- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Enrichment of Major Primary Neuroglial Cells from Neonatal Mouse Brain
(*contributed equally to this work) Published: Vol 14, Iss 2, Jan 20, 2024 DOI: 10.21769/BioProtoc.4921 Views: 4134
Reviewed by: Xi FengAchira RoyNafisa M. Jadavji

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Hanki Kim [...] Jun Young Choi
Jun 20, 2025 1353 Views
Isolation and Characterization of Cervical Cancer-Associated Mesenchymal Stem Cells From Primary Tumors Using Explant Culture
Surbhi Singla [...] Shalmoli Bhattacharyya
Jun 20, 2025 2697 Views
Generation of Intestinal Epithelial Monolayers From Single-Cell Dissociated Organoids
Neta Felsenthal and Danijela Matic Vignjevic
Oct 5, 2025 2220 Views
Abstract
The central nervous system (CNS) relies on the complex interaction of neuroglial cells to carry out vital physiological functions. To comprehensively understand the structural and functional interplay between these neuroglial cells, it is essential to establish an appropriate in vitro system that can be utilized for thorough investigation. Traditional protocols for establishing primary neuronal and mixed glial cultures from prenatal mice or neural stem cells require sacrificing pregnant mice and have the drawback of yielding only specific types of cells. Our current protocol overcomes these drawbacks by utilizing the brain from day-0 pups to isolate CNS resident neuroglial cells including astrocytes, microglia, oligodendrocytes [oligodendrocyte precursor cells (OPCs) and differentiated oligodendrocytes], and meningeal fibroblasts, as well as hippocampal neurons, avoiding sacrificing pregnant mice, which makes this procedure efficient and cost effective. Furthermore, through this protocol, we aim to provide step-by-step instructions for isolating and establishing different primary neuroglial cells and their characterization using cell-specific markers. This study presents an opportunity to isolate, culture, and establish all major CNS resident cells individually. These cells can be utilized in various cell-based and biochemical assays to comprehensively investigate the cell-specific roles and behaviors of brain resident cells in a reductionist approach.
Key features
• Efficient isolation of major neuroglial cells like meningeal fibroblasts, neurons, astrocytes, oligodendrocytes, and microglia from a single day-0 neonatal mouse pup’s brain.
• Circumvents the sacrifice of pregnant female mice.
• Acts as a bridging experimental method between secondary cell lines and in vivo systems.
• Isolated cells can be used for performing various cell-based and biochemical assays.
Graphical overview
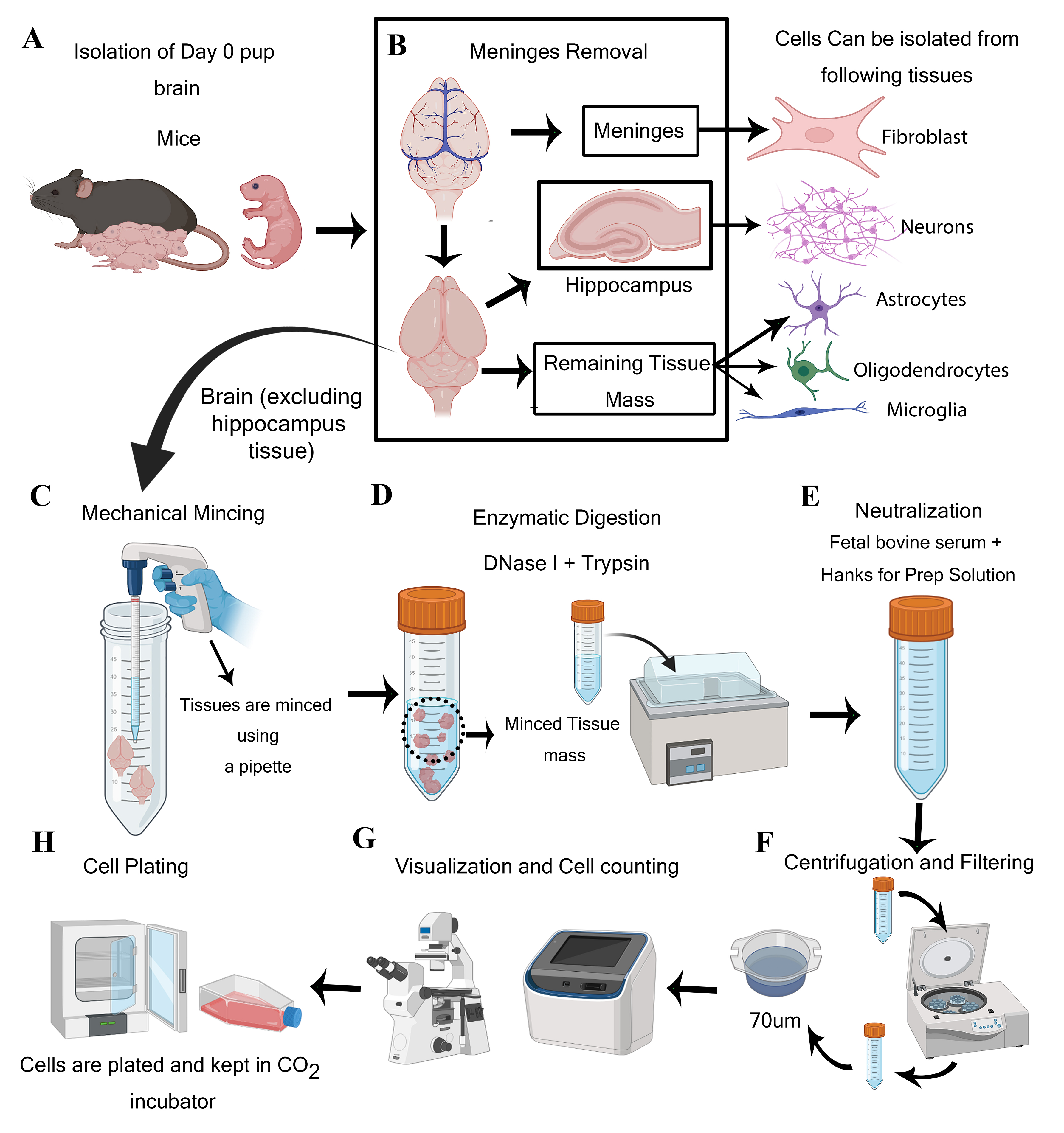
Steps for isolation of meningeal fibroblast and neuroglial cells from day 0 pups of mice (Created using BioRender.com)
Background
Intricate neuro-glial interactions, as an intertwined system of brain circuitry, play a vital role in various central nervous system (CNS) functions and in the maintenance of homeostasis [1, 2]. Contemporary research indicates that the role of glial cells is not limited to mere supportive cells for neurons; instead, glial cells carry out complex physiological functions such as recycling neurotransmitters, regulation of the blood–brain barrier, myelin sheath formation, immunological functions, and many more. Impairment or dysfunction of glial cell function frequently disrupts CNS cell communication, potentially leading to diverse pathological conditions [3–5]. To understand this complex cellular and molecular crosstalk in various pathological conditions, diverse experimental animal models have been developed [6, 7]. For instance, transgenic mice expressing mutated forms of amyloid protein precursor and presenilin-1 serve as a disease model for Alzheimer's [8], while transgenic mice overexpressing α-synuclein are widely employed to study Parkinson's disease [9]. Additionally, experimental autoimmune encephalitis and virus-induced demyelination models [mouse hepatitis virus (MHV) and Theiler's murine encephalomyelitis virus] have been extensively used to elucidate the etiology of multiple sclerosis [10, 11].
While in vivo models provide unique insights into neuropathological studies, investigating complex cellular interactions in an in vivo setup to emphasize the cell's individualistic role is an intricate process [12]. However, relying on secondary cell lines may be insufficient to understand the diverse functions of neuroglial cells, as they are transformed cells, and continuous cell divisions accumulate mutations [13, 14]. That is why a suitable in vitro primary culture system is necessary to elucidate the complex array of neuroglial crosstalk and, at the same time, overcome the limitations mentioned above. Existing primary neuroglial cell isolation procedures routinely involve using a certain developmental stage of pups to isolate a specific cell (8–12 pups), which can often be exhausting and time-consuming. Cells like astrocytes and meningeal fibroblasts are usually isolated from day-0 pups (P0) [15–17] whereas oligodendrocytes precursor cells’ (OPCs) isolation is done from 5–7-day-old pups [18]. However, neurons are terminally divided cells, and their isolation requires embryonic pups (E14–E15) [19], where the mice mother must be sacrificed.
Our current protocol overcomes these limitations by using day-0 pups to separate a variety of neuroglial cells. This procedure involves the collection of meninges and hippocampal tissue to isolate meningeal fibroblasts [16] and neurons [20], respectively. The remaining brain tissues (left after isolating meninges and hippocampus) can either be used to establish a mixed glial culture with subsequent isolation of enriched astrocytes [21] and microglia [22] or be used for OPCs culture, using serum-free oligodendrocyte-specific medium. OPCs can later be differentiated into mature oligodendrocytes using chemically defined differentiation medium [17]. In addition, this protocol avoids the sacrifice of female mice, as day-0 pups are used to harvest the cells. Astrocytes and fibroblasts isolated using this protocol can be passaged, whereas oligodendrocytes, neurons, and microglia should be seeded as per the experimental requirement. Therefore, if RNA or proteins from these cells are required, more pups are needed to obtain a larger number of these cells. Microglial cells isolated through this protocol can only be used for immunofluorescence experiments. For gene-level expression studies, it is advisable to use the CD11b microbeads–based magnetic cell sorting procedure to get a high yield of microglial cells [22].
The precision and handling can influence the yield of cells during the isolation process, which is discussed in detail in the procedure section. To mitigate such variability, it is essential that all steps be executed consistently and with the utmost care. This method allows for isolating all major CNS resident neuroglial cells without using fluorescence-activated cell sorting. These cells can be used to understand individual neuroglial cell–specific roles in CNS biology and pathology. This approach also increases the experimental opportunities for the scientific community more efficiently and cost effectively.
Materials and reagents
Biological materials
Animals
Mice: Day-0 mice pups, C57BL/6 (Strain #000664, common name B6) obtained from The Jackson Laboratory
Viruses: RSA59, an isogenic recombinant strain of murine beta corona virus (MHVA59) [23]
Reagents
Hank’s balanced salt solution (HBSS) (10×) (Gibco, catalog number: 14185-052)
Phosphate buffered saline (PBS) pH 7.4 (10×) (Gibco, catalog number: 70011-044)
Sodium bicarbonate (NaHCO3) (7.5%) (Gibco, catalog number: 25080-094)
CAUTION: NaHCO3 is skin and eye irritant. Wear suitable gloves, goggles, and protective clothing.
Dulbecco’s modified Eagle medium (DMEM) (powder) (Gibco, catalog number: 12100-046)
Trypsin 0.25% (1×) (Gibco, catalog number: 15050-057)
L-Glutamine 200 mM (100×) (Gibco, catalog number: 25030-081)
0.5% Trypsin-EDTA (10×) (Gibco, catalog number: 15400-054)
CAUTION: Components containing Trypsin may produce an allergic reaction. EDTA may cause irritation to the eyes and skin. Use respiratory protection as well as protection for skin and eyes.
Heat inactivated horse serum (origin: New Zealand) (Gibco, catalog number: 26050-070)
Penicillin-streptomycin (Pen-Strep) (Gibco, catalog number: 15140-122)
Distilled water (Gibco, catalog number: 15230-147)
Sterile deionized H2O
F12 nutrient mixture (Ham) medium (Gibco, catalog number: 11765-054)
Heat inactivated fetal bovine serum (FBS) (Gibco, catalog number: 10082-147)
D-(+)-Glucose, Hybri-Max, ≥99.5% (Sigma-Aldrich, catalog number: G5146)
Trypsin from bovine pancreas (Sigma-Aldrich, catalog number: T9201-5G)
Laminin from Engelberth-holm swarm murine sarcoma basement membrane (Sigma-Aldrich, catalog number: L2020-1MG)
MEM non-essential amino acids solution (Sigma-Aldrich, catalog number: M7145)
HEPES buffer (1 M) (Gibco, catalog number: 15630-080)
CAUTION: It may cause irritation to the skin, eyes, and upper respiratory tract. Wear protective gloves, goggles, and mask.
