- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Optical Modulation of the Blood-Brain Barrier for Glioblastoma Treatment
Published: Vol 14, Iss 2, Jan 20, 2024 DOI: 10.21769/BioProtoc.4920 Views: 2370
Reviewed by: Mary Luz UribeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
NanoPDLIM2-Based Combination Therapy for Lung Cancer Treatment in Mouse Preclinical Studies
Thi Hoa Le [...] Zhaoxia Qu
Sep 5, 2025 2231 Views
Combining Microwave Ablation With CAR-T-Cell Therapy in Tumor-Bearing Mouse Models
Bihui Cao [...] Jia Shen
Oct 20, 2025 2319 Views
Stimulation-Guided AAV Delivery and Longitudinal Assessment of Optogenetic Expression in Rat Motor Nerves
Emma M. Moravec and Jordan J. Williams
Dec 20, 2025 593 Views
Abstract
The blood–brain barrier (BBB) is a major obstacle to the diagnostics and treatment of many central nervous system (CNS) diseases. A prime example of this challenge is seen in glioblastoma (GBM), the most aggressive and malignant primary brain tumor. The BBB in brain tumors, or the blood–brain–tumor barrier (BBTB), prevents the efficient delivery of most therapeutics to brain tumors. Current strategies to overcome the BBB for therapeutic delivery, such as using hyperosmotic agents (mannitol), have impeded progress in clinical translation limited by the lack of spatial resolution, high incidences of complications, and potential for toxicity. Focused ultrasound combined with intravenously administered microbubbles enables the transient disruption of the BBB and has progressed to early-phase clinical trials. However, the poor survival with currently approved treatments for GBM highlights the compelling need to develop and validate treatment strategies as well as the screening for more potent anticancer drugs. In this protocol, we introduce an optical method to open the BBTB (OptoBBTB) for therapeutic delivery via ultrashort pulse laser stimulation of vascular targeting plasmonic gold nanoparticles (AuNPs). Specifically, the protocol includes the synthesis and characterization of vascular-targeting AuNPs and a detailed procedure of optoBBTB. We also report the downstream characterization of the drug delivery and tumor treatment efficacy after BBB modulation. Compared with other barrier modulation methods, our optical approach has advantages in high spatial resolution and minimally invasive access to tissues. Overall, optoBBTB allows for the delivery of a variety of therapeutics into the brain and will accelerate drug delivery and screening for CNS disease treatment.
Key features
• Pulsed laser excitation of vascular-targeting gold nanoparticles non-invasively and reversibly modulates the blood–brain barrier permeability.
• OptoBBTB enhances drug delivery in clinically relevant glioblastoma models.
• OptoBBTB has the potential for drug screening and evaluation for superficial brain tumor treatment.
Graphical overview
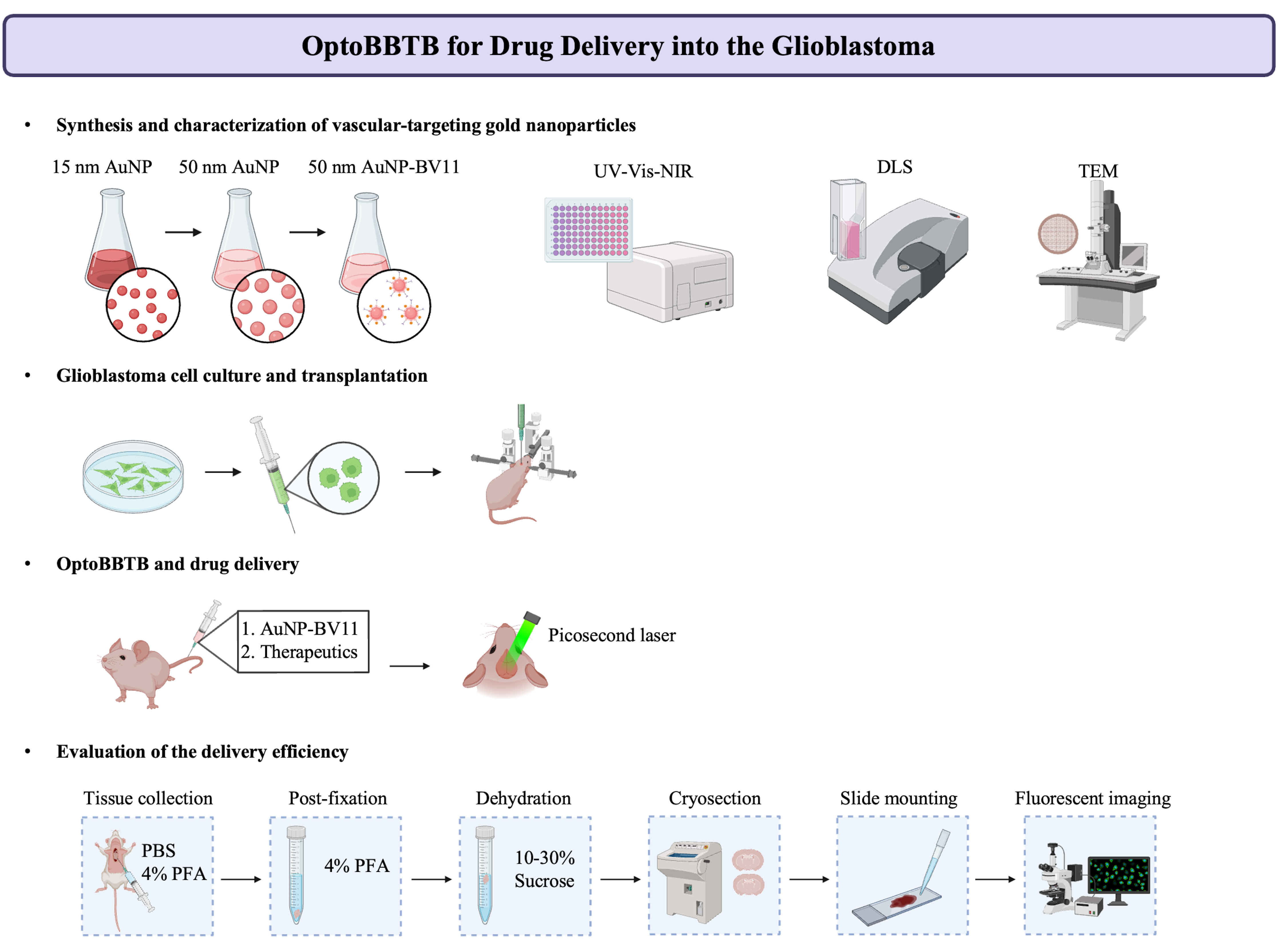
