- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Seed Collection in Temperate Trees—Clean, Fast, and Effective Extraction of Populus Seeds for Laboratory Use and Long-term Storage
Published: Vol 14, Iss 3, Feb 5, 2024 DOI: 10.21769/BioProtoc.4927 Views: 2030
Reviewed by: Samik BhattacharyaSimab KanwalTie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Dark Respiration Measurement from Arabidopsis Shoots
Jose P. Fonseca [...] Kirankumar S. Mysore
Oct 5, 2021 4178 Views
Proteoliposomes for Studying Lipid-protein Interactions in Membranes in vitro
Helmut Kirchhoff
Oct 20, 2021 3346 Views
A Novel Method for Measuring Mitochondrial Respiratory Parameters in Wheat Paleae (Paleae Superior) Using the XF24 Analyzer
Daniel Schniertshauer and Jörg Bergemann
Aug 5, 2023 1348 Views
Abstract
Seeds ensure the growth of a new generation of plants and are thus central to maintaining plant populations and ecosystem processes. Nevertheless, much remains to be learned about seed biology and responses of germinated seedlings to environmental challenges. Experiments aiming to close these knowledge gaps critically depend on the availability of healthy, viable seeds. Here, we report a protocol for the collection of seeds from plants in the genus Populus. This genus comprises trees with a wide distribution in temperate forests and with economic relevance, used as scientific models for perennial plants. As seed characteristics can vary drastically between taxonomic groups, protocols need to be tailored carefully. Our protocol takes the delicate nature of Populus seeds into account. It uses P. deltoides as an example and provides a template to optimize bulk seed extraction for other Populus species and plants with similar seed characteristics. The protocol is designed to only use items available in most labs and households and that can be sterilized easily. The unique characteristics of this protocol allow for the fast and effective extraction of high-quality seeds. Here, we report on seed collection, extraction, cleaning, storage, and viability tests. Moreover, extracted seeds are well suited for tissue culture and experiments under sterile conditions. Seed material obtained with this protocol can be used to further our understanding of tree seed biology, seedling performance under climate change, or diversity of forest genetic resources.
Key features
• Populus species produce seeds that are small, delicate, non-dormant, with plenty of seed hair. Collection of seed material needs to be timed properly.
• Processing, seed extraction, seed cleaning, and storage using simple, sterilizable laboratory and household items only. Obtained seeds are pure, high quality, close to 100% viability.
• Seeds work well in tissue culture and in experiments under sterile conditions.
• Extractability, speed, and seed germination were studied and confirmed for Populus deltoides as an example.
• Can also serve as template for bulk seed collection from other Populus species and plant groups that produce delicate seeds (with no or little modifications).
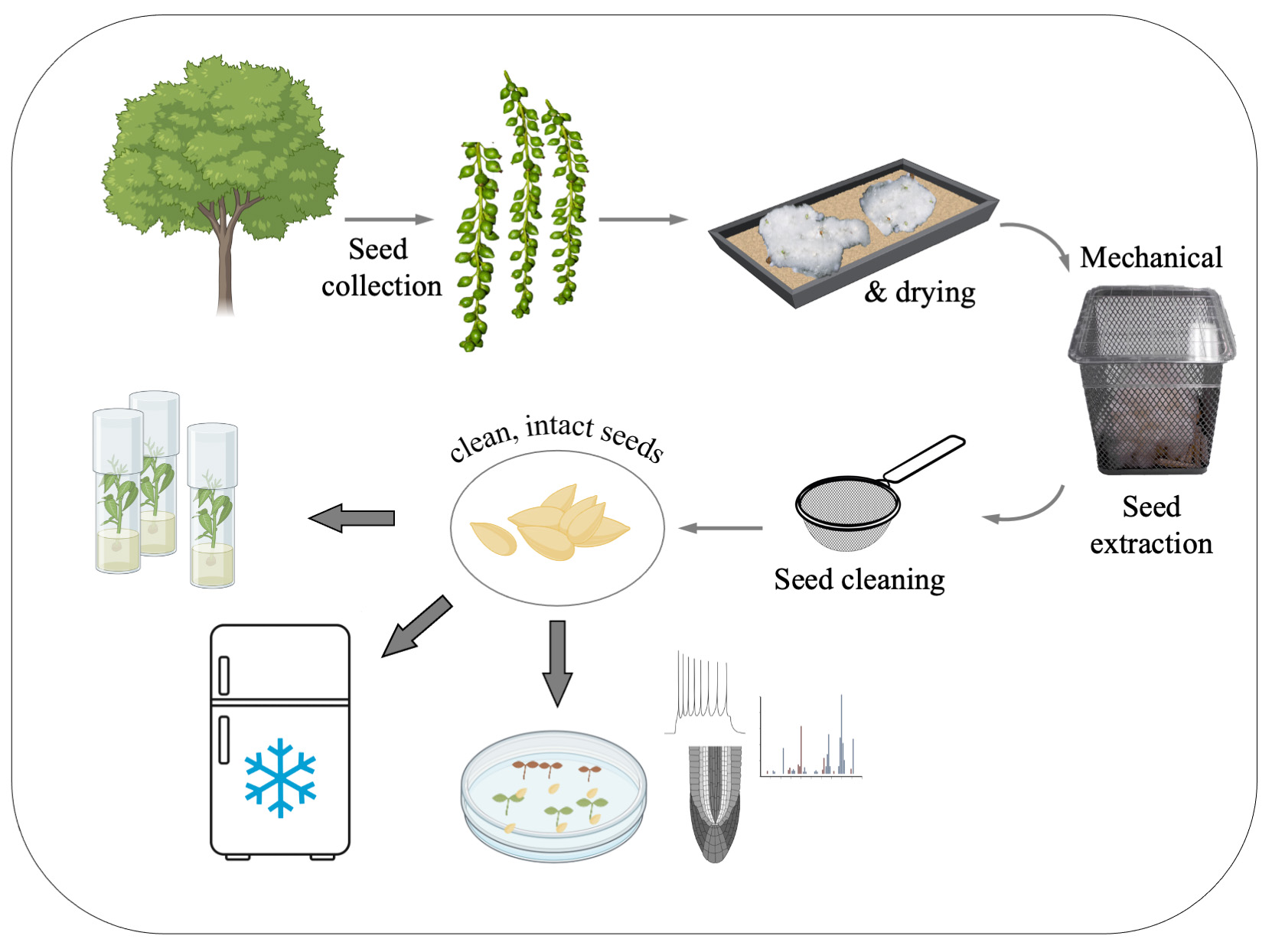
Graphical overview
Background
Seeds are an innovation of Spermatophyta (seed plants). They are specialized dispersal units that safely package the plant embryo to allow for dispersal in space and time. Seeds are critical for successful propagation of the individual and maintenance of populations within a species but also for terrestrial ecosystems including forest ecosystems. Seeds can vary drastically in their characteristics, such as size, mass, color, or durability [1]. The smallest orchid seeds, for example, weigh less than 1 μg, while the large seeds of the double coconut palm (Lodoicea maldivica) can reach up to 25 kg in weight [2–4]. Poplar seeds lose viability within a few days or weeks after release, while seeds of a date palm from an archeological site were reported to have germinated after 2,000 years [5,6]. This means that seed collection, handling, processing, and storage need to be tailored carefully to the species or taxonomic group of interest to ensure seed viability and successful plant growth from the seed. Here, we focus on the seeds of temperate forest trees of the genus Populus.