Insulin from bovine pancreas (Sigma-Aldrich, catalog number: I6634-50MG)
Poly-D-Lysine hydrobromide (Sigma-Aldrich, catalog number: P7886-100MG)
Neurobasal medium (NB) (Gibco, catalog number: 21103-049)
Deoxyribonuclease I (DNase I) from bovine pancreas (Sigma-Aldrich, catalog number: D5025-15KU)
CAUTION: May cause allergy or asthma symptoms or breathing difficulties if inhaled. Use protective clothing, gloves, and masks while using. Do not vortex, as DNase I is prone to physical denaturation.
B27 supplement 50× (Gibco, catalog number: 17504-044)
Magnesium chloride (MgCl2·6H2O) (SISCO Research Laboratories PVT LTD, catalog number: 1349130)
Calcium chloride (CaCl2·2H2O) (SRL, catalog number: 10035-04-8)
L-Thyroxine sodium salt pentahydrate (T4 thyroxine) (Sigma-Aldrich, catalog number: T0397-1MG)
Apo-Transferrin human (transferrin) (Sigma-Aldrich, catalog number: T-4382)
Putrescine dihydrochloride (Sigma-Aldrich, catalog number: P5780-5G)
Progesterone (Sigma-Aldrich, catalog number: P8783-5G)
Selenium dioxide (Sigma-Aldrich, catalog number: 325473)
CAUTION: Toxic if swallowed or inhaled. May cause damage to organs through prolonged or repeated exposure. Very toxic to aquatic life with long-lasting effects. Avoid contact with skin, eyes, and clothing. Use a face shield, safety glasses, gloves, and respiratory protectant.
Biotin (Sigma-Aldrich, catalog number: B-4501)
0.15 M Borate (Sigma-Aldrich, catalog number: B6768-1KG)
99.9% Ethanol, absolute, analytical CSS reagent (Changshu song sheng fine chemicals, catalog number: GB678-90)
CAUTION: Highly flammable liquid may cause drowsiness and nausea.
Sodium chloride (NaCl) (SRL, catalog number: 7647-14-5)
Potassium chloride (KCl) (MERCK, catalog number: 7447-40-7)
di-Sodium hydrogen phosphate (Na2HPO4) (MERCK, catalog number: 7558-79-4)
Potassium dihydrogen phosphate (KH2PO4) (MERCK, catalog number: 7778-77-0)
Trypan Blue (0.4%) (Gibco, catalog number: 15250061)
Growth factors
Nerve growth factor (NGF) (Invitrogen, catalog number: 13257-019)
Fibroblast growth factor (FGF) (R&D System, catalog number: 133-FB/CF)
Platelet-derived growth factor (PDGF) (R&D System, catalog number: 221-AA)
Neurotrophin-3 (NT3) (PeproTech, catalog number: 450-03-10UG)
Reagents for immunofluorescence
Triton X-100 (Sigma-Aldrich, catalog number: T8787-100ml)
CAUTION: Harmful if swallowed. Causes skin irritation and serious eye damage. Use skin and eye protectants.
Bovine serum albumin, for molecular biology (BSA) (HIMEDIA, catalog number: MB083-25G)
CAUTION: Causes skin and eye irritation; may cause an allergic response. Avoid contact with skin and eyes and wear respiratory protection, gloves, and goggles.
Antibodies
Primary antibodies against various cell markers are diluted with 0.1% BSA (prepared from 1% BSA) as per the given ratio (Table 1).
Table 1. Primary antibodies
Antibody name Dilution Cellular markers for Recommended storage condition Mouse anti-GFAP (Sigma, catalog number: G3893) 1:100 Astrocytes Stored at -20 °C freezer Rabbit anti-Vimentin (CST, catalog number: D21H3) 1:200 Meningeal fibroblasts Rabbit anti-Iba1 (SIGMA Wako, catalog number: 019-19741) 1:600 Microglia Mouse anti-NG2 (MERCK, catalog number: MAB5384-I) 1:50 OPCs Rabbit anti-MAP 2 (SIGMA, catalog number: M3696) 1:200 Neurons Mouse anti-NFM (SIGMA, catalog number: N5389) 1:100 Neurons Mouse anti-Gal C (H8H9) (Homemade) 1:20 Mature oligodendrocytes Mouse anti-A2B5 (Homemade) 1:20 OPCs Rabbit anti-Cx43 (SIGMA, catalog number: C6219) 1:1,000 a gap junction protein Fluorescently tagged secondary antibodies raised against mouse or rabbit are diluted in 0.1% BSA (prepared from 1% BSA) as per the given ratio (Table 2).
Table 2. Secondary antibodies
Antibody name Dilution Recommended storage condition Donkey anti-Rabbit Alexa Fluor 568 (Invitrogen, catalog number: A10042) 1:1,000 Stored at 4 °C Goat anti-Mouse Alexa Fluor 488 (Invitrogen, catalog number: A11001) 1:1,000 Goat anti-Mouse Alexa Fluor 546 (Invitrogen, catalog number: A11003) 1:1,000
Solutions
DMEM solution (see Recipes)
Astrocyte-specific medium (see Recipes)
Hanks for prep solution (see Recipes)
Serum for prep solution (see Recipes)
1× PBS (see Recipes)
HBSS solution (see Recipes)
NB/B27 medium (see Recipes)
Oligodendrocytes-specific media (see Recipes)
Neuron-specific media (see Recipes)
DM supplement (see Recipes)
T4 thyroxine (see Recipes)
30% glucose (1.66 M) (see Recipes)
Insulin (see Recipes)
Biotin (see Recipes)
Oligodendrocyte differentiation media (see Recipes)
DMEM Minus (-) (see Recipes)
DMEM Plus (+) (see Recipes)
HBSS (+) or 1% glucose HBSS solution (see Recipes)
DNase I solution (see Recipes)
Trypsin (see Recipes)
Poly-D-Lysine (PDL) coating (see Recipes)
Wash buffer (see Recipes)
Blocking serum (1% BSA) (see Recipes)
Recipes
DMEM solution
Prepare by dissolving DMEM powder in 1 L of double-autoclaved Milli Q water; then, add NaHCO3. Follow the table below for preparation. Mix properly (so that no clumps remain) and then adjust the pH to 7.2–7.4. Sterilize the solution by passing through a 0.22 µm filter paper. The solution can be stored at 4 °C for up to one month.
Components Quantity Final concentration Recommended storage condition DMEM powder 1 pouch 13.4 g/L 4 °C NaHCO3 3.7 g/L 44.04 mM Room temperature (20–25 °C) Double-autoclaved Milli Q H2O 1 L NA Room temperature Final volume 1 L 4 °C Astrocyte-specific medium
Sterilize the solution by passing through a 0.22 µm filter paper. Always prepare fresh; the solution can be stored for approximately one month at 4 °C.
Components Quantity Final concentration Recommended storage condition FBS 10 mL 10% (v/v) -20 °C Pen-Strep 1 mL 1% (v/v) -20 °C L-Glutamine 0.1 mL 0.2 mM -20 °C DMEM solution 89.9 mL NA 4 °C Final volume 100 mL 4 °C Hanks for prep solution
Sterilize the solution by passing through a 0.22 µm filter paper before use. The solution can be stored at 4 °C for up to 1–2 weeks.
Components Quantity Final concentration Recommended storage condition HBSS (10×) 10 mL 10% (v/v) 4 °C 1 M HEPES buffer 2.5 mL 25 mM 4 °C 7.5% NaHCO3 1 mL 1% (v/v) 4 °C Pen-Strep 1 mL 1% (v/v) -20 °C Double-autoclaved Milli Q H2O 85.5 mL NA Room temperature Final volume 100 mL 4 °C Serum for prep solution
Sterilize the solution by passing through a 0.22 µm filter paper before use. The solution can be stored at 4 °C for up to one week.
Components Quantity Final concentration Recommended storage condition Heat-inactivated FBS 10 mL 10% (v/v) -20 °C Non-essential amino acid solution 1 mL 1% (v/v) 4 °C L-Glutamine 1 mL 2 mM -20 °C Pen-Strep 1 mL 1% (v/v) -20 °C DMEM 87 mL NA 4 °C Final volume 100 mL 4 °C 1× PBS
Dissolve the following reagents as per the given quantities in 800 mL of distilled water and adjust the pH to 7.2–7.4; then, make up the volume up to 1 L and filter sterilize the solution by passing through a 0.22 µm filter paper.
Components Quantity Final concentration Recommended storage condition NaCl 8 g 137 mM Room temperature KCl 0.2 g 2.7 mM Room temperature Na2HPO4 0.24 g 10 mM Room temperature KH2PO4 1.44 g 1.8 mM Room temperature Distilled water 1 L NA Room temperature Final volume 1 L 4 °C HBSS solution
100× Ca2+ (90 mM)
Dissolve 1.324 g of CaCl2·2H2O in 100 mL of distilled water.
100× Mg2+ (104.8 mM)
Dissolve 2.13 g of MgCl2·6H2O in 100 mL of distilled water.
HBSS with Ca2+ and Mg2+
Sterilize the solution by passing through a 0.22 µm filter paper. This solution can be stored for up to 1–2 months at 4 °C.
Components Quantity Final concentration Recommended storage condition HBSS (10×) 10 mL 10% (v/v) 4 °C Ca2+ (100×) 1 mL 0.9 mM 4 °C Mg2+ (100×) 1 mL 1.05 mM 4 °C Double-autoclaved Milli Q H2O 88 mL NA Room temperature Final volume 100 mL 1% (v/v) 4 °C NB/B27 medium
Sterilize the solution by passing through a 0.22 µm filter paper. Always prepare fresh and do not store the solution for more than two weeks.
Components Quantity Final concentration Recommended storage condition B27 Supplement 50× 2 mL 2% (v/v) 4 °C Pen-Strep 2.5 mL 2.5% (v/v) -20 °C L-glutamine 2.5 mL 5 mM -20 °C NB 93 mL NA 4 °C Final volume 100 mL 4 °C Oligodendrocytes-specific media
Always prepare fresh and use it according to your experimental planning.
Critical step: Do not filter the growth factors. Always add the growth factors instantly in the NB/B27 incomplete media just before use for your experiments and put growth factors back in the -20 °C freezer without any delay.
Components Quantity Final concentration Recommended storage condition PDGF (1 µg/mL) 100 µL 2 ng/mL -20 °C FGF (10 µg/mL) 50 µL 10 ng/mL -20 °C NT3 (1 µg/mL) 50 µL 1 ng/mL -20 °C NB/B27 medium 50 µL NA 4 °C Final volume 50 µL 4 °C Neuron-specific media
Always prepare the solution fresh just before the experiment.
Critical step: Do not filter the growth factors. Caution should be followed as aforesaid for oligodendrocytes-specific medium.
Components Quantity Final concentration Recommended storage condition NGF (100 µg/mL) 7 µL 50 ng/mL -20 °C NB/B27 medium 15 mL NA 4 °C Final volume mL 4 °C DM supplement
Filter sterilize the mixture after combining all components using a 0.2 µm filter. Store 1 mL aliquots at -20 °C for approximately two months.
Components Quantity Final concentration Recommended storage condition Transferrin 5 mL 250 mg/5 mL DMEM -20 °C Putrescine 5 mL 26.85 mg/5 mL DMEM (33.3 mM) Room temperature Progesterone 10 mL 3 mg/5 mL [of 95% EtOH (1.908 mM)] (dilute 25 µL of this in 10 mL DMEM) Room temperature Selenium dioxide 5 mL 3.3 mg/10 mL DMEM (2.974 mM) (dilute 40 µL of this in 5 mL DMEM) Room temperature Final volume 25 mL -20 °C T4 thyroxine
Aliquot and store at 4 °C.
