euthanasia, the colon, small intestine, and mesenteric lymph nodes are collected for further analysis.
The colon length is also a parameter that indicates the severity of the chronic inflammation. Using a ruler, the colon length is determined. Shorter colons relative to mice with the same age and sex are associated with severe gut inflammation (Igalouzene et al., 2022 and Figure 5).
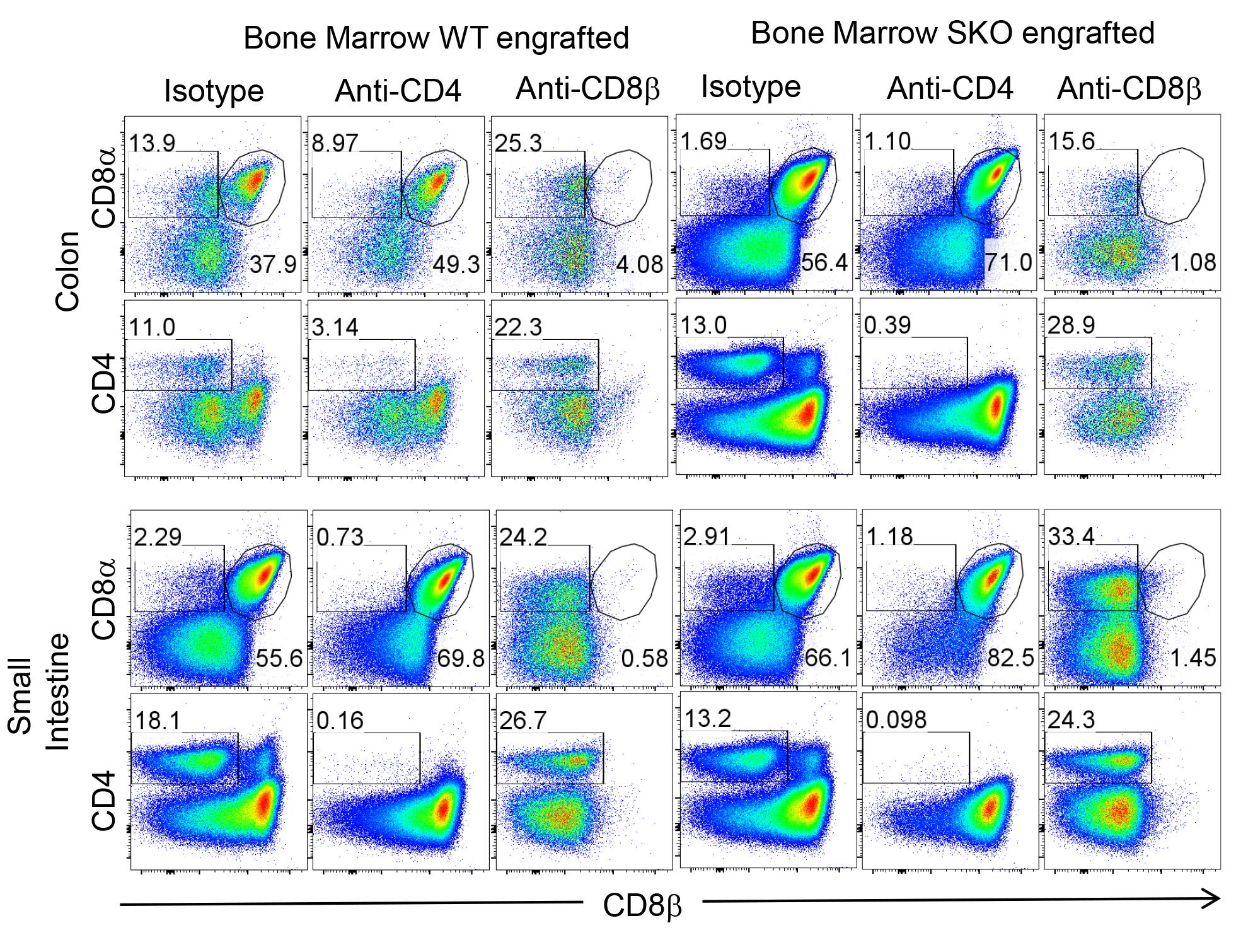
Figure 4. Validation of specific deletion within the colon and small intestine. Representative flow cytometry plots showing the frequency of CD4+ and CD8αβ+ T cells and CD8αα+ T cells within the colon and small intestine of irradiated RAG2KO mice reconstituted with WT or SMAD4-deficient bone marrow cells and injected with isotype or anti-CD8β (clone 53-5.8) or anti-CD4 (clone GK1.5) depleting antibodies.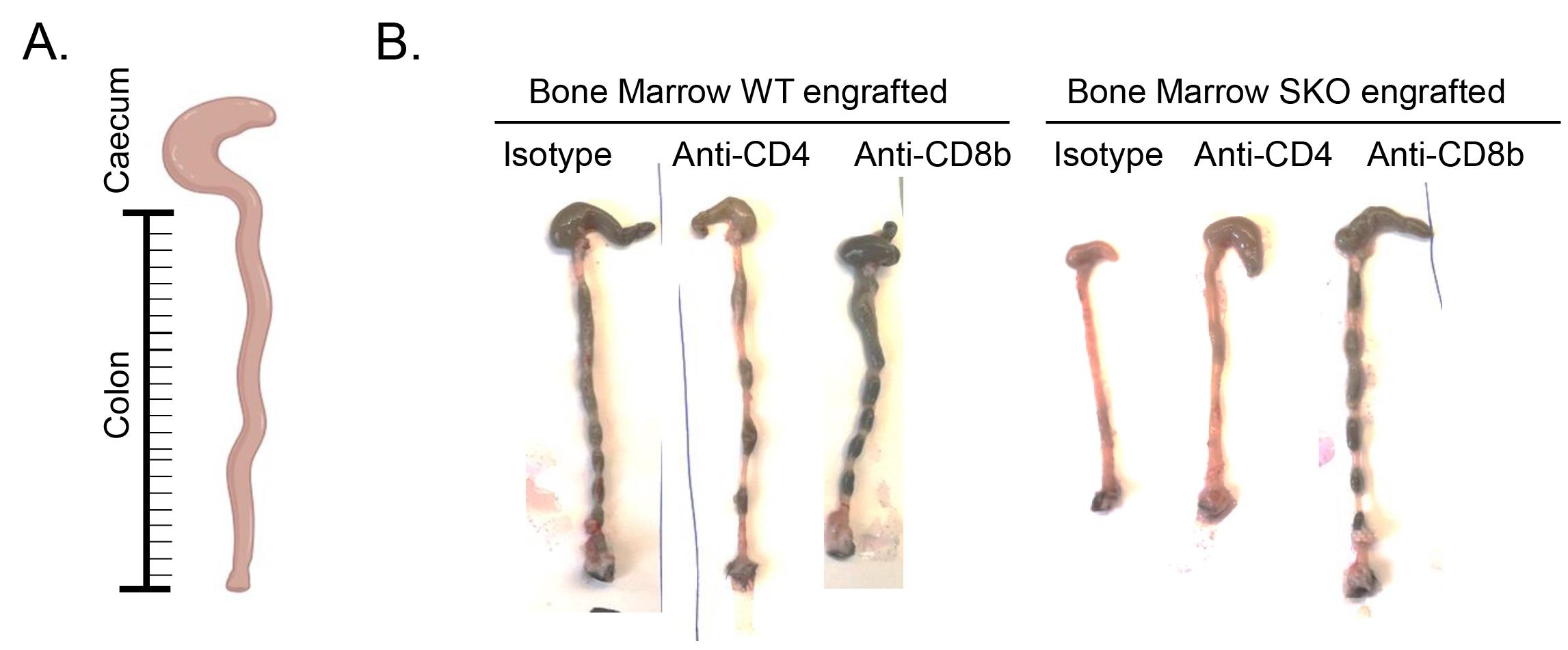
Figure 5. Analysis of gut inflammation in bone marrow–engrafted mice: colon length. Photo of the colon of irradiated RAG2KO mice reconstituted with WT or SMAD4-deficient bone marrow cells and injected with isotype or anti-CD8β or anti-CD4 depleting antibodies. The reduction of the size of the colon is a sign of a severe chronic gut inflammation.Histological assessment of inflammation can also be performed as proposed by Erben et al. (2014). Briefly, the colon and small intestine were treated with 2% formaldehyde (VWR) for fixation, followed by embedding in paraffin and sectioning. Tissues sections were subjected to hematoxylin and eosin staining. We evaluated intestinal inflammation in a blinded manner using a systematic scoring system based on the following criteria: colon length, extent and distribution of inflammatory cell infiltration, crypt hyperplasia, presence of neutrophils within crypts, occurrence of crypt abscesses, erosion, formation of granulation tissues, and villous blunting. Please refer to Igalouzene et al. (2022) to see some examples of images and Erben et al. (2014) if you are eager to learn more on the procedure. In Igalouzene et al. (2022), histological examination demonstrated a reduction in immune cell infiltration and absence of hyperplasia and crypt abscesses in SKO BM/engrafted mice treated with anti-CD8β (Igalouzene et al., 2022, Figure 2D and 2E, and Supplemental Figure 4B).