Despite their importance for healthy forests, much remains to be learned about seed traits and biology in forest trees, ranging from the molecular mechanisms of seed production or seed performance under climate change to applications in forest genetic resources preservation and long-term forest management [7–11].
Trees in the genus Populus are among the most widespread trees in North America, including some of the largest and fastest growing hardwoods in this large geographic area [5,12,13]. This taxonomically complex genus comprises poplars, cottonwoods, and aspens. They are early successional in natural plant communities but are also used widely in intensive culture for biomass, pulp, and paper production, for lumber, as shelterbelts or urban trees, or in land reclamation [5,13]. Populus species are dioecious, reach sexual maturity at 10–15 years of age, and produce seeds every year once matured. The number of seeds produced is remarkable: 28–54 million seeds have been reported for individual trees of Eastern cottonwood (P. deltoides) and European aspen (P. tremula) [5,14,15]. The seeds themselves are small and covered with a significant amount of hair (cotton or white fluff) that allows for easy wind dispersal. Seeds do not show dormancy, germinate quickly under suitable conditions, and can lose viability rapidly under natural conditions [5,16]. These characteristics impose challenges on seed collection, cleaning, and storage. Seed collection, therefore, needs to be timed precisely with the seasons and seed ripening on the trees. Moreover, extraction of seeds from the difficult-to-separate seed hair requires careful handling to prevent damage to the thin seed coat. Finally, seeds need to be stored properly to ensure longer lasting viability.
Agroforestry equipment such as seed macerators or grinders and fanning mills can be used to extract large numbers of Populus seeds [5,17]; however, seed viability declines strongly within two days, possibly due to damage of the delicate seed coat. Similarly, brushing against a coarse screen released seeds from the white cotton, but the extraction efficiency was low (20%), and seeds remained viable only for a few days [18]. Smaller quantities can be collected using a vacuum cleaner, and separation from cotton can be done in a nested set of soil sieves and applying a stream of air [19–21]. Based on this rationale, we established a method for seed extraction that leaves the thin seed coat intact and that yields very clean seeds, suitable for molecular analyses. The method is fast, simple, and effective. It uses household items that can be obtained easily, that are used in every lab, and importantly, that can be cleaned and sterilized easily. Storage of the extracted seeds was tested under different conditions. Germination tests confirmed high viability of seeds directly after extraction as well as after extended storage time. Growth in tissue culture without contamination furthermore confirmed purity of seeds.
Due to the high quality, intactness, and purity of seeds extracted with this protocol, these lend themselves well to analyses of seed responses under controlled conditions in tissue culture, also germinating well on soil and other substrates. The protocol was specifically developed for Populus to separate seeds from the attached tufts of hair but could equally be adjusted for the extraction of small, delicate seeds in other species that need separation from attached structures or impurities.
Materials and reagents
Biological materials
Female trees from the genus Populus that carry catkins with fruits that are near maturity (see Figure 1). Nearly mature catkins develop between late spring and earlier summer in temperate climates. The precise timing of seed maturation will depend on geographic location and local weather conditions.
The reproductive material used in this protocol was collected from Populus deltoides Bartr. ex Marsh. trees growing in Ontario, Canada in the Greater Toronto Area (GTA) and at the University of Toronto Mississauga campus. Details on location of the trees and on seed collection are given below in Table 1.
Table 1. Trees and material used in this study
Genotype (tree) Species Latitude Longitude Elevation (m) Collection date POP31 P. deltoides 43.6311319 -79.4715803 75 2023 POP32 P. deltoides 43.6310720 -79.4713711 75 2023 POP50 P. deltoides 43.6294418 -79.474405 70 2023 POP50 P. deltoides 43.6294418 -79.474405 70 2022 POP29 P. deltoides 43.5464906 -79.6599571 110 2021 POP19 P. deltoides 43.5461274 -79.6605435 114 2023
Reagents
Murashige & Skoog (MS) basal salt mixture (PhytoTech Labs, catalog number: M524)
Plant preservative mixture (PPM) (Plant Cell Technology, catalog number: PPM)
Agar (BioShop, catalog number: AGR003)
Potassium hydroxide (KOH) (BioShop, catalog number: PHY202)
Solutions
MS medium (100 mL) (see Recipes)
Recipes
MS medium (100 mL)
Adjust pH to 5.8 using 2.5 M KOH and autoclave. Cool medium to approximately 60 °C and add 200 μL of PPM (0.2% final concentration) immediately before pouring plates. The given volume yields four plates.
Reagent Final concentration Quantity or Volume MS 0.5× 0.2166 g Agar 0.7% (w/v) 0.7 g H2O n/a to 100 mL PPM 0.2% (v/v) 200 μL Total n/a 100 mL
Laboratory supplies
Petri dish, round, 100 mm × 15 mm (Fisherbrand, catalog number: 2071-FB0875712)
Petri dish, square, 100 mm × 15 mm (Fisherbrand, catalog number: 08-757-11A)
Borosilicate glass vial (VWR, catalog number: 66011-085)
50 mL conical centrifuge tube (FroggaBio, catalog number: TB50-500)
Splinter forceps (Almedic, catalog number: 7737-A10-600)
Micropore tape (3M Micropore, catalog number: 1533-0)
Household items
Paper lunch bags (1.88 L) (Canadian Tire, catalog number: 053-0200-8)
Plant propagation tray with lid (The Grow Depot, model: Mondi Propagation Tray 10 × 20)
Wire mesh pencil cup (10 cm × 8 cm × 8 cm); pencil cups can be obtained at any office supply store (e.g., Grant & Toy, Dollarstore)
Round bamboo skewer, 5 mm in diameter and cut into 2–2.5 cm pieces. Round bamboo sticks can be obtained at any retail or grocery store
Stainless steel fine mesh kitchen strainer (mesh size 30 and 40)
White paper sheets
Equipment
Micro bead sterilizer (Fisherbrand, catalog number: 14-955-342)
Laminar flow hood (Thermo Scientific, model: Heraguard ECO)
Growth chamber (Conviron, model: MTPS144)
Software and datasets
R Studio v 2023.03.0+386 [22]
R v4.3.x [23]
Procedure
Seed collection
Identify mature female trees and monitor reproductive development carefully.