Components Quantity Final concentration Recommended storage condition T4 thyroxine 1 mg 22.5 µM -20 °C DMEM 50 mL NA 4 °C Final volume 50 mL 4 °C 30% glucose (1.66 M)
Dissolve 30 g of glucose in 100 mL of sterile ddH2O. Sterilize the solution by passing through a 0.22 µm filter paper and store at 4 °C.
Insulin
Mix the provided quantity of insulin in DMEM and then add 1 N HCl dropwise until the cloudy solution clears. Filter sterilize the solution and store 1 mL aliquots at 4 °C.
Components Quantity Final concentration Recommended storage condition Insulin 25 mg 4.36 µM -20 °C DMEM 10 mL NA 4 °C 1 N HCl NA NA Room temperature Final volume 10 mL 4 °C Biotin
Dissolve biotin in doubled-distilled water (ddH2O) (5 mg in 100 mL of ddH2O) to make a stock of 204.65 µM. Then, prepare a working stock of 40.93 µM as per the instructions below and sterilize the solution by passing through a 0.2 µm filter paper; store at 4 °C.
Components Quantity Final concentration Recommended storage condition Biotin 20 mL 40.93 µM 4 °C DD H2O 80 mL NA Room temperature Final volume 100 mL 4 °C Oligodendrocyte differentiation media
Mix all the given components and store the solution at 4 °C.
Components Quantity Final concentration Recommended storage condition T4 thyroxine 4 mL 0.9 µM -20 °C 30% glucose 2 mL 33.4 mM 4 °C L-Glutamine 2 mL 4 mM -20 °C Pen-Strep 2 mL 2% v/v -20 °C Insulin 1 mL 4.36 µM 4 °C DM supplement 1 mL 1% v/v -20 °C Biotin 200 µL 81.86 µM 4 °C DMEM 94 mL NA 4 °C F12 medium 94 mL NA 4 °C Final volume 200 mL 4 °C DMEM Minus (-)
Sterilize the solution by passing through a 0.22 µm filter paper.
Critical: Always prepare fresh and store at 4 °C for no more than 1–2 weeks.
Components Quantity Final concentration Recommended storage condition 1 M HEPES buffer 1 mL 10 mM 4 °C Pen-Strep (100×) 1 mL 1% (v/v) -20 °C Non-essential amino acid solution 1 mL 1% (v/v) 4 °C DMEM 97 mL NA 4 °C Final volume 100 mL 4 °C DMEM Plus (+)
Sterilize the solution by passing through a 0.22 µm filter paper.
Critical: Prepare fresh and store at 4 °C refrigerator for not more than 1–2 weeks. FBS and HS should be filtered first to prevent the clogging of filter paper.
Components Quantity Final concentration Recommended storage condition 1 M HEPES buffer 1 mL 10 mM 4 °C Pen-Strep (100×) 1 mL 1% (v/v) -20 °C Non-essential amino acid solution 1 mL 1% (v/v) 4 °C Heat inactivated FBS 5 mL 5% (v/v) -20 °C Heat inactivated horse serum 5 mL 5% (v/v) -20 °C DMEM 87 mL NA 4 °C Final volume 100 mL 4 °C HBSS (+) or 1% glucose HBSS solution
Sterilize the solution by passing through a 0.22 µm filter paper. The solution can be stored for up to one month at 4 °C.
Components Quantity Final concentration Recommended storage condition HBSS (1×) 100 mL NA 4 °C Glucose 1 g (w/v) 55.5 mM Room temperature Final volume 100 mL 4 °C DNase I solution
DNase I enzyme is commercially available as a powder. Sterilize the solution by passing through a 0.2 µm filter paper before use. Aliquot the solution to 2 mL tubes and store at -20 °C.
Components Quantity Final concentration Recommended storage condition DNase I 3 mg 0.1 mg/mL -20 °C 1× PBS 30 mL NA 4 °C Final volume 30 mL -20 °C Trypsin
Sterilize the solution by passing through a 0.2 µm filter before use. Aliquot the solution in 2 mL tubes and store at -20 °C.
Components Quantity Final concentration Recommended storage condition Trypsin from bovine pancreas 300 mg 10 mg/mL -20 °C 1× PBS 30 mL NA 4 °C Final volume 30 mL -20 °C Poly-D-Lysine (PDL) coating
Dissolve 5 mg of PDL in 100 mL of distilled water, then add 400 mL of distilled water to make up the volume.
Sterilize the solution by passing through a 0.2 µm filter paper. Store at 4 °C.
To coat:
Add 1 mL of 0.15 M borate (in 0.15 M NaOH, pH 8.4) per 100 mL of PDL solution and filter coat flasks with the PDL-borate for at least 2 h at room temperature.
Suction the liquid off the plastic and dry at 37 °C overnight.
Reagent setup for immunofluorescence
Wash buffer
Critical: Always prepare fresh just before using and store at 4 °C.
Components Quantity Final concentration Recommended storage condition 1× PBS 49 mL NA 4 °C Ca2+ (100×) 0.5 mL 0.9 mM 4 °C Mg2+ (100×) 0.5 mL 1.04 mM 4 °C Final volume 50 mL 4 °C Blocking serum (1% BSA)
Mix properly using the vortex and keep the solution for some time to settle the frothing.
Critical: Do not vortex for a long period, otherwise it will cause too much frothing in the solution.
Instead of BSA, goat serum can also be used for the preparation of blocking serum and antibody diluents.
Components Quantity Final concentration Recommended storage condition BSA 1 g 1% (w/v) 4 °C Wash buffer 100 mL NA 4 °C Final volume 100 mL 4 °C
Laboratory supplies
Cell strainer (70 and 100 μm) (Falcon, catalog numbers: 352350 and 352360)
15 mL centrifuge tubes (Tarsons, catalog number: 546021)
50 mL centrifuge tubes (Tarsons, catalog number: 546041)
Serological pipette (5 mL and 10 mL) (Thermo scientific Nunc, catalog numbers: 170355N and 7128)
6-well plate (Nunc Thermo, catalog number: 140675)
35 mm disc (Nunc Thermo Scientific, catalog number: 150460)
60 mm disc (Nunc Thermo Scientific, catalog number: 150288)
100 mm disc (Nunc Thermo Scientific, catalog number: 150350)
Bacterial Petri dish (Tarson, catalog number: 460095)
Pipette controller (Gilson, catalog number: 101300)
Syringes (Dispo Van, Hindustan Syringes and Medical devices, catalog number: 840054SM1)
Pipette tips [Tarson, catalog numbers: 521020 (200–1,000 µL), 521010 (2–200 µL), 521000 (0.2–10 µL)]
0.22 µm filter (Merck, Life Sciences, catalog number: B20345)
4 chambers (mounted on permanox) slides (Nunc, catalog number: 120075LE 1007)
Equipment
Biosafety hood (Thermo Scientific, Heraguard, catalog number: 41237696)
Centrifuge (Eppendorf, model: 5702R, catalog number: 5703YH207703)
CO2 incubator (Thermo Scientific, model: Heracell 150i, catalog number: G-3168)
Dissection microscope (Nikon SMZ 745 Model C-LEDS, catalog number: 226763)
Microscope (Nikon, model: Eclipse Ts2, catalog number: 137714)
Analytical balance (Sartorius PRACTUM213-10IN, catalog number: S/N 003670729)
Shaker incubator (ZHICHENG, catalog number: G-1377 ZHWY-103B)
Water bath with shaker (Jio Tech, catalog number: G-3179)
Forceps (Fine Science Tools, catalog number: 00649-11)
Fine forceps (Fine Science Tools, catalog numbers: 11251-33, 11252-40, 11252-30)
Software and datasets
For image processing
ImageJ (NIH, USA)
Zen 2010 software (Carl Zeiss, Germany)
Procedure
Experimental design
Isolation of meningeal fibroblasts, neurons, and various glial cells such as astrocytes, oligodendrocytes, and microglia from day-0 mouse pups’ brain (and meninges) indicates the potential of the current protocol for mechanistic and translational studies [16, 17, 21, 22]. This protocol relies on the precise separation of meninges (rich in fibroblast cells) and hippocampus (rich in pyramidal neurons) from the brain of any mouse and even rat strain.
The protocol requires one litter (>6–8 pups) to harvest adequate CNS resident cells. In the institute animal facility, one 7–9-weeks-old male and one 7–9-weeks-old female mouse are bred. After visual confirmation of the pregnancy, separate the female mice from the male and keep them under observation for pups. Collect the neonatal pups at day 0 to isolate neuroglial and fibroblast cells. Carefully decapitate the pups and immediately place the head in ice-cold Hanks for prep solution. Under the dissection microscope, remove the cranium and collect the intact brain tissue in another ice-cold Hanks for prep solution. Remove the meninges as much as possible from brain tissue using fine forceps. The tissue should then be mechanically minced using a 5 mL serological pipette (refer to Graphical overview Panel C) and through enzymatic digestion using DNase I and trypsin for 30 min.
To calculate the number of cells, thoroughly mix the cell suspension, take out 20 µL of cell suspension in an Eppendorf tube, and mix it with trypan blue dye. Then, count the cells using a cell counter. After counting the cells, seed them as per the experimental requirement.
Astrocytes, OPCs (P0), and fibroblasts can be plated directly on the cell culture flask, while plates for oligodendrocytes (P1) and neurons need to be coated beforehand with Poly-D-Lysine and laminin for experiments (see Recipes). Plate microglial cells directly on precoated chambered slides for experiments.
Isolated neuroglial cells, except neurons, should be kept in serum for prep solution (refer to steps 45–47 of the Procedure) for 24 h at 37 °C (in 5% CO2), followed by adding the chemically defined medium as per the cell types. Neurons will be plated with DMEM+ medium for 24 h at 37 °C (in 5% CO2) before changing into a chemically defined medium (steps 31–33). Fibroblasts can be plated directly in an astrocyte-specific medium (steps 15–16) (see Recipes) (Figure 1).