Background
High-grade gliomas represent the most malignant primary brain tumors in adults. Among these, grade IV astrocytomas, also referred to as glioblastoma multiforme (GBM), constitute over 50% of all primary brain tumors (Straehla et al., 2022). Despite standard-of-care treatment that includes maximal safe resection, fractionated radiation to 60 Gy with concurrent daily temozolomide (TMZ) followed by up to six months of adjuvant TMZ, as well as the progress made in developing new treatment approaches, GBM continues to remain predominantly incurable (Stupp et al., 2005). In addition to the heterogeneous and immunosuppressive tumor microenvironment, a significant challenge for GBM therapy lies in the difficulty of delivering sufficient drug concentrations in the tumor due to the protective blood–brain barrier (BBB), which is also known as blood–brain–tumor barrier (BBTB) in brain tumors. The BBB governs the exchange of ions, molecules, and cells between the bloodstream and the brain, ensures optimal neuronal functioning, and shields brain tissue from harmful toxins and pathogens. Nevertheless, the BBB also prevents more than 98% of small-molecule drugs and all macromolecular therapeutics from entering the brain, resulting in a challenge in drug delivery for brain disease treatment. Although a variety of pathologic conditions, including brain tumors, can disrupt the integrity of the BBB (Sarkaria et al., 2018), the disruption is heterogeneous or inadequate to facilitate clinically meaningful drug penetration. Additionally, evidence suggests that GBM tumor cells infiltrate neighboring tissues without disrupting the BBB, leading to inevitable recurrence (Belykh et al., 2020). To date, numerous strategies have been developed to overcome the BBB for drug delivery (van Tellingen et al., 2015; Belykh et al., 2020; Rosenblum et al., 2018; Brunner and Borchard, 2021). Nonetheless, GBM is still considered incurable, and many promising results from preclinical studies do not translate successfully to clinical trials. Thus, it is of utmost importance to create and validate treatment strategies using clinically relevant GBM models to achieve a more efficient transition from preclinical testing to clinical implementation.
Here, we describe an optical method to open the BBTB (optoBBTB) to modulate the BBB for drug delivery in two clinically relevant genetically engineered mouse models (GEMMs) that recapitulate key GBM characteristics (e.g., PS5A1 GEMM for infiltrative tumor margin with an intact BBTB and 73C GEMM for angiogenic tumor core with a gradually disrupted BBTB) (Cai et al., 2023). We show that optoBBTB facilitates the delivery of paclitaxel (Taxol) to both GEMMs, which further reduces tumor growth and prolongs the mice’s survival. These results demonstrate that optoBBTB is effective for drug delivery and GBM treatment in these two clinically relevant GEMMs.