Notes:
Trees in the genus Populus are dioecious. Copious amounts of seeds are produced on mature female trees (Figure 1A).
Flowers are arranged in flower clusters (catkins). After fertilization, catkins lengthen, and flowers develop into green fruit capsules carrying the developing and maturing seed (Figure 1B). Capsules split open when ripe and release seed with trichomes (cotton) for wind dispersal (Figures 1C and 1D).
Depending on geographic location and prevailing weather conditions, trees flower in spring, and seeds mature within 4–6 weeks [13,16].
Collect maturing catkins when the first few capsules begin to open (Figure 1C). Gently pick catkins from branches by breaking them off at the base. Store catkins in paper bags.
Notes:
If catkins are collected too early (Figure 1B), seeds will not mature properly, and the extracted seeds will not be viable. If catkins are left too long on the tree (Figure 1D), wind can disperse the whole seed crop in a very short period of time (e.g., an afternoon).
Therefore, close monitoring is required when catkins mature in late spring or early summer, ideally on a daily basis.

Figure 1. Tree habit and seed-bearing structures in Populus. A. Populus deltoides tree (eastern cottonwood) in late spring carrying female catkins (flower clusters). Each catkin comprises several capsules; each developed from an individual flower. B–D. P. deltoides catkins at different stages of capsule opening. B. Immature catkins with developing seeds fully enclosed in capsules. C. Catkin with first capsules split open upon gradual maturation, exposing trichomes and seeds for wind dispersal. D. All capsules on the catkin are fully open. They release copious amounts of seeds attached to tufts of long hair (cotton). Scale bars: 2 cm.When catkin collection is completed, bring material indoors and spread out in trays. Evenly distribute catkins in a single layer and avoid overlap of catkins to allow for rapid drying. Keep in a dry place. Drying in the lab, a general storage area, or a climatized office at room temperature (20–24 °C) works well. No special drying room is required in temperate climates.
Note: Without drying, the material can become moldy and seeds will not mature properly.
Cover trays very loosely with a lid to keep emerging cotton inside the tray while allowing for proper ventilation.
Note: It will take between one and seven days for seeds to shed, i.e., for cotton to emerge.
Seed extraction should be done as soon as all capsules are open and cotton emerges.
Note: Tufts of long cellulose-rich hair are attached to the small, light seeds to enable wind dispersal. The hair needs to be separated from the seeds without damaging the delicate seed coat.
Yield: Harvesting and processing 15–25 catkins will yield approximately 10,000 clean seeds (see also Validation section 2 of this protocol).
Note: Seed production per catkin can vary between individual trees (Figure 3).
Seed extraction—mechanical extraction by shaking
Disinfect the surface of the work area and cover it completely with clean sheets of paper. This will help later with gathering the extracted seeds.
Assemble the seed shaker (Figures 2A and 2B) by placing 10–15 fresh pieces of bamboo sticks inside an autoclaved mesh pencil cup.
Notes:
The added bamboo stick pieces facilitate the mechanical seed extraction.
Various objects of different shape, density, and material have been tested for use in mechanical seed extraction. These included pieces of wood, small aquarium rocks, steel balls, or bamboo sticks. Moreover, bamboo pieces of variable length have been tested. Pieces of bamboo sticks 2–2.5 cm in length yielded best results.

Figure 2. Materials required for seed extraction, cleaning, and storage. A. Our protocol was designed to use exclusively (i) simple and (ii) easy-to-clean items that are (iii) readily available in every household and lab. B. Our assembled seed shaker. Cotton from dried and mature catkins (seeds embedded in hair) is placed inside a mesh pencil cup and covered with the lid of a Petri dish. Added bamboo stick fragments aid with the mechanical extraction. Shaking easily releases seeds, some of which are shown around the cup. C. Intact, clean seeds are stored in glass vials. Scale bars: 2 cm.Transfer 0.5–1 g of cotton into the shaker and cover it with a Petri dish lid (Figure 2B).
Notes:
The cotton is the white fluff released from dry and mature catkins (Figure 1D, A.4), which comprises seeds and attached hair.
Avoid adding parts of the seed capsules or any other hard pieces from the catkin to the shaker.
Keep the fluff loose and avoid pressing it down. Compressed cotton will reduce the efficiency of seed extraction.
Adding more than 1 g of cotton will also reduce extraction efficiency and prolong the shaking and clean-up process.
Tilt the seed shaker (Figure 2B) to the side and shake vigorously by hand. Seeds will fall out of the shaker and onto the paper sheets. Seeds’ hair (white cotton with most seeds removed) will stay inside.
Continue shaking until only a few seeds are being extracted or until the trichomes inside the seed shaker form small, felt-like clumps.
Gather the extracted seeds from the paper sheets on the work surface, place them in a small container, and set them aside for subsequent cleaning steps.
Cover the work surface again with the paper sheets and remove the clumped trichomes from the mesh cup but leave bamboo sticks inside the pencil cup.
Repeat steps B3–B7 until all cotton from one genotype (tree) has been processed.
To avoid sample mix-up and cross-genotype contamination, process cotton from one genotype at a time. Sterilize mesh pencil cups before processing a new genotype, use a fresh set of bamboo stick pieces, and thoroughly clean the working area.
Seed cleaning
Place the extracted seeds into a fine mesh kitchen strainer and sieve the material in a gentle circular motion. Small debris will pass through the sieve.
Note: We tested several household items with different mesh sizes for suitability. A typical fine mesh kitchen strainer worked best. It retains Populus seeds but allows small debris to pass through.
Next, place seeds onto a single sheet of paper and manually remove larger pieces of debris such as residual trichomes or fragments of dry capsules with tweezers.
Some very small pieces of debris can be static and will adhere to the seed surface. These are removed by transferring seeds from one sheet of paper to another. Small static debris stays behind and remains on the sheet of paper. Repeat two or three times.
Transfer the seeds into a clean glass vial and store at -20 °C.
Notes: Seed storage conditions are critical.
The seeds have a delicate seed coat, hardly contain endosperm, and do not exhibit dormancy.
Fresh mature Populus seeds germinate rapidly under favorable conditions, but seed quality deteriorates quickly within weeks when released from the capsule under natural conditions or when seeds are stored at room temperature [17,18,24–26].
Based on seed storage characteristics, Populus seeds are considered suborthodox, and dry mature seeds can be kept at cold or subfreezing temperatures for prolonged preservation of seed viability [27].
Seeds extracted with our protocol that were stored immediately at -20 °C retain high viability for weeks and years (see Validation section 5). They had a seed moisture content (SMC) of approximately 10%, which is comparable to reported SMC values for mature, dry Populus seeds [27].