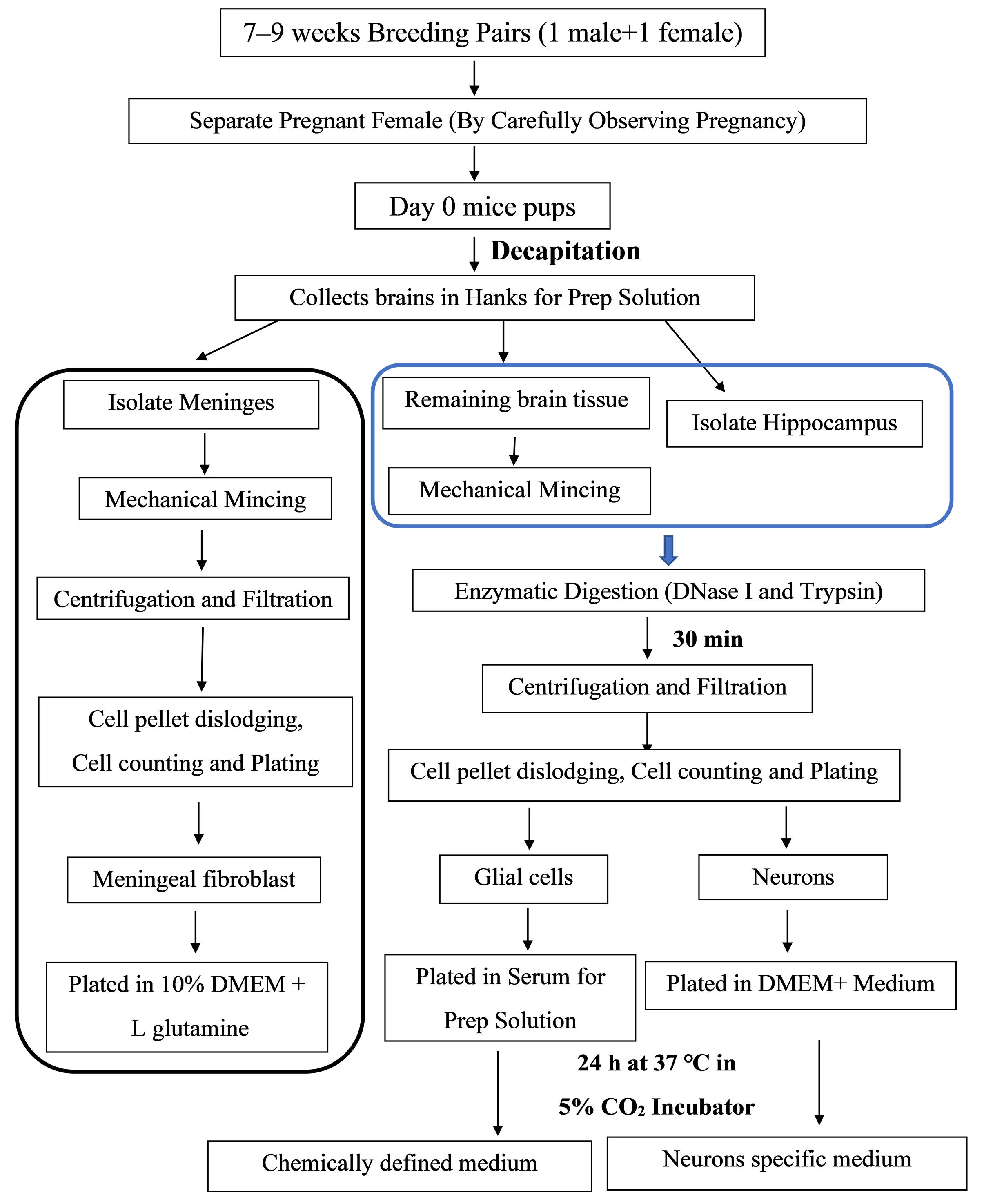
Figure 1. Schematic representation showing the experimental design for the isolation procedure
Preparation of reagents and equipment (Timing ~15 min)
Keep the stock solutions ready before proceeding with experiments (refer to the Recipes section). All the necessary reagents should be kept on ice or at 4 °C (e.g., DNase I and Trypsin for thawing).
Turn on the shaker water bath and set it to 37 °C for pre-heating.
Turn on the laminar hood and do the UV sterilization for 30 min before starting the procedure. Then, after putting all items required for the procedure (centrifuge tubes, plates, pipette tips, 100 mm plates, cell strainer etc.) inside, repeat the UV sterilization for 30 min.
CRITICAL STEP: Wipe all the dissecting tools required for the procedure with 70% (v/v) ethanol and then do the UV sterilization on the laminar hood.
Aliquot 10–15 mL of Hanks for prep solution in 50 mL centrifuge tubes (for meninges, collect in a 15 mL centrifuge tube) and keep on ice. For neurons, aliquot HBSS+ glucose or 1% glucose HBSS solutions in a 15 mL centrifuge tube.
Tissue collection (Timing ~30 min)
Collect the day-0 pups and keep them in a Whitman filter paper before proceeding with experiments.
Initial procedures for all the glial cells, meningeal fibroblasts, and neurons are considerably the same.
Carefully decapitate the pups and place their head immediately in ice-cold Hanks for prep solution to efficiently isolate the neuroglial cells.
Caution: Animal sacrifice must be performed as per the institute's approved protocols.
Remove the skin and cranium carefully with minimum damage to the brain using a pair of serrated fine forceps while observing under the microscope.
Once the cranium is removed, scoop out the brain (Figure 2A) and transfer it to a fresh 100 mm Petri dish containing Hanks for prep solution.
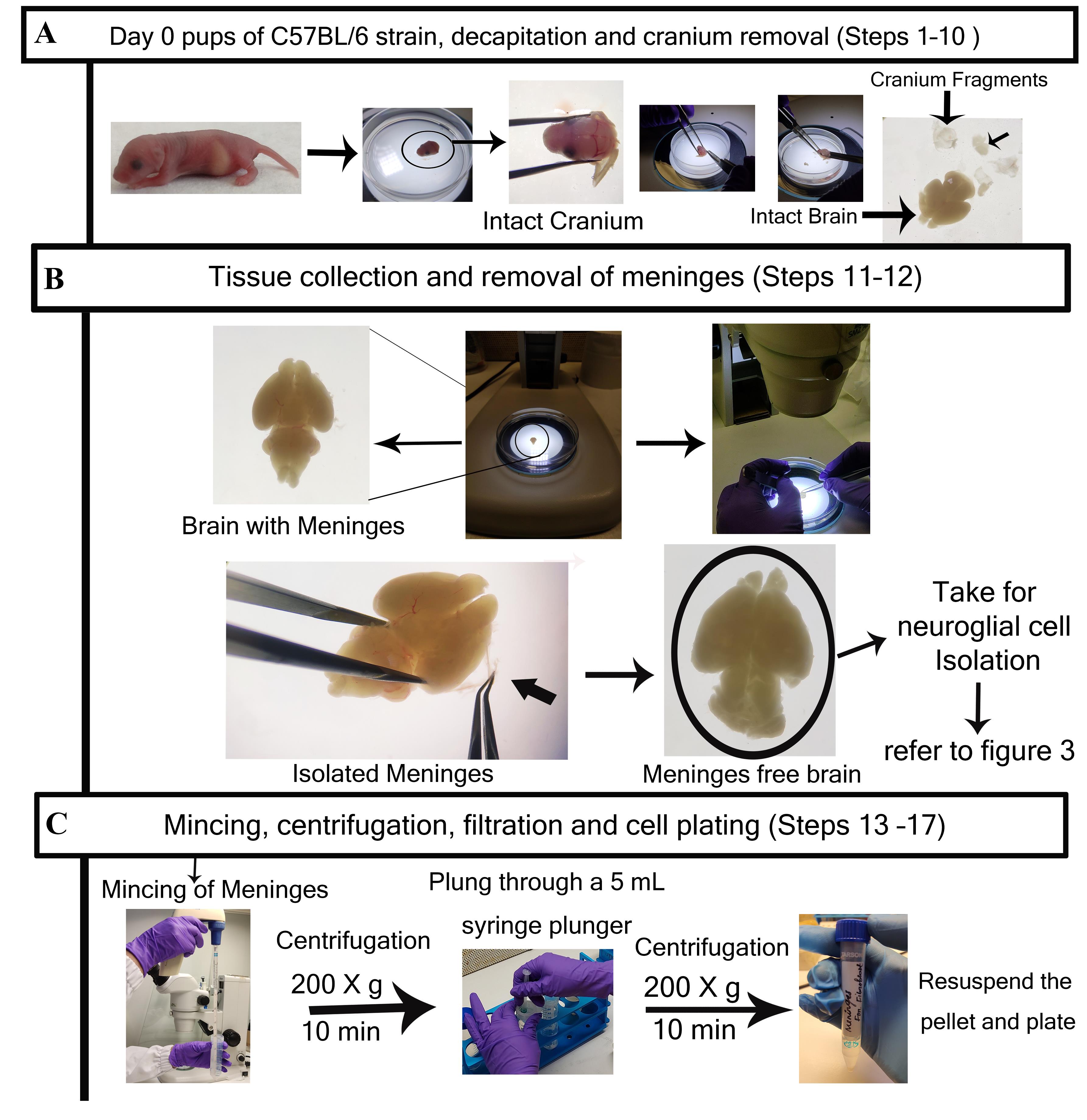
Figure 2. Detailed step-by-step visualization of meningeal fibroblast cells isolation from meninges. A. Representative image of brain tissues dissected from a day-0 mice pup. Brain with intact meninges. B. The meninges are isolated from the brain and kept separately for fibroblast culture. Post meninges collection, the meninges-free brain is taken for neuroglial cell and neuron isolation (refer to Figure 3). C. Meninges are subjected to mechanical mincing and subsequently centrifuged at 200× g for 10 min. After re-suspending with HBSS, collected cell pellets are subjected to filtration through a 70 µm cell strainer and centrifuged again at 200× g for 10 min. The final re-suspended pellet is collected for cell counting and then plated according to individual experimental requirements.
Meninges removal (Timing ~10 min/brain)
Carefully remove the meninges from the brain while observing under a dissection microscope, using extra fine forceps. Meninges can be differentiated from brain tissue because of blood vessels that look reddish, whereas the brain is milky white.
Caution: Meninges should be removed carefully without disturbing the brain tissue mass, otherwise isolating the hippocampus for neuronal isolation will be tough.
CRITICAL STEP: This is a crucial step because inadequate removal of meninges can cause cross-contamination of fibroblast cells in astrocyte culture (as the growth medium for both the cells is the same).
Collect the meninges in a 15 mL centrifuge tube containing 13 mL of HBSS.
Pause Point: The collected meninges can be instantly used for fibroblast isolation or can be kept on ice for a few minutes (~30–45 min).
Isolation of meningeal fibroblast cells (Timing ~30 min)
Homogenize the collected meninges using a 5 mL serological pipette; pass it through a 70 μm cell strainer. (Use a syringe plunger to pass cells through.)
Collect the filtrate in a 15 mL centrifuge tube, rinse the strainer with HBSS, and centrifuge at 200× g for 10 min. The pellet is meningeal cells.
Add HBSS again to wash the cells, re-suspend the pellet, strain through a 70 µm strainer, and again centrifuge at for 200× g 10 min. (Repeat this step two times.)
After the washing is done, re-suspend the pellet in astrocyte-specific medium.
Plate the cells and allow to grow for 72 h. We prefer to plate in T75 flasks, but you can also plate in T25 flasks and other cell culture plates based on experimental requirements.
After 72 h, observe the attached cells under a microscope.
Gently wash with 1× HBSS (you can also use 1× PBS) to remove the non-adherent cells and debris and add fresh medium.
Change the culture medium every 2–3 days until confluency.
Isolation of hippocampus (Timing ~20 min/brain)
Observe the meninges-free brain under the microscope and remove the olfactory bulbs and cerebellum. Keep these tissues in a 50 mL centrifuge tube containing Hanks for prep solution and finally separate the cerebral hemisphere.
Cut the cerebral hemisphere into two halves and cut the hippocampus away from the medial surface of the cortex (refer to Figure 3A). Place in a 15 mL centrifuge tube containing 13 mL of HBSS+ on ice.
Caution: The hippocampus is a densely neuron-rich region. Inefficient isolation of the hippocampus can lead to contamination of other glial cells.
TROUBLESHOOTING: The hippocampus looks translucent under the light microscope so, for better visualization, use a black surface beneath the Petri dish or use DMEM- to keep the cerebral hemisphere as it will make the surroundings reddish.
After isolation of the hippocampus, collect the remaining brain tissue mass in the same 50 mL centrifuge tube where you have earlier collected cerebellum and olfactory bulb tissues.
These remaining tissues will be used for the glial cell (astrocyte, oligodendrocytes, and microglia) isolation.