To validate the effectiveness of specific T-cell depletion and assess inflammation levels, flow cytometry analysis can be performed. Use distinct antibody clones from those applied in the in vivo depletion process. Specifically, anti-CD8β (clone YTS156.7.7) and anti-CD4 (clone RM4.5) can be employed to label the cells. For the analysis of neutrophil infiltration, indicative of inflammation, use anti-Ly6g and anti-CD11b APC antibodies. Acquire the cells using a flow cytometry machine. Gate the events based on FSC-A and SSC-A, followed by gating on FSC-A and FSC-H to exclude doublets. Eliminate dead cells by gating on cells negative for LIVE/DEAD fixable staining. Then, focus on CD45+ cells, which correspond to immune cells. For instance, CD45+CD8αβ+ T cells represent CD8αβ+ T cells. Successful depletion of CD8αβ T cells is evidenced by the complete absence of CD8αβ T cells in mice treated with anti-CD8β (see Figure 2). Importantly, the elimination of other populations (TCRγδ+ and CD8aa+ T cells) is not observed, indicating the specificity of the depletion (Figure 4).
By using this protocol, we identified CD8αβ+ T cells as an important effector driving intestinal severe inflammation in SMAD4-deficient mice (Igalouzene et al., 2022), providing new insights into the role of T-cell populations in this complex disease. The depletion of conventional CD8αβ+ T cells alleviates the onset of inflammation (weight loss, colon length reduction) evidenced by the reduction of neutrophils (CD11b+Ly6g+) recruitment within the epithelium and the reduction in colon length (Figure 5–6) (see Igalouzene et al. 2022 for further details). Listed below are a few examples of how one can analyze the effect of T-cell depletion on inflammation; these examples are not exhaustive.
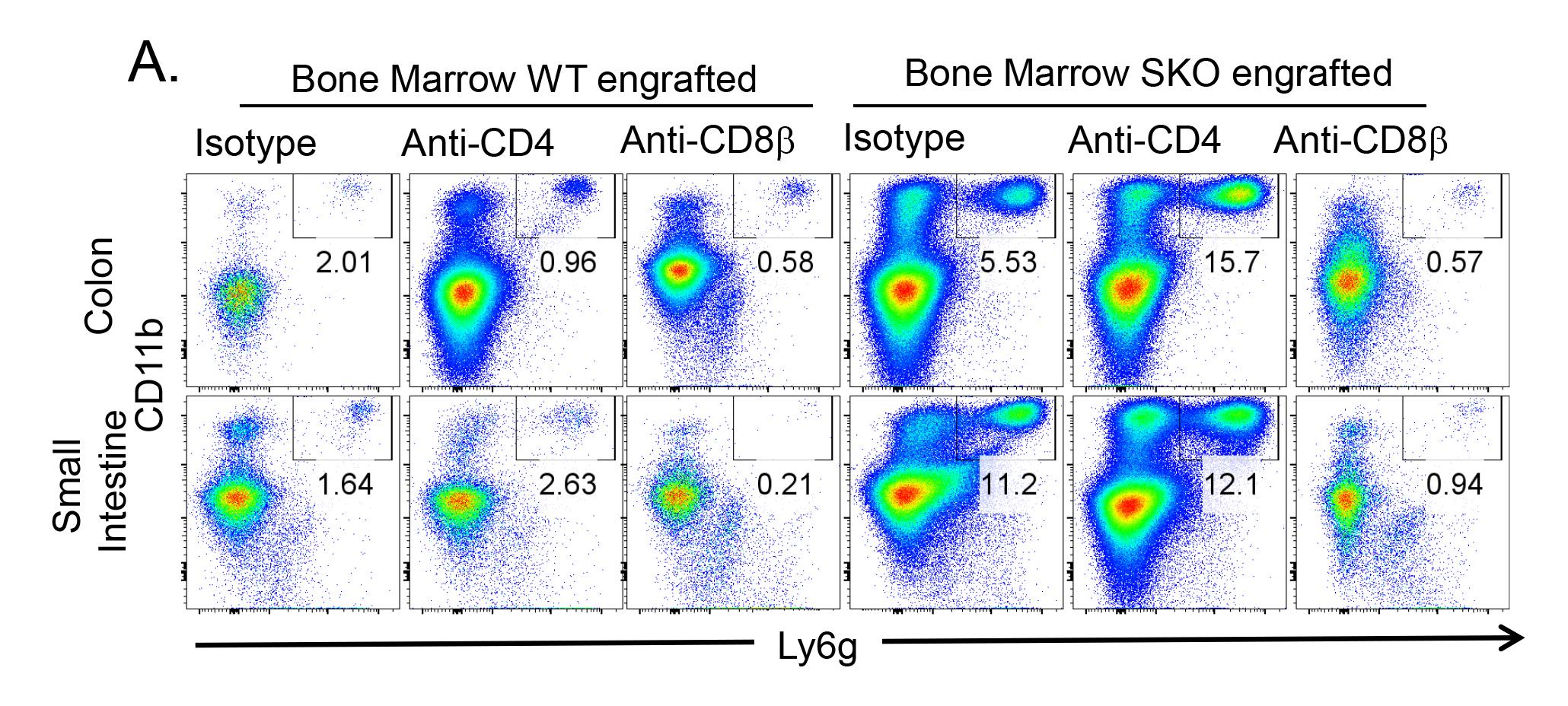
Figure 6. Analysis of gut inflammation in bone marrow–engrafted mice: neutrophil infiltration. Representative flow cytometry plots showing the frequency of neutrophils CD11b+Ly6g+ mobilized within the epithelium of the colon and the small intestine.