Germination test—to assess seed quality
Prepare MS plates as detailed under Recipes. Work in a laminar flow hood and allow plates to solidify and dry.
Notes:
Poplar seeds can be damaged easily by surface sterilization. Instead of surface sterilization, 0.2% PPM (final concentration) is added to the medium to prevent microbial contamination.
Alternatively, moist filter paper can be used for germination tests.
Set aside at least 100 seeds per condition for the germination test.
Sterilize tweezers using a micro bead sterilizer and let it cool down prior to handling seeds. Place seeds on a plate using sterilized tweezers and seal plates with micropore tape. Here, we used 50 seeds per plate and included at least two replicates. Work under a laminar flow hood.
Place plated seeds in a climate-controlled chamber under long-day conditions (16:8 h light/dark and 21/19 °C day/night).
Count germinated seeds three days after plating and calculate germination efficiency as number of germinated seeds per total seeds plated multiplied by 100.
Notes:
A seed is considered germinated when the radicle (embryonic root) has penetrated the seed coat.
Seed lots collected in previous years were included to study the effect of longer-term seed storage.
Plant growth and survival after germination was further recorded (see Validation section 5).
Data analysis
All data presented in the validation section were visualized using R and R Studio.
Validation of protocol
Selection of biological material
To test our seed extraction protocol, we selected the eastern cottonwood (Populus deltoides Bartr. ex Marsh.), a typical species from the genus Populus that is native to eastern North America. P. deltoides plants grow quickly into large trees on moist, well-drained sites, provide ecosystem services, and are used in commercial forestry and urban environments. Female mature trees were identified and closely monitored for seed development and maturation during spring and early summer between May and June of 2023 (Figure 1).
Characterization of reproductive structures and seed production in P. deltoides
To assess our seed extraction protocol for effectiveness, we first aimed to characterize reproductive structures and seed production in P. deltoides by studying three trees from our local study population in detail (Table 1, Materials and Reagents section). Seeds develop in capsules on female catkins. We counted the number of seeds per capsule and the number of capsules per catkin and determined the number of seeds per entire catkin (Figure 3). This was done by manually picking and individually counting all seeds that had developed inside a capsule, for each capsule on each of the catkins studied.
On average, we counted 13, 14, and 19 seeds per capsule for each of the three genotypes, with minimum and maximum values for seeds per individual capsule ranging from 1 to 38 (Figure 3A). Note that not every ovule will eventually produce a mature seed, leading to variable numbers of seeds per capsule. We further observed on average 41, 44, and 40 capsules per catkin, depending on genotype (Figure 3B). This amounted to approximately 516, 631, and 768 seeds per catkin on average for each of the three genotypes, with extreme values for individual catkins ranging from 391 to 921 seeds at maturity (Figure 3C). For comparison, Bessey [14] reported 32 seeds per capsule and 27 capsules per catkin for a single mature P. deltoides tree. This would amount 864 seeds per catkin. Similarly, our observations are well within the range described for P. deltoides in the Flora of North America [28]. The characterization of our material (Figure 3) provides now an exact reference point for our seed extraction protocol.
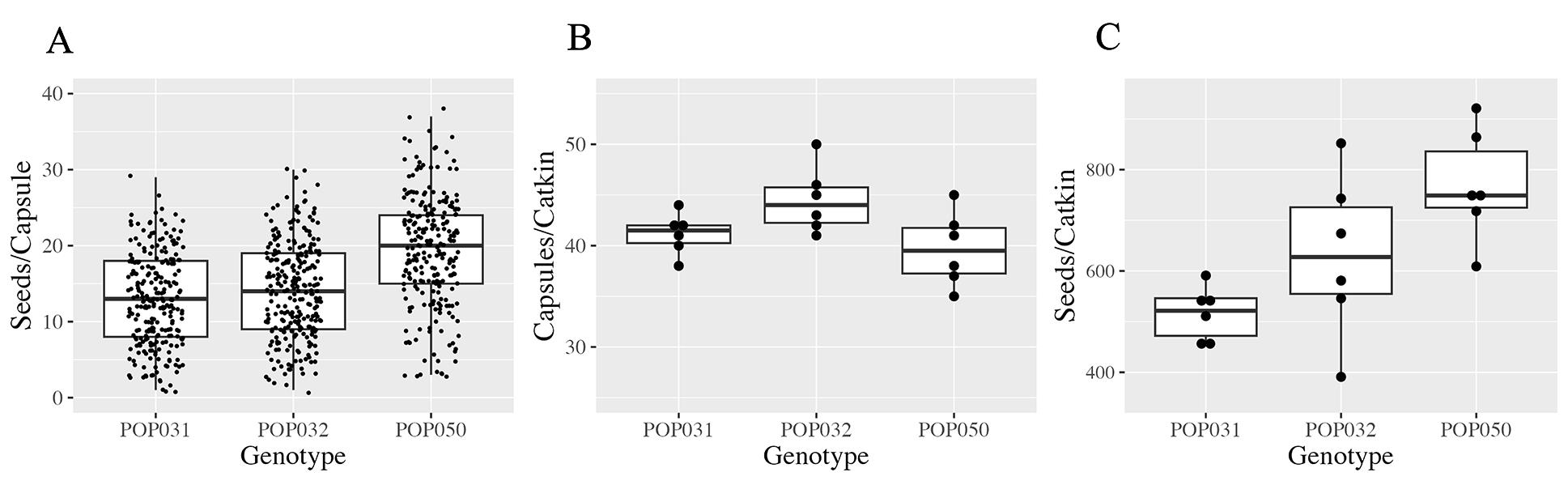
Figure 3. Characterization of seed production in eastern cottonwood (P. deltoides). Trees in the genus Populus produce catkins carrying several capsules and each capsule contains multiple seeds. The reproductive material was characterized thoroughly by manual counting. A. Number of seeds per individual capsule. B. Capsules per catkin. C. Seeds per entire catkin. Data is shown for three different genotypes (trees) and six randomly selected catkins per tree. Each dot represents an individual data point. Within each box in the boxplot, horizontal lines indicate the median, and boxes extend from the 25th to 75th percentile (i.e., the 1st and 3rd quartile). Whiskers represent 1.5 times the interquartile range between the 25th and 75th percentile.Seeds are extracted effectively from P. deltoides reproductive structures using our protocol
Not all produced seeds can be extracted easily. Maisenhelder [18] reports, for example, that only 20% of eastern cottonwood seeds were readily extractable from seed-containing structures in Populus using the method described in his publication. Despite its potential relevance, effectiveness of extraction procedures for Populus seeds are, however, rarely reported.