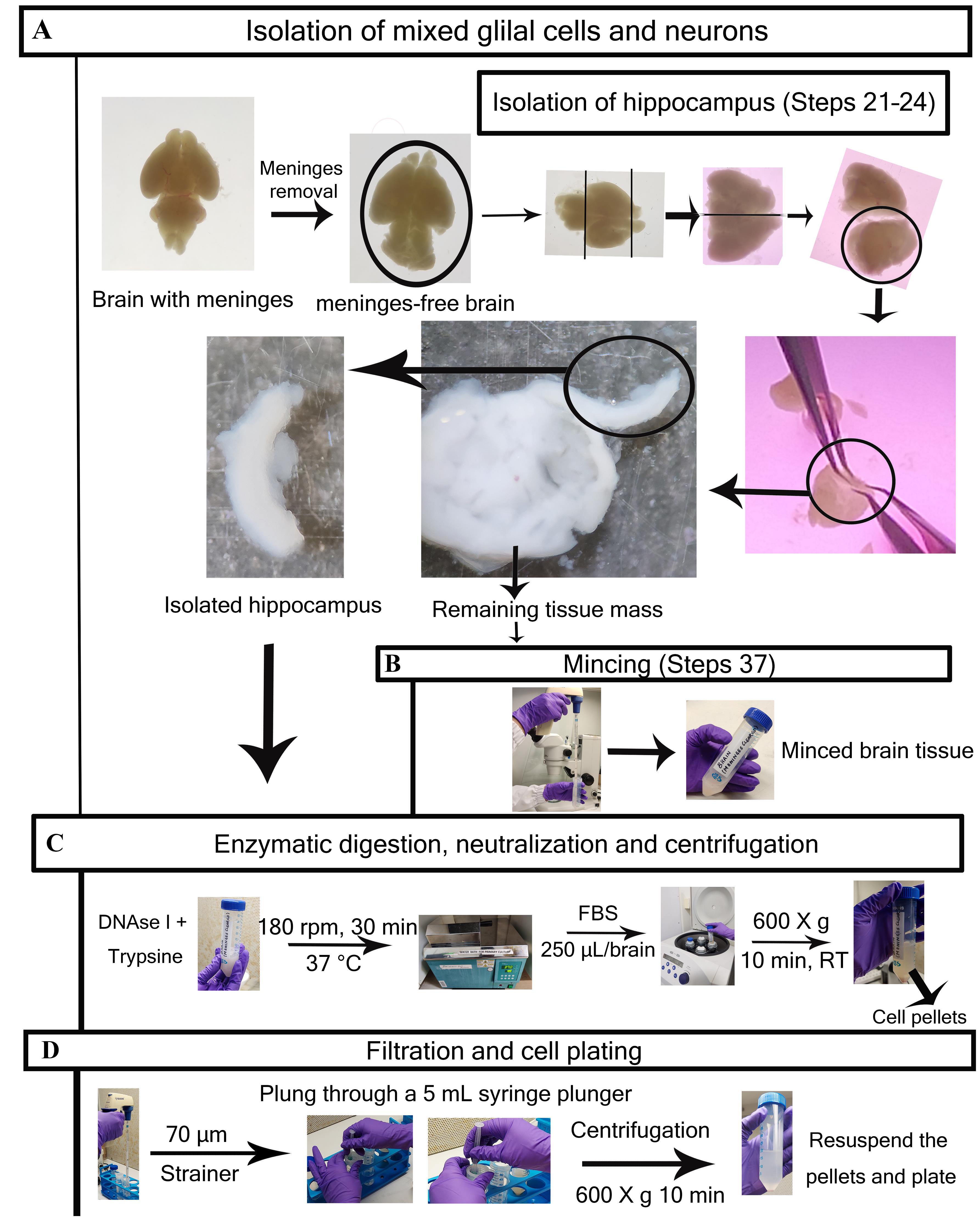
Figure 3. Detailed step-by-step visualization of neuroglial cells isolation procedure. A. Brain with intact meninges (previous steps are described in Figure 2A and 2B); the meninges are removed (for fibroblast culture), and meninge-free brains are taken for major glial cells and neuron culture. The hippocampus region is collected for neuron isolation, while the remaining tissue mass (after isolating meninges and hippocampus) is taken for major glial cell isolation. B. Tissues kept for glial cell isolation are subjected to mechanical mincing (hippocampus will not be minced). C. Minced tissues for glial cells and hippocampus tissues are incubated with DNase I + Trypsin at 180 rpm for 30 min at 37 °C in a shaking water bath. The enzymatically digested tissues are subjected to neutralization using FBS and subsequent centrifugation at 600× g for 10 min at RT. D. The collected cell pellet, after re-suspending with Hanks for prep solution, is subjected to filtration through a 70 µm cell strainer. Final yields are counted and plated according to individual experimental requirements. Step numbers are not mentioned in Figure 3 for enzymatic digestion, neutralization, centrifugation, filtration, and cell plating procedure as they will vary for specific cell types (refer to “Procedure” section).
Isolation of neurons (Timing ~30 min)
Take the 15 mL centrifuge tube containing HBSS+ along with previously collected hippocampus tissues and centrifuge at 100× g for 5 min at 4 °C.
Discard the supernatant and add 1 mL of trypsin and 300 µL of DNase I to the tube. Mix properly and incubate for 15 min at room temperature.
Add 3 mL of DMEM+ media to stop the reaction (neutralization of enzymes).
CRITICAL STEP: Enzymatic over-digestion could damage the culture so do not keep it for a longer time than required.
Centrifuge at 400× g for 5 min at 4 °C.
Discard the supernatant and add DMEM+; gently pipette up and down until it makes a cell suspension.
Then, pass the cell suspension through a 70 µm cell strainer on a 50 mL centrifuge tube and rinse the strainer with an equal volume of media (e.g., 5 mL suspension/5 mL media).
Count the cell number using a cell counter and dilute it to 106 cells/mL with DMEM+.
Seed in PDL/Laminin-coated (see Recipes) tissue culture plates with an appropriate number of cells.
Incubate plates at 37 °C and 5% CO2.
After 24 h, change the medium to neuron-specific media (see Recipes) and observe the neurons for axon outgrowth.
Every 2–3 days, change the medium. After ~5 days, neurons will be ready for experiment.
Isolation of brain resident glial cells (astrocytes, oligodendrocytes, microglia) (Timing: vary according to cell type)
Take the 50 mL centrifuge tube consisting of Hanks for prep solution (5 mL for multiple brains) along with the remaining brain tissue mass (after isolation of meninges and hippocampus).
CRITICAL PERIOD: Isolate meninges (refer to step 10) from brain parenchyma and keep them separate to avoid cross-contamination of fibroblast cells in astrocyte culture.
Pipette up and down 10 times using a 5 mL serological pipette to break up tissue (tissue mincing).
Add 0.25 mL of trypsin and 0.25 mL of DNase I per brain.
Incubate in a shaking water bath at 37 °C for 30 min (refer to the enzymatic digestion step).
After the digestion, add 0.25 mL of FBS per brain and 5 mL of Hanks for prep solution.
Mix properly using a pipette and centrifuge at 600× g for 10 min.
Take the supernatant with a gentle vacuum.
Caution: Do not disturb the pellet while discarding the supernatant, otherwise it will affect the cell amount.
Add 5–10 mL of Hanks for prep solution and centrifuge again at 600× g for 10 min.
Again, take the supernatant with a gentle vacuum.
Add 5–10 mL of Hanks for prep solution, re-suspend the pellet, and put through a 70 µm cell strainer. Centrifuge again at 600× g for 10 min (repeat the step one more time).
Re-suspend the pellet in serum for prep solution.
Count the cells in a cell counter and plate the cells in non-coated tissue culture flasks (usually three brains per one T-75 flask).
Incubate the seeded cells in serum for prep solution for 24 h in a 5% CO2 incubator at 37 °C.
Cell-specific media change
After 24 h, wash cells with HBSS with Ca2+ and Mg2+ and put astrocyte-specific medium (see Recipes) in the flask where astrocytes are to be grown and oligodendrocyte-specific medium in the flask where OPCs are to be grown (go to step 55 for OPCs).
Enrichment of astrocytes and isolation of microglia from mixed glial cells
Once the mixed glial cells form a confluent monolayer, stop the addition of fresh medium for 10 days (starvation) to allow differential adhesion of astrocytes and microglia. Starvation leads to significant microglial growth. In these cultures, it peaked at 12–14 days.
After the starvation is complete, thoroughly agitate the culture flask in an orbital incubator shaker at 180 rpm for 45 min at 37 °C to remove the less adherent microglial cells from tightly adhered astrocytes.
Quickly remove the media with non-adherent cells and collect it in 15 mL tubes (go to step 54).
Microglial isolation (Timing ~10–12 days)
Take the cells suspended in the culture medium in a 15 mL tube and centrifuge at 300× g for 5 min at 4 °C.
Re-suspend the centrifuged pellet and dilute it with fresh astrocyte-specific medium, bringing the cells to a final concentration of 8 × 105 cells/mL; add 0.5 mL to each well of a 4-well chamber slide or 2 mL/well of a 6-well plate.
Astrocyte enrichment (Timing ~10–12 days)
After microglia has been shaken, maintain the flask in astrocyte-specific medium following a wash with sterile PBS. For astrocyte isolation, trypsinize the remaining adherent monolayers of astrocytes and use them as enriched astrocyte cultures for further experimentation.
OPCs isolation (Timing ~2 h)
After 24 h (step 47), remove non-adherent cells by washing with HBBS with Ca2+ and Mg2+.
Add oligodendrocyte-specific medium (see Recipes) to the cells 24 h after removal of serum for prep media.
Maintain these mixed glial cultures in oligodendrocyte-specific medium until confluent (approximately one week).
OPCs, which mainly grow on top of the mixed glia culture (mainly astrocyte layer) and are comparatively less adherent than astrocytes, should be dislodged by washdown method.
Plate the dislodged oligodendrocytes onto Poly-D-Lysine-coated coverslips (see Recipes) and maintain in oligodendrocyte-specific medium until the culture reaches 80% confluence.
Differentiation of OPCs (Timing ~7–10 days)
Remove the media once the OPCs are fully confluent; then, wash the OPCs with 1× HBSS with Ca2+ Mg2+ and add oligodendrocyte differentiation medium (see Recipes) for 7–10 days.
After day 7 onward, these can be used for experimentation.
Data analysis
Perform immunofluorescence experiments using the previously described protocol [24]. Fix 70%–80% confluent culture using 4% PFA). Then, permeabilize the cells with PBS containing 0.5% Triton X-100, followed by blocking with 1% BSA. Incubate the cells with primary antibodies in 0.1% BSA solution for 1 h, followed by washing using PBS with Ca2+ and Mg2+ and then labeling with secondary antibodies in 0.1% BSA for 1 h (see Recipes). Wash the cells and mount them with DAPI (VectaShield, Vector Laboratories) and visualize using the epifluorescence microscope [NIKON eclipse Ti2 microscope and DS Qi2 device camera (TYO)] or confocal microscope (Zeiss confocal microscope LSM710). Images are processed using ImageJ and Zen 2010 software (Carl Zeiss, Germany).
Meningeal fibroblast cells are the largest isolated cells, appearing transparent when viewed under a brightfield microscope. Growing microglial cells have short processes and a fusiform shape, while astrocyte cells form a monolayer in culture with a dense spread-out cell body. The morphological architecture of differentiated oligodendrocytes and OPCs differ significantly; OPCs are typically small cells with bright cytoplasm and short processes, whereas differentiated oligodendrocytes are multi-branched structures. Throughout their growth phase, neural cells exhibit a variety of morphologies; during their early stage, they are less branched and small in size, but with time, they develop networks with other neurons.
Validation of protocol
Experimental validation
Isolated and enriched primary neuroglial cells from day-0 mice pups’ brain show characteristic morphology throughout their growth period. These cells can be further characterized through immunolabeling using their cell-specific markers to verify their distinctiveness (Figures 4 and 5). Meningeal fibroblasts require astrocyte-specific medium as a growth medium. These cells show their elongated structure (largest among all other cells) around day 5–7 post-plating. These cells look transparent under the brightfield microscope (Figure 4A) and express type III intermediate filament protein vimentin (Figure 5A) [16, 25].