Materials and reagents
Biological materials
Immunodeficient nude mice Foxn1nu, 7 weeks old, female, 20–25 g (Jackson Laboratory, catalog number: Nu/J 002019)
PS5A1 cell line, generated from Dr. Robert Bachoo’s laboratory at the University of Texas Southwestern Medical Center (Singh et al., 2017; Gao et al., 2020)
73C cell line, generated from Dr. Robert Bachoo’s laboratory at the University of Texas Southwestern Medical Center (Singh et al., 2017; Gao et al., 2020)
Anti-JAM-A antibodies BV11, provided by Dr. Monica Giannotta at FIRC Institute of Molecular Oncology Foundation
Reagents
Reagents for glassware cleaning
Hydrochloric acid (HCl) 37% w/w (Fisher Scientific, catalog number: 7647-01-0)
Nitric acid (HNO3) 70% w/w (Fisher Scientific, catalog number: 7697-37-2)
Reagents for gold nanoparticle (AuNP) synthesis and antibody conjugation
Gold (III) chloride trihydrate (HAuCl4·3H2O) (Sigma-Aldrich, catalog number: 520918-1G)
Sodium citrate tribasic dihydrate (Sigma-Aldrich, catalog number: S4641-500G)
Hydroquinone (Sigma-Aldrich, catalog number: H9003-100G)
OPSS-PEG-SVA 3400 Da (Laysan Bio, Inc., catalog number: OPSS-SVA-3400)
mPEG-thiol 1,000 Da (Laysan Bio, Inc., catalog number: M-SH-1K)
Cytiva HyCloneTM phosphate-buffered saline (PBS) (Fisher Scientific, catalog number: SH3025801)
PierceTM borate buffer (20×) (Fisher Scientific, catalog number: PI28341)
Endotoxin-free ultrapure water (Sigma-Aldrich, catalog number: TMS-011-A)
Reagent for cell culture
GibcoTM DMEM high glucose GlutaMAXTM supplement (Fisher Scientific, catalog number: 10-566-016)
GibcoTM DMEM/F-12, no glutamine (Fisher Scientific, catalog number: 21-331-020)
GibcoTM Hanks' Balanced Salt Solution, no calcium, no magnesium, and no phenol red (HBSS) (Fisher Scientific, catalog number: 14-175-095)
Epidermal growth factor (EGF) (Sigma-Aldrich, catalog number: E4127)
Recombinant human fibroblast growth factor basic (FGF2) (Sigma-Aldrich, catalog number: F0291)
Progesterone (Sigma-Aldrich, catalog number: P6149)
GibcoTM B-27TM supplement (50×), serum-free (Fisher Scientific, catalog number: 17-504-044)
GibcoTM insulin-transferrin-selenium-ethanolamine 100× (Fisher Scientific, catalog number: 51-500-056)
GibcoTM fetal bovine serum (FBS) (Fisher Scientific, catalog number: A3160502)
GibcoTM penicillin-streptomycin (Fisher Scientific, catalog number: 15-140-122)
GibcoTM StemProTM AccutaseTM cell dissociation reagent (Fisher Scientific, catalog number: A1110501)
GibcoTM Trypsin-EDTA (0.25%), phenol red (Fisher Scientific, catalog number: 25-200-056)
Reagents for optoBBTB and drug delivery
0.9% saline solution, sterile (Fisher Scientific, catalog number: S5815)
Isoflurane USP (Covetrus, catalog number: NDC 66794-017-25)
Cremophor® EL (Millipore Sigma, catalog number: 61791-12-6)
Ethanol absolute (Fisher Scientific, catalog number: BP2818100)
Taxol Janelia Fluor® 646 (Tocris Bioscience, catalog number: 6266)
Paclitaxel (Sigma-Aldrich, catalog number: T7191)
Buprenorphine SR-LAB 5 mL (1 mg/mL) (ZooPharm, catalog number: BSRLAB1)
Reagents for tissue collection and ex vivo drug delivery analysis
Paraformaldehyde, 4% in PBS (Fisher Scientific, catalog number: AAJ61899AP)
Sucrose (Millipore Sigma, catalog number: 57-50-1)
Hoechst 33342 solution (Fisher Scientific, catalog number: PI62249)
Scigen Tissue-PlusTM O.C.T. compound (Fisher Scientific, catalog number: 23-730-571)
InvitrogenTM Fluoromount-GTM mounting medium (Fisher Scientific, catalog number: 50-187-88)
Solutions
HAuCl4 solution, 1 M (see Recipes)
HAuCl4 solution, 25 mM (see Recipes)
Sodium citrate solution, 112.2 mM (see Recipes)
Sodium citrate solution, 15 mM (see Recipes)
Hydroquinone solution, 25 mM (see Recipes)
2 mM borate buffer stock solution, pH = 8.5 (see Recipes)
10% sucrose stock solution (see Recipes)
20% sucrose stock solution (see Recipes)
30% sucrose stock solution (see Recipes)
Recipes
HAuCl4 solution, 1 M
*Note: Upon unsealing the bottle, rapidly transfer the HAuCl4·3H2O into a tube to measure the weight. Aliquot the stock solution into 0.5 mL Eppendorfs and snap-freeze the solution with liquid nitrogen for future utilization. The stocks can be stored at -80 °C.