We randomly assigned catkins to either manual seed counting (see also Figure 3) or mechanical extraction by shaking as described herein. Shaking allowed to extract on average 402, 591, and 635 seeds per catkin for the three genotypes studied (Figure 4, left panel). This means that between 78% and 83%–94% of all the seeds per catkin were extracted easily by shaking from the genotypes studied. Across all genotypes, 85% of the seeds present in a catkin were extracted easily with our protocol (Figure 4, right panel).
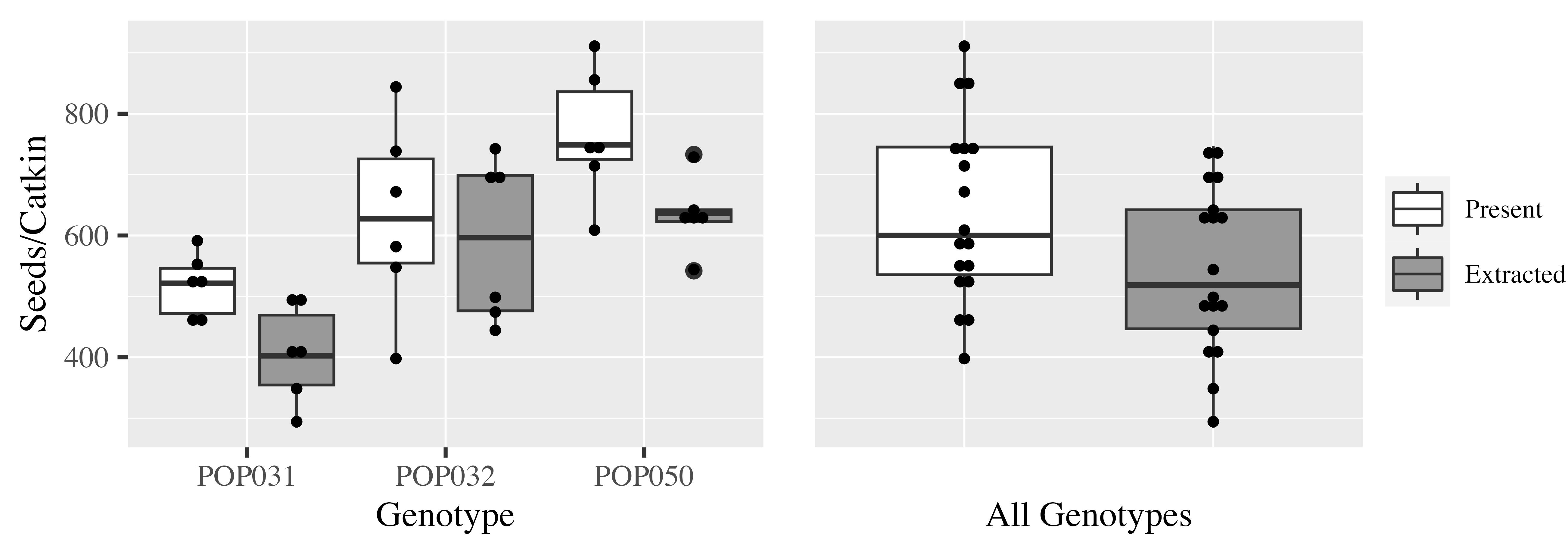
Figure 4. Extraction of seeds from P. deltoides reproductive structures using our protocol. The protocol presented herein allows to extract clean and healthy seeds effectively from P. deltoides cotton (dark grey, label: “Extracted”). For comparison, the total number of seeds that is present in the reproductive material was determined by manual handpicking and counting (white, label: “Present”). Catkins were used as reference unit for reproductive material. Data is given for three different genotypes (trees) and six randomly selected catkins per tree and extraction method (left panel). The right plot summarizes the data across the different genotypes.Clean seeds are extracted quickly and efficiently from P. deltoides cotton
Time requirements are also of practical relevance. Therefore, we recorded the time spent on seed extraction and seed cleaning when using our protocol. This was compared with the time needed for manual seed picking. We selected one genotype, POP50, and documented extraction times in parallel for several lab members with different levels of work experience (undergraduate, graduate, research scientist). Very clean seeds were obtained, at a rate of 36 seeds per minute on average when using our protocol compared to only five seeds per minute, approximately, when seeds were picked manually (Figure 5).
When testing different materials for our manual shaker (Figure 2B), we observed that the shape of the mesh pencil holder had a clear influence on both seed yield and seed extraction speed. Round cups were less efficient than square mesh pencil cups. Therefore, we proceeded with square cups and recommend them for future work.
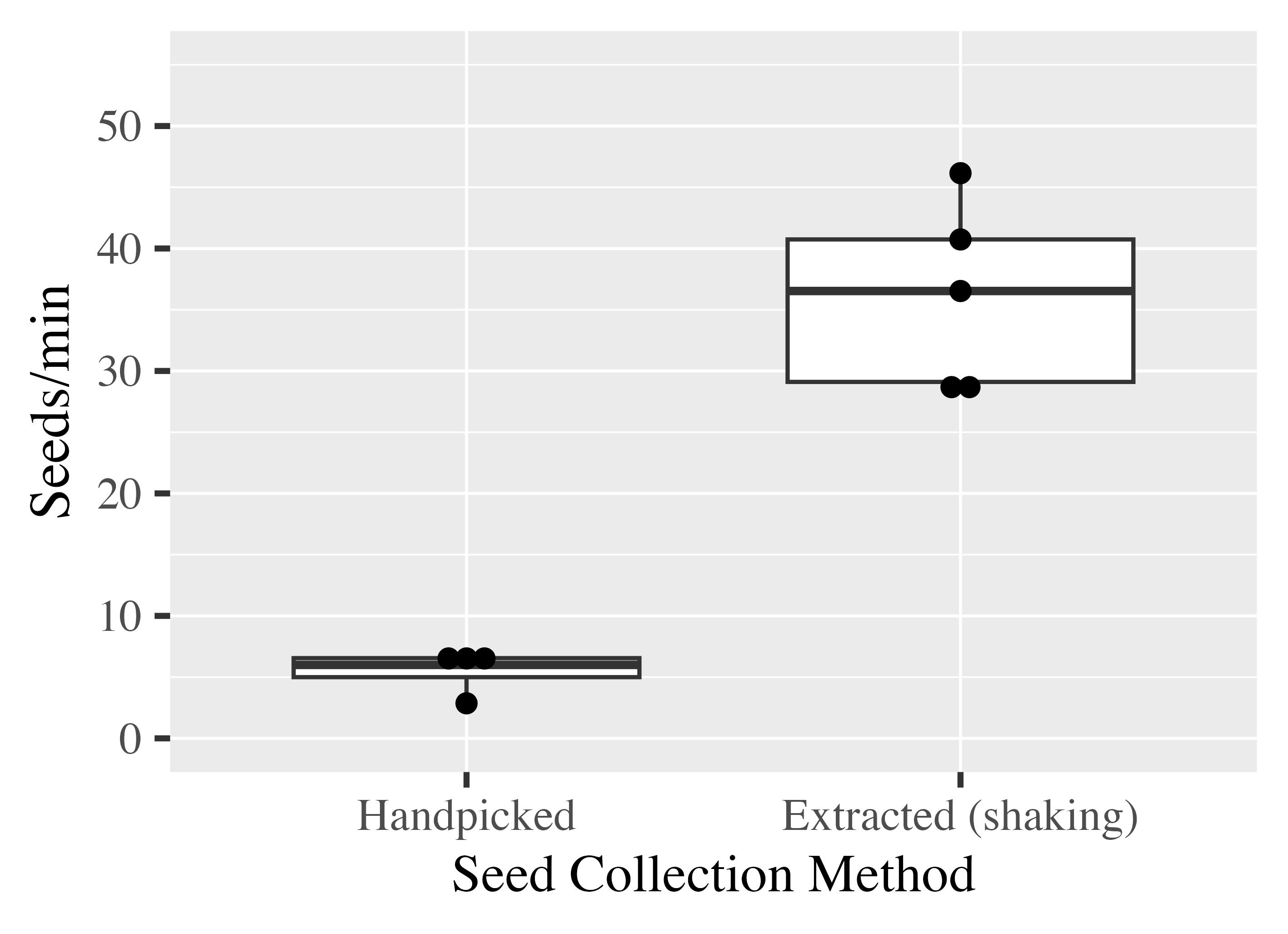
Figure 5. Clean seeds are extracted quickly from P. deltoides cotton. Seed extraction was timed for our extraction protocol and for handpicking using cotton from genotype POP50. Four to five extractions each were timed. The timing included both extraction and cleaning steps.Extracted seeds are viable and suitable for tissue culture and long-term storage