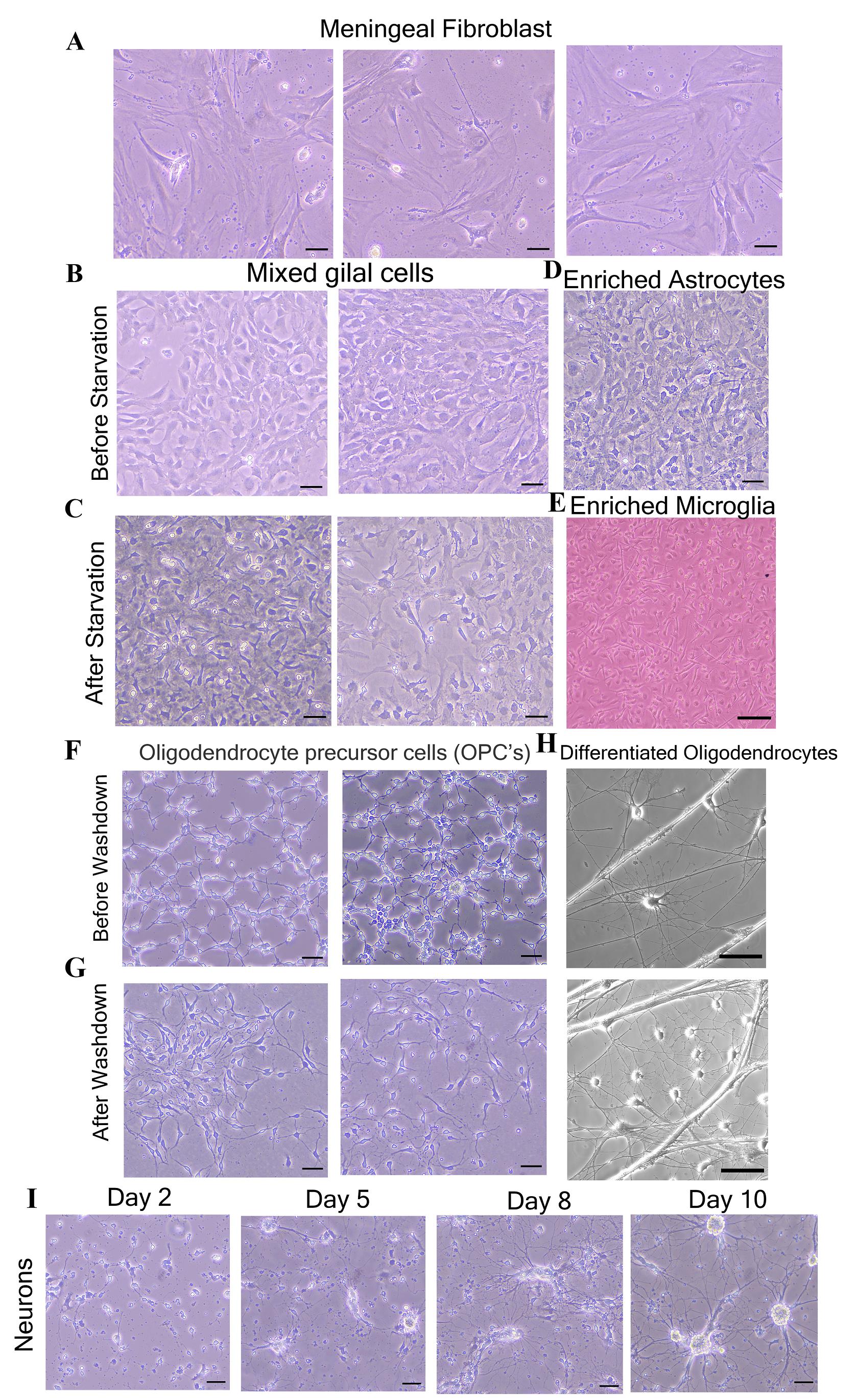
Figure 4. Morphological characterization of isolated primary neuroglial and meningeal fibroblast cells. Brightfield images show the specific neuroglial and fibroblast cells observed during the procedural steps of the cultures. A. Meningeal fibroblast cells (after five days of plating). B. Mixed glial cells before starvation. C. After starvation. D, E. Enriched astrocytes and microglia. F, G. Oligodendrocyte precursor cells before and after wash-down, respectively. H. Differentiated oligodendrocytes. I. Neurons at different stages of development (Day 2–Day 10). Scale bars are 50 µm for all brightfield images and 20 µm for Figure 3E).
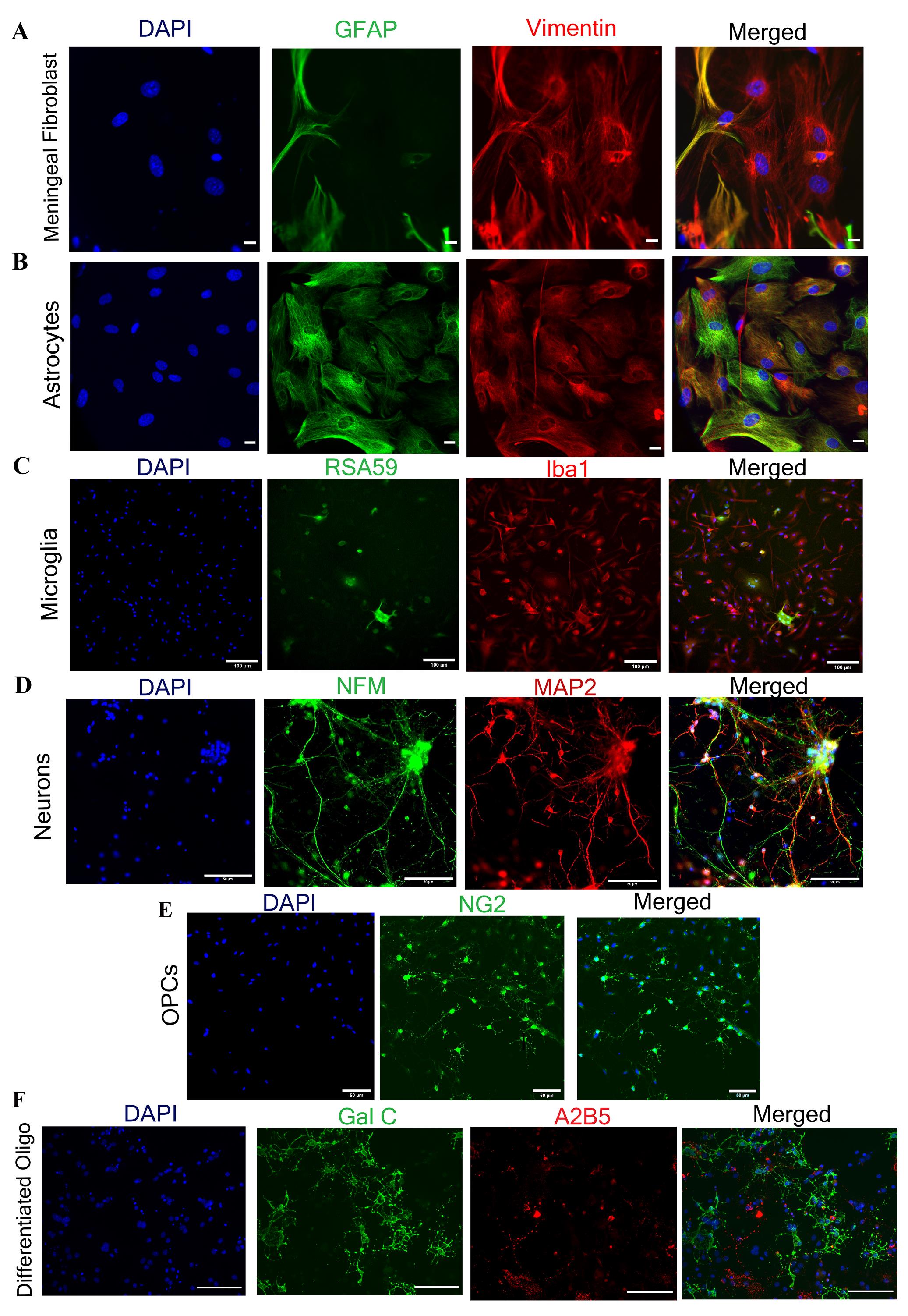
Figure 5. Immunofluorescence characterization of isolated primary neuroglial and meningeal fibroblast cells. A. Meningeal fibroblast cells. B. Astrocytes are labeled with vimentin (in red) and GFAP (in green). Astrocyte cells are dual positive for both GFAP and vimentin, while meningeal fibroblast cells are only positive for vimentin and negative for GFAP. C. Microglial cells are marked with Iba1 (in red). D. Neuronal cells show double-positive for NFM (in green) and MAP2 (in red). E, F. OPCs and differentiated oligodendrocytes are characterized using NG2 (in green) and Gal C+ A2B5- (Gal C in green, A2B5 in red). For double-immunostaining, Alexa Fluor 488, 546, and 568 secondary antibodies were used (see Recipes), and nuclei were counter-stained with DAPI (in blue). Scale bars are 20 µm in A and B, 50 µm in D and E, and 100 µm in C and F.
After starvation, plate astrocytes as per the experimental requirement in astrocyte-specific medium. Astrocytes are star-shaped cells characterized by dense spread-out cell bodies (darker in color) forming a monolayer around day 10–12 post-plating (Figure 4B and 4D). When the mixed glial cells are fully confluent and subjected to starvation (starvation facilitates microglial growth) [22] for 10–12 days, small bright microglial cells are visible over the monolayer (Figure 4C). After isolation, microglial cells should be plated on a chambered slide. These cells show a characteristic spindle (fusiform) shape with short processes (Figure 4E) and are positive for ionized calcium-binding adaptor molecule 1 (Iba1) protein (Figure 5C) [26, 27]. Enriched astrocytes are characterized by double-immunolabeling with anti-GFAP (glial fibrillary acidic protein) and anti-vimentin antibodies. Astrocyte cells are positive cells for both markers [21, 28] (Figure 5B).
As previously described, both astrocytes and oligodendrocytes are isolated from the same CNS tissues only by growing them in separate cell-specific chemically defined media. OPCs are characterized by a small structure with bright cytoplasm and two processes emerging from the central cytoplasm (Figure 4F and 4G). Once OPCs are differentiated into mature oligodendrocytes, they attain myelinating behavior and show a more branched structure; these branches radiate from the central bright cytoplasm (Figure 4H). The OPCs and differentiated oligodendrocytes positively express proteoglycan nerve/glial antigen 2 (NG2) [29, 30] and galactocerebroside (GalC) [31] respectively (Figure 5E and 5F).
Neurons may take 1–2 weeks post-plating for growth. During the initial days, these cells look small with bright cytoplasm and few processes, but gradually they develop a more branched structure with increased network connections (Figure 4I). These neurons can be characterized by the presence of neuronal-specific marker neurofilament (NFM) and microtubule-associated protein 2 (MAP 2) (Figure 5D) [32, 33].
These isolation procedures enable us to carry out a myriad of downstream experiments, e.g., protein localization studies such as Connexin 43 localization on fibroblast cells (Figure 6A), protein trafficking, or gene expression (RNA and protein) studies. There are numerous prior studies where these cells have been used to understand neuroglial tropism of a hepato-neurotrophic β coronavirus (RSA59 virus: an isogenic recombinant strain of MHVA59) [10] and also virus-induced alteration of protein expression in primary cells [16, 21]. Our findings imply that at MOI 2, the RSA59 virus infects all neuroglial cells (Figure 6B–6E) as well as meningeal fibroblasts (Figure 6A). These infected neuroglial cells can be harvested for downstream RNA and proteins, which then can be examined in accordance with the needs of each individual investigation.