Validation of protocol
For representative data including weight loss, colon length, histological scoring, and cytometry, we refer readers to Igalouzene et al., (2022), Figures 2 and 8 as well as Supplemental Figure 2, 3, and 4.
General notes and troubleshooting
General notes
All solutions, media, materials, and buffers should be sterile and prepared according to standard protocols. It is essential to perform the isolation procedure under sterile conditions to avoid contamination. Additionally, work fast and always keep buffers and cell suspensions at 4 °C to prevent cell death.
The use of a bone marrow–engrafted mouse model accelerates the intestinal pathologies, allowing researchers to address questions without the need of long-term treatments. This approach significantly minimizes potential side effects associated with prolonged antibody treatment, as the pathology emerges in short term; therefore, there is no need to treat the mice for a long period. Additionally, this facilitates the synchronization of the chronic inflammation development in mice of similar sex and age, thereby enhancing the precision and comparability in investigation.
The elimination of T cells from the donor bone marrow cells is performed to prevent the transfer of T cells that have been conditioned in the donor, potentially accelerating and exacerbating the pathology by bypassing the natural initiation of inflammation in the engrafted mice. Novel T cells will be generated from the stem cells present in the donor bone marrow cells within the reconstituted mice, thereby providing a more faithful natural recapitulation of the onset of inflammation.
Notably, for the precise depletion of conventional CD8αβ T cells while sparing other cell types that express the common marker CD8α, researchers can take advantage of using of CD8β depleting antibodies (clone: 53-5.8) as performed in this protocol, in contrast to other studies using CD8α depleting antibodies that deplete a lot of unspecific immune populations (Figure 4).
Acknowledgments
The protocol described here was adapted from our previously published work (Igalouzene et al., 2022). This work was supported by the Association pour la Recherche sur le Cancer (ARC) (PJA20181207928-PJA20151203509-ARCDOC42020070002550). We would like to thank the P-PAC animal platform for taking care of the mice and the flow cytometer platform of the CRCL.
Competing interests
The authors declare no competing financial interest.
Ethical considerations
The experiments were conducted in accordance with the guidelines for animal care of the European Union (ARRIVE) and were validated by the local Animal Ethic Evaluation Committee (CECCAPP) and the French Ministry of Research.
References
- Erben, U., Loddenkemper, C., Doerfel, K., Spieckermann, S., Haller, D., Heimesaat, M.M., Zeitz, M., Siegmund, B., and Ku¨hl, A.A. (2014). A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 7, 4557.
- Hahn, J. N., Falck, V. G. and Jirik, F. R. (2011). Smad4 deficiency in T cells leads to the Th17-associated development of premalignant gastroduodenal lesions in mice. J. Clin. Invest. 121(10): 4030–4042.
- Igalouzene, R., Hernandez-Vargas, H., Benech, N., Guyennon, A., Bauché, D., Barrachina, C., Dubois, E., Marie, J. C. and Soudja, S. M. (2022). SMAD4 TGF-β–independent function preconditions naive CD8+ T cells to prevent severe chronic intestinal inflammation. J. Clin. Invest. 132(8): e1172/jci151020.
- Kim, B. G., Li, C., Qiao, W., Mamura, M., Kasperczak, B., Anver, M., Wolfraim, L., Hong, S., Mushinski, E., Potter, M., et al. (2006). Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 441(7096): 1015–1019.
- Kim, E. R., and Chang, D. K. (2014). Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 20(29): 9872–9881.
- Monteleone, G., Kumberova, A., Croft, N. M., McKenzie, C., Steer, H. W. and MacDonald, T. T. (2001). Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J. Clin. Invest. 108(4): 601–609.
- Zhao, M., Gönczi, L., Lakatos, P.L., and Burisch, J. (2021). The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 1573–1587.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Aoudi, A., Labiad, O., Igalouzene, R., Mejri, O., Sanchez, M. and Soudja, S. (2024). Addressing the Role of Conventional CD8αβ+ T Cells and CD4+ T Cells in Intestinal Immunopathology Using a Bone Marrow–Engrafted Model. Bio-protocol 14(4): e4934. DOI: 10.21769/BioProtoc.4934.
Category
Immunology > Immune cell staining > Flow cytometry
Cell Biology > Cell Transplantation > Immune cell adoptive transfer
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