Reagent Final concentration Quantity or Volume HAuCl4·3H2O 1 M 1 g (see note*) Ultrapure H2O n/a 2.539 mL Total (optional) n/a n/a HAuCl4 solution, 25 mM
Note: This solution should be prepared freshly.
Reagent Final concentration Quantity or Volume HAuCl4 (1 M) 25 mM 0.05 mL Ultrapure H2O n/a 1.95 mL Total (optional) n/a 2 mL Sodium citrate solution, 112.2 mM
Note: This solution should be prepared freshly.
Reagent Final concentration Quantity or Volume Sodium citrate 112.2 mM 0.165 g Ultrapure H2O n/a 5 mL Total (optional) n/a n/a Sodium citrate solution, 15 mM
Note: This solution should be prepared freshly.
Reagent Final concentration Quantity or Volume Sodium citrate 15 mM 0.022 g Ultrapure H2O n/a 5 mL Total (optional) n/a n/a Hydroquinone solution, 25 mM
Note: This solution should be prepared freshly.
Reagent Final concentration Quantity or Volume Hydroquinone 25 mM 0.0138 g Ultrapure H2O n/a 5 mL Total (optional) n/a n/a 2 mM borate buffer stock solution, pH = 8.5
Reagent Final concentration Quantity or Volume Borate buffer (20×) 2 mM 0.2 mL Ultrapure H2O n/a 99.8 mL Total (optional) n/a 100 mL 10% sucrose stock solution
*Note: PBS should be added in increments of 10 mL. Allow the solution to mix completely before adding any more PBS.
Reagent Final concentration Quantity or Volume Sucrose 10% (w/v) 10 g PBS (1×) n/a see note* Total (optional) n/a 100 mL 20% sucrose stock solution
*Note: PBS should be added in increments of 10 mL. Allow the solution to mix completely before adding any more PBS.
Reagent Final concentration Quantity or Volume Sucrose 20% (w/v) 20 g PBS (1×) n/a see note* Total (optional) n/a 100 mL 30% sucrose stock solution (100 mL)
*Note: PBS should be added in increments of 10 mL. Allow the solution to mix completely before adding any more PBS.
Reagent Final concentration Quantity or Volume Sucrose 30% (w/v) 30 g PBS (1×) n/a see note* Total n/a 100 mL
Laboratory supplies
Supplies for AuNP synthesis and antibody conjugation
FisherbrandTM polygon stir bars (Fisher Scientific, catalog number: 14-512-127)
FisherbrandTM IsotempTM stirrer (Fisher Scientific, catalog number: S88850200)
PYREXTM short-neck heavy-wall round-bottom boiling flasks, standard taper joints (Fisher Scientific, catalog number: 10-068-1C)
PYREXTM narrow-mouth heavy-duty glass Erlenmeyer flask 250 mL (Fisher Scientific, catalog number: 10-040F)
DWK Life Sciences KimbleTM KIMAXTM west condensers (Fisher Scientific, catalog number: K452000-2430)
PYREXTM crystallizing dishes (Fisher Scientific, catalog number: 08-741-D)
FisherbrandTM sterile polystyrene disposable serological pipettes with magnifier stripe 10 mL (Fisher Scientific, catalog number: 13-678-11E)
FisherbrandTM sterile polystyrene disposable serological pipettes with magnifier stripe 50 mL (Fisher Scientific, catalog number: 13-678-11F)
FisherbrandTM premium microcentrifuge tubes 2.0 mL (Fisher Scientific, catalog number: 05-408-138)
FisherbrandTM premium microcentrifuge tubes 1.5 mL (Fisher Scientific, catalog number: 05-408-129)
Thermo ScientificTM low protein binding microcentrifuge tubes 1.5 mL (Fisher Scientific, catalog number: PI90410)
Thermo ScientificTM NuncTM 15 mL conical sterile polypropylene centrifuge tubes (Fisher Scientific, catalog number: 12-565-269)
Thermo ScientificTM NuncTM 50 mL conical sterile polypropylene centrifuge tubes (Fisher Scientific, catalog number: 12-565-271)
0.22 μm syringe filters (VWR, catalog number: 76479-030)
SpectrumTM trial size kits for biotech-grade CE dialysis membrane tubing (1 m), MWCO: 20 kd (Fisher Scientific, catalog number: 08-801-251)