Seeds need to be viable for assays and downstream experiments. To assess seed quality, viability was tested in a standard germination test. Populus seeds do not enter dormancy and germinate rapidly under favorable conditions, often within 24 h. However, seed longevity is limited in nature [5].
Seeds extracted with our protocol were subjected to two different storage conditions and tested at different time intervals (Figure 6). Fresh seeds that were tested directly after extraction and cleaning exhibited a high germination efficiency of 98% (Figure 6B), and the emerging seedlings were healthy and strong (Figure 6A). Viability declined by 5% when seeds were stored at room temperature for 15 days (93% germination efficiency), and the trend continued. After 30 days of seed storage at room temperature, germination efficiency dropped to 84% (Figure 6A and 6B). In contrast, seeds stored at -20 °C for the same period of time (30 days) retained a high germination efficiency of 94%, and seeds germinated vigorously (Figure 6A and 6B). Longer-term effects on seed viability were only studied for -20 °C storage conditions (Figure 6C and 6D). After one year of seed storage at subfreezing temperatures, seeds retained viability and germinated with a high efficiency of 95%. A germination efficiency of 79% was still observed after two years of storage at -20 °C, although for a different tree. It should also be noted that, after successful germination, almost all plants survived, continued to grow well, and established healthy plants (Figure 7), further highlighting seed quality and seed health.
The germination data confirm that the seeds extracted with our protocol are, despite of their delicate nature, intact and viable and germinate vigorously. They are very clean and suitable for plating, tissue culture, and experiments under sterile conditions. Seeds lose viability quickly under ambient conditions and should not be stored at room temperature. Storage at -20 °C, however, retains viability well for weeks, months, and years.
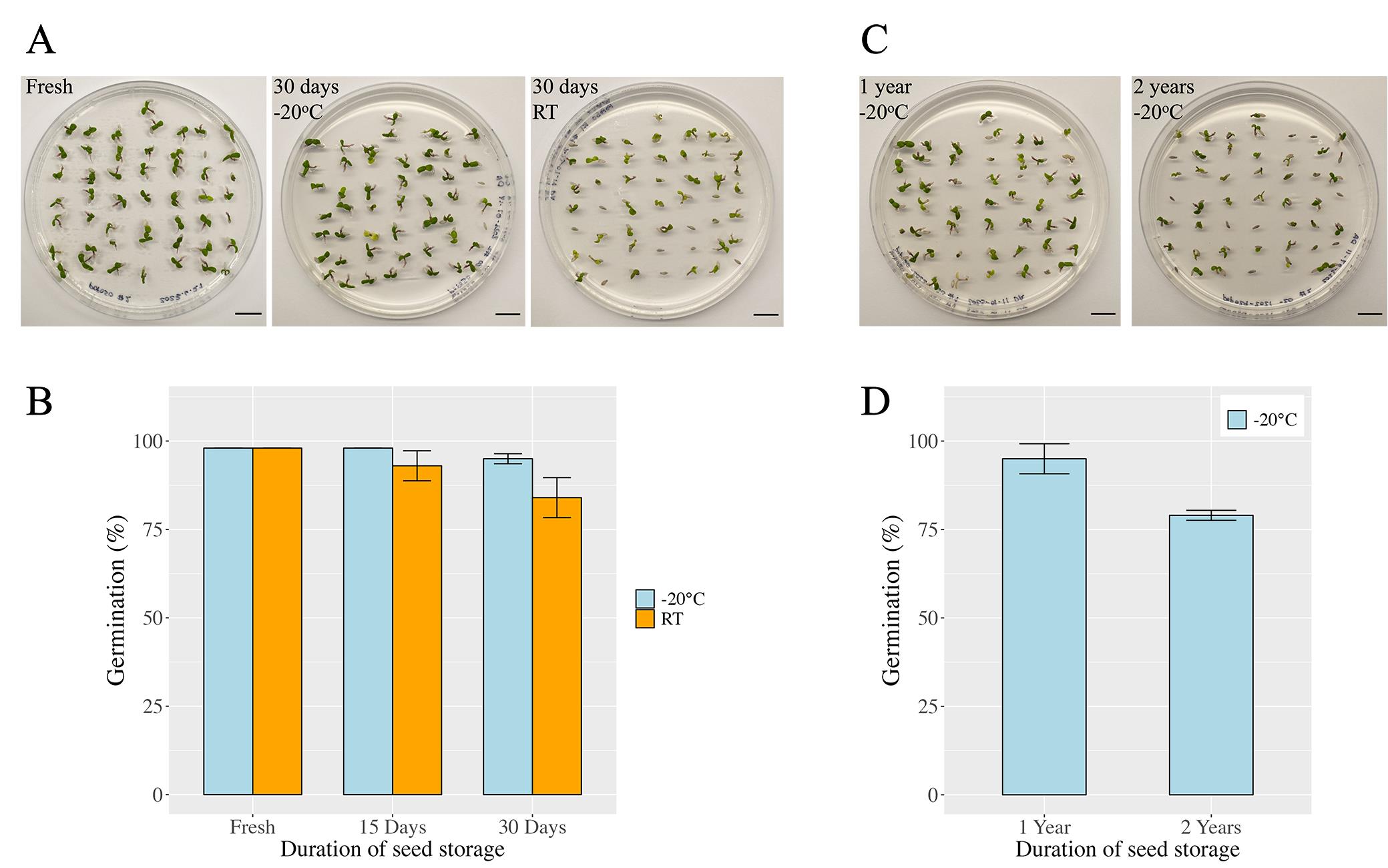