Validation from literature
Astrocytes, being the most abundant glial cell in the CNS, help in regulating pH and K+ buffering and communicate with other glial cells for the maintenance of CNS homeostasis. Failure of neuroglial cells to sustain this homeostasis is fatal for CNS [1, 34]. Astrocytes are involved in several neurodegenerative and neuroinflammatory diseases like Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis, and multiple sclerosis. Using the current protocol, studies indicate that murine coronavirus (MHVA59) infection of primary astrocytes alters the expression of the astrocytic gap junction protein Cx43 by obstructing the protein’s microtubular trafficking [refer to Figure 2 and Figure 7 (Basu et al., 2017)] [35]. It was also found that the downregulation of Cx43 in vivo corresponds to the depletion of the oligodendrocytic gap junction protein Cx47 [refer to Figure 10 and Figure 11 (Basu et al., 2017)] [21, 35]. &Bgr;-amyloid (Aβ) peptides impede functional gap junction’s activity in primary astrocytes [refer Figure 1 (Maulik et al., 2020)] [36]. Similarly, MHV-A59 infection also reduces Cx43 expression in meningeal fibroblasts, resulting in altered gap junction communication [refer Figure 5 (Bose et al., 2018)] [16], which could affect the integrity of the blood–brain barrier [16, 37]. Therefore, this procedure offers a platform to study blood–brain barrier integrity and protein localization, as well as protein trafficking in neuroglial and fibroblast cells. It also helps in the development of targeted therapies for Alzheimer’s disease, emphasizing Cx43 channels [16, 36]. MHV-A59 infection in primary astrocytes increases Endoplasmic reticulum (ER) stress along with downregulation of the ER-resident chaperone protein, Erp29. Additionally, it has been demonstrated that 4-PBA treatment reduces ER stress by enhancing the expression of the chaperone Erp29 [refer to Figures 1, 2, and 3 (Bose et al., 2023)] [38]. This suggests that the current isolation procedure can also be used to comprehend neuro-glial-cells-specific stress responses [38].
Compromised oligodendroglial lineages are found to be involved in various neuropathologies, including demyelinating diseases and metabolic diseases [39, 40]. In primary culture, the mouse corona viruses RSA59 (a demyelinating strain) and RSMHV2 (a non-demyelinating strain) infect OPCs and mature oligodendrocytes differently. RSA59 infects both lineages (OPCs along with mature oligodendrocytes); on the contrary, RSMHV2 only infects OPCs [refer to Figure 3 and 4 (Kenyon et al., 2015)] [17]. Oligodendrocyte lineages assist in the myelination of neurons and enhance neuronal functionality [41]. Recent investigations have also demonstrated that OPCs are important cytotoxic targets during inflammatory demyelination, participate in antigen presentation, and have immunological attributes [42]. Therefore, this procedure will also help to dissect the cellular mechanism behind virus-induced demyelination through in vitro studies using primary oligodendrocytes.
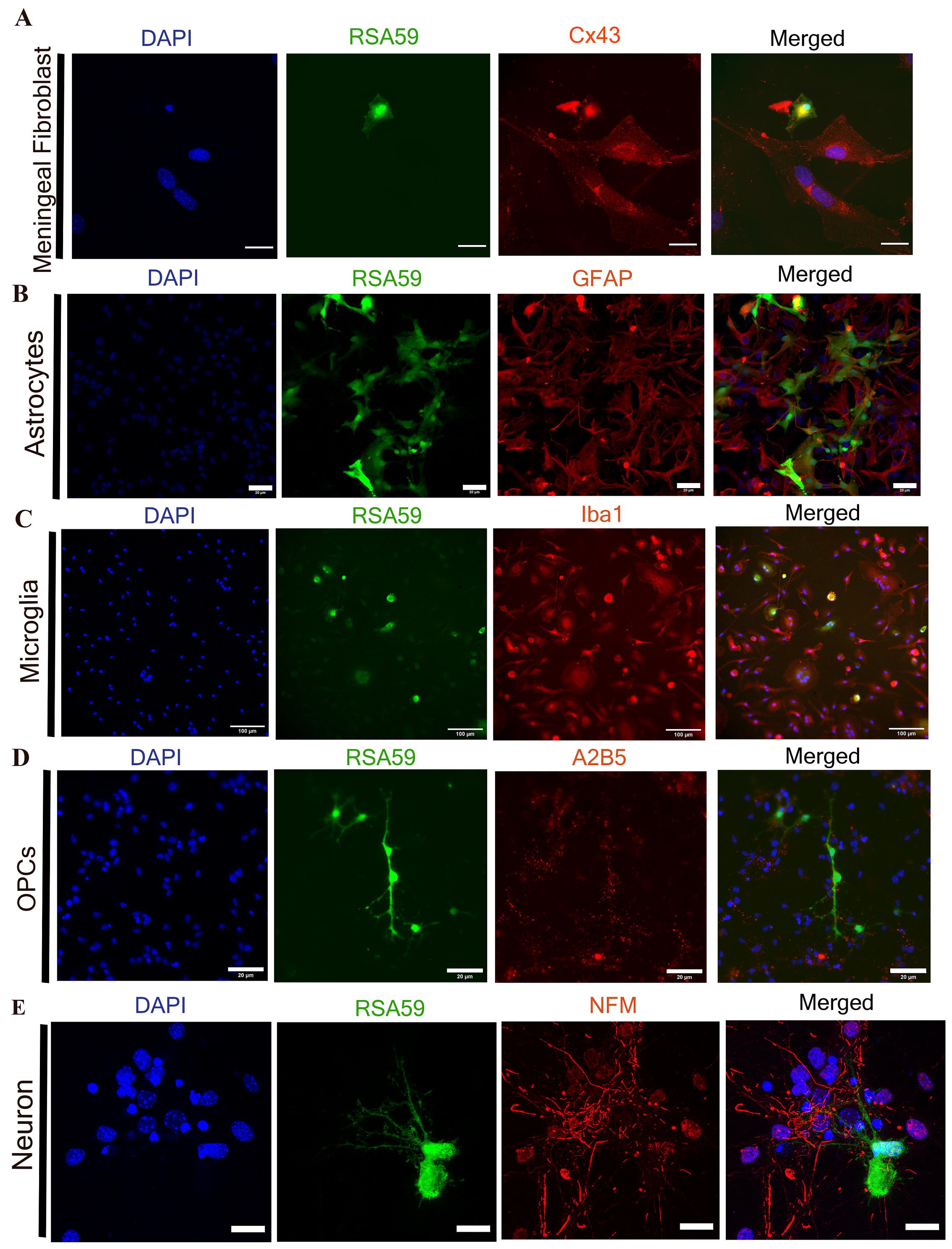
Figure 6. Downstream applications of isolated neuroglial and meningeal fibroblast cells. All neuroglial cells are getting infected with a recombinant murine coronavirus (RSA59), which shows EGFP expression (green) post-infection of cells (Panel A–E). Infected cells were fixed with 4% PFA and proceeded for an immunofluorescence experiment. A. Cx43, a surface gap junction forming protein (in red), got internalized post RSA59 (in green) infection in meningeal fibroblast cells. B–D. RSA59 virus (in green) shows tropism for astrocytes, OPCs, and microglial cells, confirmed using cell-specific markers GFAP, A2B5, and Iba1 (in red), respectively. E. Confocal image shows the RSA59 virus (in green) also has neuronal tropism marked by neuronal specific marker NFM (in red). For double-immunostaining, Alexa Fluor 488, 546, and 568 secondary antibodies were used as previously described (see Recipes); DAPI marks the cell nuclei (in blue). Scale bars are 20 µm in A, B, D, and E, and 100 µm in C.
Microglia are the brain-resident myeloid cells that perform a variety of dynamic functions such as antigen presentation, phagocytosis, and cytokine generation [43]. Primary astrocytes and microglia treated with TNF α result in differential functional activation of both cells [refer Figure 5 (Marek et al., 2008)] and demonstrate that specific isolation of primary cells is suitable for differential functional activity analysis [22]. In addition to their distinctive function in the brain, several reports also suggest that interacting nexus of neurons with glial cells, oligodendrocytes, and astrocytes enhances myelination in vitro [44]. According to earlier reports, embryonic prenatal rat pups’ hippocampus [19, 45] and adult rats’ hippocampus [46] are required for neuronal culture; however, with this isolation procedure, from a single day-0 post-natal pup brain, neurons can also be isolated along with a wide variety of CNS glial cells and meningeal fibroblasts, bypassing the sacrifice of female mice. The primary neurons also have been used to study differential infectivity of four isogenic recombinant strains of murine beta coronaviruses (RSA59PP, RSA59P, RSMHV2PP, and RSMHV2 P) [refer to Figure 1 (Safiriyu et al., 2023)] [20].
The presented protocol has been extensively employed for several published research works (references given below) in order to perform various cell-based and biochemical experiments including immunofluorescence and gene expression (RNA and protein) studies through RT-qPCR, western blot, and ELISA.
Isolation of meningeal fibroblast was done in Bose et al. (2018) [16]; isolation of astrocytes was done in Basu et al. (2016 and 2017) [21, 35]; isolation of OPCs and differentiated oligodendrocytes was done in Kenyon et al. (2015) [17]; isolation of microglia was done in Marek et al. (2008) [22]; and isolation of primary neurons was done in Safiriyu et al. (2023) [20].
General notes and troubleshooting
Troubleshooting (Table 3)
Table 3. Troubleshooting suggestions are listed here
| Step | Problem | Possible reason | Possible solution |
|---|---|---|---|
| 11 | Low yield of meningeal fibroblast | Improper removal of meninges, contamination with brain tissues | Properly isolate meninges using a pair of sharp fine forceps, kept separately to reduce astrocyte contamination |
| 35 | Fibroblast contamination in astrocyte culture | Inadequate removal of meninges | Carefully remove meninges as much as possible |
| 36 | The brain tissues are too big | Poor mincing, diminished scissor functioning | Mince for a longer duration, use sharp scissors |
| 41 | Low yield of neuroglial cells | Removal of cell pellets along with the supernatant (contain viscous myelin debris) | Carefully discard the supernatant by observing the pellets; in case of doubt, refilter the supernatant and again centrifuge |
| 44 | Clogging of mesh | Improper mincing results in larger tissue fragments | Refilter using a new 70 µm cell strainer |
Acknowledgments
Author Contributions: Manuscript writing S.K.S., M.S. and J.D.S. Figure Preparation S.K.S., M.S. and J.D.S. All authors contributed to the article and read and approved the submitted version.
We would like to thank CSIR for providing fellowships to S.K.S. and M.S. We also thank the central imaging facility and Animal Facility of IISER Kolkata for all the necessary support for our experiments. We also want to thank SERB-POWER grant: SPG/20220/000454 (Promoting opportunities for Women in Exploratory Research) program for providing research funds to JDS for a structured effort toward enhanced diversity in research to ensure equal access and weighed opportunities for Indian women scientists engaged in research and development activities. The funders had no role in study design, data collection, and analysis, the decision to publish, or manuscript preparation.
This procedure has been extensively used in several previous research works: isolation of meningeal fibroblast was done in Bose et al. (2018) [16], isolation of astrocytes was done in Basu et al. (2016 and 2017) [21, 35], isolation of OPCs and differentiated oligodendrocytes was done in Kenyon et al. (2015), isolation of microglia was done in Marek et al. (2008) [22], and isolation of primary neurons was done in Safiriyu et al. (2023) [20].
Competing interests
The authors declare no competing interests.
Ethical considerations
All animal experiments were approved by the ethical committee of the Indian Institute of Science Education and Research (IISER) Kolkata. Animal experiments are performed in strict accordance with the Institute Animal Ethical Committee guidelines. IAEC Protocol number: IISERK/IAEC/AP/2019/29.01 SL NO-43.