Figure 6. Viability of extracted P. deltoides seeds and effect of storage conditions. Seeds extracted with our protocol were subjected to standard germination tests. A. Images of representative plates with germinated cottonwood seedlings. Seeds were stored for 0, 15, and 30 days at either -20 °C or room temperature (RT). B. Germination efficiency is shown for the conditions described in A. C, D. Seeds collected in previous years were tested after storage at -20 °C over longer periods of time: one and two years. Again, typical plates with germinated seedlings as well as germination efficiencies are given. A–D. Seeds were plated on MS medium, and germination (radicle emergence) was scored three days after plating. Fifty seeds per plate were prepared in duplicate for the germination test. All data represent genotype POP50, except for the seeds that were stored for two years (POP29). Of note: fresh seeds and seeds stored at -20 °C for 15 days gave identical germination values across replicates. Thus, error bars are not visible for these conditions.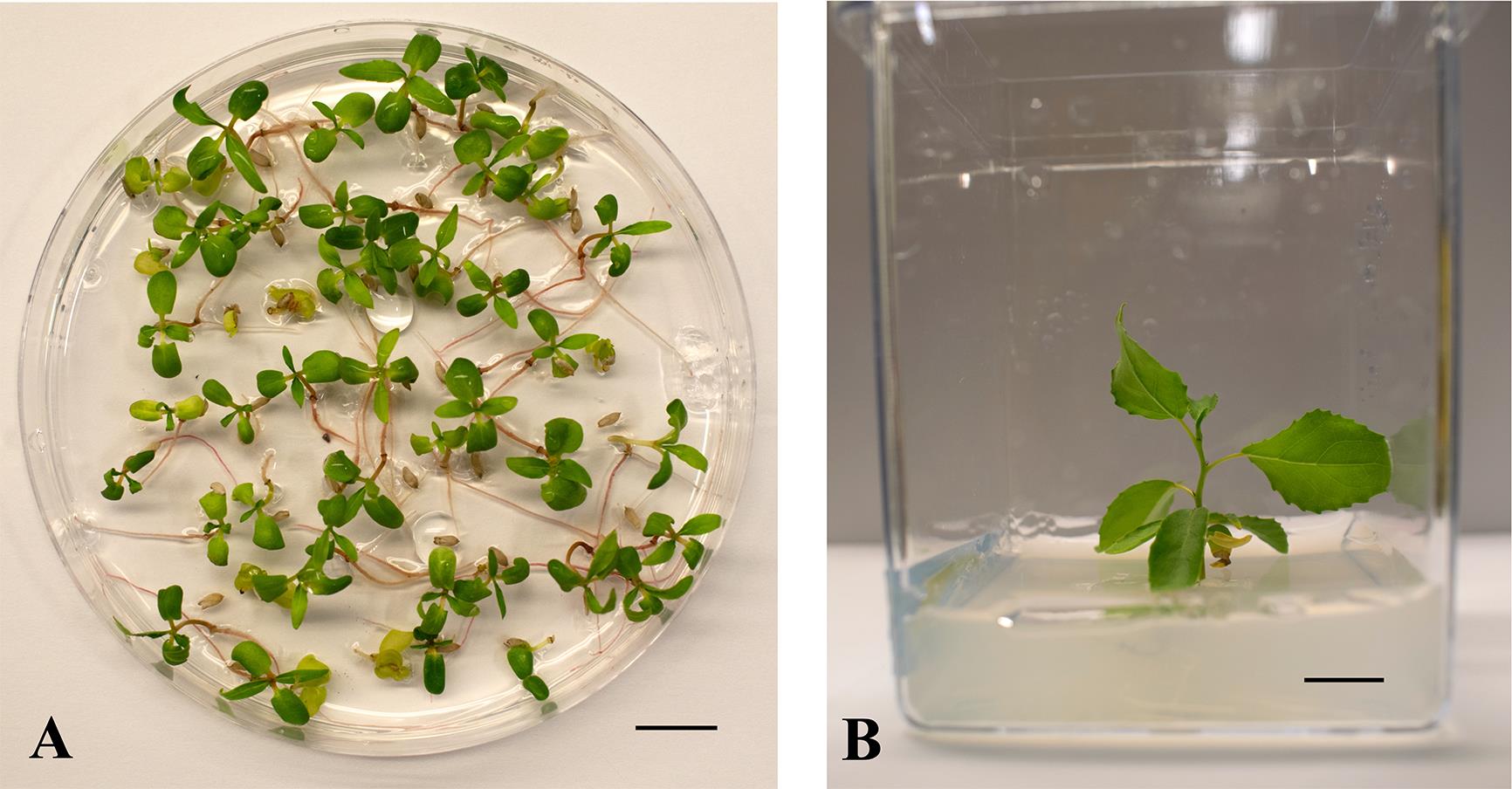
Figure 7. Populus deltoides plants continue to grow well after successful germination. Following a standard germination test, plants show healthy seedling growth on plates (A) and in tissue culture (B). A. Two-week-old plants, genotype POP50. B. Eight-week-old plant, genotype POP19. Scale bar: 1 cm.
Concluding remarks
Most plant life starts with a seed. Here, we describe a protocol for the collection and storage of seeds for Populus trees. This genus is of economic and ecological importance and serves as scientific model for deciduous trees. Seeds are delicate, short-lived, and attached to dense tufts of hair, needing to be separated and processed quickly. Our protocol describes a fast and effective way of collecting, extracting, and cleaning these seeds using simple laboratory and household items only. All equipment used in this protocol can be cleaned and sterilized easily. Seed quality and, critically, storage conditions have been tested. Germination tests confirm lasting quality of the obtained seeds for weeks, months, and up to years of storage. The obtained seeds are very clean and suitable for tissue culture and experiments under sterile conditions.
Acknowledgments
We thank Dana Al Refai and Stefan Heinen for their contributions to the seed counting data and seed plating. This work was generously supported by competitive funding awarded to KB from the National Sciences and Engineering Research Council of Canada (NSERC) (RGPIN-2017-06552), the Canadian Foundation of Innovation (CFI, 36678) and the University of Toronto (RSAF).