References
- Verkhratsky, A., Krishtal, O. A. and Burnstock, G. (2009). Purinoceptors on Neuroglia. Mol. Neurobiol. 39(3): 209–209. doi: 10.1007/s12035-009-8070-3
- Gomes, F., Spohr, T., Martinez, R. and Moura Neto, V. (2001). Cross-talk between neurons and glia: highlights on soluble factors. Braz. J. Med. Biol. Res. 34(5): 611–620. doi: 10.1590/s0100-879x2001000500008
- Fields, R. D. and Stevens-Graham, B. (2002). New Insights into Neuron-Glia Communication. Science 298(5593): 556–562. doi: 10.1126/science.298.5593.556
- Garden, G. A. and La Spada, A. R. (2012). Intercellular (Mis)communication in Neurodegenerative Disease. Neuron 73(5): 886–901. doi: 10.1016/j.neuron.2012.02.017
- Schiera, G., Di Liegro, C. M. and Di Liegro, I. (2019). Cell-to-Cell Communication in Learning and Memory: From Neuro- and Glio-Transmission to Information Exchange Mediated by Extracellular Vesicles. Int. J. Mol. Sci. 21(1): 266. doi: 10.3390/ijms21010266
- Ransohoff, R. M. (2012). Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat. Neurosci. 15(8): 1074–1077. doi: 10.1038/nn.3168
- Domínguez-Oliva, A., Hernández-Ávalos, I., Martínez-Burnes, J., Olmos-Hernández, A., Verduzco-Mendoza, A. and Mota-Rojas, D. (2023). The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 13(7): 1223. doi: 10.3390/ani13071223
- Drummond, E. and Wisniewski, T. (2016). Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 133(2): 155–175. doi: 10.1007/s00401-016-1662-x
- Beal, M. F. (2001). Experimental models of Parkinson's disease. Nat. Rev. Neurosci. 2: 325–334. doi:10.1038/35072550
- Das Sarma, J. (2010). A Mechanism of Virus-Induced Demyelination. Interdiscip. Perspect. Infect. Dis. 2010: 1–28. doi: 10.1155/2010/109239
- Constantinescu, C. S., Farooqi, N., O'Brien, K. and Gran, B. (2011). Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164(4): 1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x
- Zhang, S., Zhao, H., Liu, Z., Liu, K., Zhu, H., Pu, W., He, L., Wang, R. A. and Zhou, B. (2022). Monitoring of cell-cell communication and contact history in mammals. Science 378(6623): eabo5503. doi: 10.1126/science.abo5503
- American Type Culture Collection Standards Development Organization Workgroup A. S. N. (2010). Cell line misidentification: the beginning of the end. Nat Rev Cancer 10(6): 441–448. doi:10.1038/nrc2852
- Lorsch, J. R., Collins, F. S. and Lippincott-Schwartz, J. (2014). Fixing problems with cell lines. Science 346(6216): 1452–1453. doi: 10.1126/science.1259110
- Güler, B. E., Krzysko, J. and Wolfrum, U. (2021). Isolation and culturing of primary mouse astrocytes for the analysis of focal adhesion dynamics. STAR Protoc. 2(4): 100954. doi: 10.1016/j.xpro.2021.100954
- Bose, A., Basu, R., Maulik, M. and Das Sarma, J. (2018). Loss of Cx43-Mediated Functional Gap Junction Communication in Meningeal Fibroblasts Following Mouse Hepatitis Virus Infection. Mol. Neurobiol. 55(8): 6558–6571. doi: 10.1007/s12035-017-0861-3
- Kenyon, L. C., Biswas, K., Shindler, K. S., Nabar, M., Stout, M., Hingley, S. T., Grinspan, J. B. and Das Sarma, J. (2015). Gliopathy of Demyelinating and Non-Demyelinating Strains of Mouse Hepatitis Virus. Front. Cell. Neurosci. 9: e00488.
- Robinson, A. P., Rodgers, J. M., Goings, G. E. and Miller, S. D. (2014). Characterization of Oligodendroglial Populations in Mouse Demyelinating Disease Using Flow Cytometry: Clues for MS Pathogenesis. PLoS One 9(9): e107649. doi: 10.1371/journal.pone.0107649
- Tomassoni-Ardori, F., Hong, Z., Fulgenzi, G. and Tessarollo, L. (2020). Generation of Functional Mouse Hippocampal Neurons. Bio Protoc 10(15): e3702. doi: 10.21769/bioprotoc.3702
- Safiriyu, A. A., Mulchandani, V., Anakkacheri, M. N., Pal, D. and Das Sarma, J. (2023). Proline–Proline Dyad in the Fusion Peptide of the Murine β-Coronavirus Spike Protein’s S2 Domain Modulates Its Neuroglial Tropism. Viruses 15(1): 215. doi: 10.3390/v15010215
- Basu, R., Banerjee, K., Bose, A. and Das Sarma, J. (2016). Mouse Hepatitis Virus Infection Remodels Connexin43-Mediated Gap Junction Intercellular Communication In Vitro and In Vivo. J. Virol. 90(5): 2586–2599. doi: 10.1128/jvi.02420-15
- Marek, R., Caruso, M., Rostami, A., Grinspan, J. B. and Sarma, J. D. (2008). Magnetic cell sorting: A fast and effective method of concurrent isolation of high purity viable astrocytes and microglia from neonatal mouse brain tissue. J. Neurosci. Methods 175(1): 108–118. doi: 10.1016/j.jneumeth.2008.08.016
- Das Sarma, J., Scheen, E., Seo, S. H., Koval, M. and Weiss, S. R. (2002). Enhanced green fluorescent protein expression may be used to monitor murine coronavirus spread in vitro and in the mouse central nervous system. J. Neurovirol. 8(5): 381–391. doi: 10.1080/13550280260422686
- Das Sarma, J., Meyer, R. A., Wang, F., Abraham, V., Lo, C. W. and Koval, M. (2001). Multimeric connexin interactions prior to the trans-Golgi network. J. Cell Sci. 114(22): 4013–4024. doi: 10.1242/jcs.114.22.4013
- Bifari, F., Berton, V., Pino, A., Kusalo, M., Malpeli, G., Di Chio, M., Bersan, E., Amato, E., Scarpa, A., Krampera, M., et al. (2015). Meninges harbor cells expressing neural precursor markers during development and adulthood. Front. Cell. Neurosci. 9: e00383. doi: 10.3389/fncel.2015.00383
- Hopperton, K. E., Mohammad, D., Trépanier, M. O., Giuliano, V. and Bazinet, R. P. (2017). Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol. Psychiatry 23(2): 177–198. doi: 10.1038/mp.2017.246
- Saadi, F., Chakravarty, D., Kumar, S., Kamble, M., Saha, B., Shindler, K. S. and Das Sarma, J. (2021). CD40L protects against mouse hepatitis virus-induced neuroinflammatory demyelination. PLoS Pathog. 17(12): e1010059. doi: 10.1371/journal.ppat.1010059
- Chiu, F., Norton, W. T. and Fields, K. L. (1981). The Cytoskeleton of Primary Astrocytes in Culture Contains Actin, Glial Fibrillary Acidic Protein, and the Fibroblast‐Type Filament Protein, Vimentin. J. Neurochem. 37(1): 147–155. doi: 10.1111/j.1471-4159.1981.tb05302.x
- Suzuki, N., Sekimoto, K., Hayashi, C., Mabuchi, Y., Nakamura, T. and Akazawa, C. (2017). Differentiation of Oligodendrocyte Precursor Cells from Sox10-Venus Mice to Oligodendrocytes and Astrocytes. Sci. Rep. 7(1): e1038/s41598–017–14207–0. doi: 10.1038/s41598-017-14207-0
- Polito, A. and Reynolds, R. (2005). NG2‐expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J. Anat. 207(6): 707–716. doi: 10.1111/j.1469-7580.2005.00454.x
- Djelloul, M., Holmqvist, S., Boza-Serrano, A., Azevedo, C., Yeung, M. S., Goldwurm, S., Frisén, J., Deierborg, T. and Roybon, L. (2015). Alpha-Synuclein Expression in the Oligodendrocyte Lineage: an In Vitro and In Vivo Study Using Rodent and Human Models. Stem Cell Rep. 5(2): 174–184. doi: 10.1016/j.stemcr.2015.07.002
- Cheng, Y. C., Huang, C. J., Lee, Y. J., Tien, L. T., Ku, W. C., Chien, R., Lee, F. K. and Chien, C. C. (2016). Knocking down of heat-shock protein 27 directs differentiation of functional glutamatergic neurons from placenta-derived multipotent cells. Sci. Rep. 6(1): e1038/srep30314. doi: 10.1038/srep30314
- Crino, P., Trojanowski, J., Dichter, M. and Eberwine, J. (1996). Embryonic neuronal markers in tuberous sclerosis: Single-cell molecular pathology. Proc. Natl. Acad. Sci. U.S.A. 93(24): 14152–14157. doi: 10.1073/pnas.93.24.14152
- Sofroniew, M. V. and Vinters, H. V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119(1): 7–35. doi: 10.1007/s00401-009-0619-8
- Basu, R., Bose, A., Thomas, D. and Das Sarma, J. (2017). Microtubule-assisted altered trafficking of astrocytic gap junction protein connexin 43 is associated with depletion of connexin 47 during mouse hepatitis virus infection. J. Biol. Chem. 292(36): 14747–14763. doi: 10.1074/jbc.m117.786491
- Maulik, M., Vasan, L., Bose, A., Dutta Chowdhury, S., Sengupta, N. and Das Sarma, J. (2020). Amyloid-β regulates gap junction protein connexin 43 trafficking in cultured primary astrocytes. J. Biol. Chem. 295(44): 15097–15111. doi: 10.1074/jbc.ra120.013705
- Stamatovic, S. M., Johnson, A. M., Keep, R. F. and Andjelkovic, A. V. (2016). Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 4(1): e1154641. doi: 10.1080/21688370.2016.1154641
- Bose, A., Kasle, G., Jana, R., Maulik, M., Thomas, D., Mulchandani, V., Mukherjee, P., Koval, M. and Das Sarma, J. (2023). Regulatory role of endoplasmic reticulum resident chaperone protein ERp29 in anti-murine β-coronavirus host cell response. J. Biol. Chem. 299(2): 102836. doi: 10.1016/j.jbc.2022.102836
- Grinspan, J. (2002). Cells and Signaling in Oligodendrocyte Development. J. Neuropathol. Exp. Neurol. 61(4): 297–306. doi: 10.1093/jnen/61.4.297
- Narine, M. and Colognato, H. (2022). Current Insights Into Oligodendrocyte Metabolism and Its Power to Sculpt the Myelin Landscape. Front. Cell. Neurosci. 16: e892968. doi: 10.3389/fncel.2022.892968
- Mazuir, E., Fricker, D. and Sol-Foulon, N. (2021). Neuron–Oligodendrocyte Communication in Myelination of Cortical GABAergic Cells. Life (Basel, Switzerland) 11(3): 216. doi: 10.3390/life11030216
- Kirby, L., Jin, J., Cardona, J. G., Smith, M. D., Martin, K. A., Wang, J., Strasburger, H., Herbst, L., Alexis, M., Karnell, J., et al. (2019). Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 10(1): e1038/s41467–019–11638–3. doi: 10.1038/s41467-019-11638-3
- Michell-Robinson, M. A., Touil, H., Healy, L. M., Owen, D. R., Durafourt, B. A., Bar-Or, A., Antel, J. P. and Moore, C. S. (2015). Roles of microglia in brain development, tissue maintenance and repair. Brain 138(5): 1138–1159. doi: 10.1093/brain/awv066
- Ishibashi, T., Dakin, K. A., Stevens, B., Lee, P. R., Kozlov, S. V., Stewart, C. L. and Fields, R. D. (2006). Astrocytes Promote Myelination in Response to Electrical Impulses. Neuron 49(6): 823–832. doi: 10.1016/j.neuron.2006.02.006
- Kaech, S., Banker, G. (2006). Culturing hippocampal neurons. Nat Protoc 1: 2406–2415. doi:10.1038/nprot.2006.356
- Brewer, G. J. (1997). Isolation and culture of adult rat hippocampal neurons. J. Neurosci. Methods 71(2): 143–155. doi: 10.1016/s0165-0270(96)00136-7
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Kumar Samal, S., Sharma, M. and Das Sarma, J. (2024). Isolation and Enrichment of Major Primary Neuroglial Cells from Neonatal Mouse Brain. Bio-protocol 14(2): e4921. DOI: 10.21769/BioProtoc.4921.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Cell Biology > Cell isolation and culture > Monolayer culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