Competing interests
The authors declare no competing interests.
Ethical considerations
Ethics approval was not required for this study.
References
- Linkies, A., Graeber, K., Knight, C. and Leubner‐Metzger, G. (2010). The evolution of seeds. New Phytol. 186(4): 817–831. doi: 10.1111/j.1469-8137.2010.03249.x
- Arditti, J. and Ghani, A. K. A. (2000). Tansley Review No. 110. New Phytol. 145(3): 367–421. doi: 10.1046/j.1469-8137.2000.00587.x
- Tomlinson, P. B. (2006). The uniqueness of palms. Bot. J. Linn. Soc. 151(1): 5–14. doi: 10.1111/j.1095-8339.2006.00520.x
- Bellot, S., Bayton, R. P., Couvreur, T. L. P., Dodsworth, S., Eiserhardt, W. L., Guignard, M. S., Pritchard, H. W., Roberts, L., Toorop, P. E., Baker, W. J., et al. (2020). On the origin of giant seeds: the macroevolution of the double coconut (Lodoicea maldivica) and its relatives (Borasseae, Arecaceae). New Phytol. 228(3): 1134–1148. doi: 10.1111/nph.16750
- Wyckoff, G. W. and Zasada, J. C. (2008). Populus L. In: Bonner, F. T. and Karrfalt, R. P. (Eds.). The Woody Plant Seed Manual (Vol. 727, pp. 856–871). USDA Forest Service.
- Sallon, S., Cherif, E., Chabrillange, N., Solowey, E., Gros-Balthazard, M., Ivorra, S., Terral, J. F., Egli, M. and Aberlenc, F. (2020). Origins and insights into the historic Judean date palm based on genetic analysis of germinated ancient seeds and morphometric studies. Sci. Adv. 6(6): eaax0384. doi: 10.1126/sciadv.aax0384
- van der Meer, P., Jorritsma, I. and Kramer, K. (2002). Assessing climate change effects on long-term forest development: adjusting growth, phenology, and seed production in a gap model. For. Ecol. Manage. 162(1): 39–52. doi: 10.1016/s0378-1127(02)00049-x
- McKenney, D., Pedlar, J. and O’Neill, G. (2009). Climate change and forest seed zones: Past trends, future prospects and challenges to ponder. For. Chron. 85(2): 258–266. doi: 10.5558/tfc85258-2
- Havens, K., Vitt, P., Still, S., Kramer, A. T., Fant, J. B. and Schatz, K. (2015). Seed Sourcing for Restoration in an Era of Climate Change. Nat. Areas J. 35(1): 122–133. doi: 10.3375/043.035.0116
- Kijowska-Oberc, J., Staszak, A. M. and Ratajczak, E. (2021). Climate change affects seed aging? Initiation mechanism and consequences of loss of forest tree seed viability. Trees 35(4): 1099–1108. doi: 10.1007/s00468-020-02072-w
- Badano, E. I. and Sánchez-Montes de Oca, E. J. (2022). Seed fate, seedling establishment and the role of propagule size in forest regeneration under climate change conditions. For. Ecol. Manage. 503: 119776. doi: 10.1016/j.foreco.2021.119776
- Van Haverbeke, D. F. (1990). Populus deltoides. In: Burns, R. M. and Honkala, B. (Eds.). Silvics of North America (pp. 530–535). USDA Forest Service.
- Dickmann, D. I., Isebrands, J. G., Eckenwalder, J. E. and Richardson, J. (Eds.). (2001). Poplar culture in North America. NRC Research Press.
- Bessey, C. E. (1904). The number and weight of cottonwood seeds. Science 20(499): 118–119.
- Reim, P. (1929). Die Vermehrungsbiologie der Aspe auf Grundlage des in Estland und Finnland gesammelten Untersuchungsmaterial [Rudolf PE, trans. 1935. Regeneration biology of aspen on the basis of data colleted in Estonia nd Finland. Silvic. Transl. 220]. USDA Forest Service.
- Barsoum, N. (2001). Regeneration—Requirements and promotion measures. In: Lefèvre, F., Barsoum, N., Heinze, B., Kajba, D., Rotach, P., de Vries, S. M. G. and Turok, J. (Eds.). In situ conservation of Populus nigra (pp. 16–52). International Plant Genetic Resource Institute.
- Engstrom, A. (1948). Growing Cottonwood from Seed. J. For. 46(2): 130–132.
- Maisenhelder, L. C. (1951). Planting and growing cottonwood on bottomlands. Bull. 485. Mississippi Agricultural Experiment Station. 23p
- Einspahr, D. and Schlafke, D. (1957). A method of aspen and cottonwood seed extraction. Tree Planters’ Notes. 28: 10.
- Fung, M. Y. P. and Hamel, B. A. (1993). Aspen Seed Collection and Extraction. Tree Planters’ Notes. 44(3): 98–100.
- Roe, E. I. and McCain, D. P. (1962). A quick method of collectiong and cleaning aspen seeds. Tree Planters’ Notes. 51: 17–18.
- RStudio Team. (2019). RStudio: Integrated Development of R [Computer software]. Rstudio, Inc. http://www.rstudio.com/
- R Core Team. (2021). R: A language and environment for statistical computing [Computer software]. R Foundation for Statistical Computing. https://www.R-project.org/
- Hellum, A. K. (1973). Seed storage and germination of black poplar. Can. J. Plant. Sci. 53(1): 227–228. doi: 10.4141/cjps73-040
- Hardin, E. D. (1984). Variation in Seed Weight, Number per Capsule and Germination in Populus deltoides Bartr. Trees in Southeastern Ohio. Am. Midl. Nat. 112(1): 29. doi: 10.2307/2425453
- Kim, D. H. (2018). Extending Populus seed longevity by controlling seed moisture content and temperature. PLoS One 13(8): e0203080. doi: 10.1371/journal.pone.0203080
- Bonner, F. T. (2008). Storage of Seeds. In: Bonner, F. T. and Karrfalt, R. P. (Eds.). The Woody Plant Seed Manual (Vol. 727, pp. 86–95). USDA Forest Service.
- Argus, G. W., Eckenwalder, J. E. and Kiger, R. W. (2010). Salicaceae. In: Flora of North America Editorial Committee (Ed.), Flora of North America of Mexico (Vol. 7, pp. 3–164).
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Bhutta, N., Nunez-Martinez, O. F., Mei, C. and Bräutigam, K. (2024). Seed Collection in Temperate Trees—Clean, Fast, and Effective Extraction of Populus Seeds for Laboratory Use and Long-term Storage. Bio-protocol 14(3): e4927. DOI: 10.21769/BioProtoc.4927.
Category
Plant Science > Plant breeding > Seed storage
Plant Science > Plant physiology > Metabolism
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link