Supplies for AuNP characterization
FisherbrandTM 384-well polystyrene plates (Fisher Scientific, catalog number: 12-566-625)
FisherbrandTM disposable cuvettes (Fisher Scientific, catalog number: 14-955-127)
Electron microscopy sciences carbon support film 5–6 nm thick on square 200 mesh copper grid (Fisher Scientific, catalog number: NC9044609)
Supplies for cell culture
Thermo ScientificTM NuncTM EasYFlaskTM cell culture flasks 75 cm2 (Fisher Scientific, catalog number: 12-565-349)
Thermo ScientificTM NunclonTM SpheraTM cell culture flasks 75 cm2 (Fisher Scientific, catalog number: 12-566-440)
Supplies for tumor cell transplantation in mouse and optoBBTB
Micropipette puller (Sutter Instrument, catalog number: Co. P-1000)
Instech PE-20 tubing (Fisher Scientific, catalog number: 50-890-048)
BD Micro-FineTM IV insulin syringes (Fisher Scientific, catalog number: 14-829-1D)
Med Vet International Exel butterfly infusion set, with 27 G × ¾ in. needle, 12 in. tubing (Fisher Scientific, catalog number: 50-209-2293)
PuritanTM cotton-tipped applicators (Fisher Scientific, catalog number: 22-029-553)
FisherbrandTM sterile alcohol prep pads (Fisher Scientific, catalog number: 22-363-750)
World Precision Instrument mouse dissecting kit (Fisher Scientific, catalog number: 50-822-920)
Henkel Loctite 4014 medical device instant adhesive clear (Henkel Adhesives, catalog number: 202152)
Body double standard set (Reynolds Advanced Materials)
Supplies for tissue processing and ex vivo drug delivery analysis
BD VacutainerTM eccentric tip syringe (Fisher Scientific, catalog number: B300613)
FisherbrandTM SuperfrostTM plus microscope slides (Fisher Scientific, catalog number: 12-550-15)
FisherbrandTM cover glasses: rectangles (Fisher Scientific, catalog number: 12-544-18P)
Equipment
New Era Pump Systems Inc single channel syringe pump (Fisher Scientific, catalog number: NC1072839)
FisherbrandTM accuSpinTM Micro 17 microcentrifuge (Fisher Scientific, catalog number: 13-100-675)
BioTek Synergy 2 plate reader multi-mode (The LabWorld Group, catalog number: 18531)
Dynamic light scattering (DLS) zetasizer nano zs (Malvern Panalytical)
Electron microscope (JEOL Ltd., model: JEM-1400 plus)
Diode-pumped high energy picosecond Nd: YAG lasers (EKSPLA, catalog number: PL2230)
Coherent® LabMax touch power and energy meter (Coherent®, catalog number: 2256258)
Coherent® EnergyMax laser energy sensors (Coherent®, catalog number: 1110746)
Isoflurane vaporizer (Kent Scientific, catalog number: VetFlo-1231)
DC temperature controller system (FHC, catalog number: 40-90-8D)
Rectal thermistor probe (FHC, catalog number: 40-90-5D-02)
Heating pad (FHC, catalog number: 40-90-2-07)
Syringe pump controller (World Precision Instruments, catalog number: micro-2T)
Brushless micromotor system (Osada, catalog number: EXL-M40)
Dual small animal stereotaxic instrument (KOPF, catalog number: 902)
Zeiss Stemi 305 microscope (Zeiss, catalog number: STEMI305-T-B)
HM525 NX cryostat (Epredia, catalog number: HM525 NX)
Virtual slide microscope (Olympus, model: VS120)
Spinning disk super-resolution microscope (Olympus, model: SD-OSR)
IVIS® Lumina Series III (PerkinElmer, catalog number: CLS136334)
Software and datasets
ImageJ (version 1.53c, 6/27/2020)
GraphPad Prism (version 9.5.0, 12/6/2022)
Procedure
General preparation for nanoparticle synthesis and conjugation
Prepare fresh aqua regia in the chemical fume hood, by gently mixing 3:1 (v/v) HCl: HNO3.
Soak the glassware, magnetic stir bar, and condenser in aqua regia for at least 30 min.
CAUTION: Mishandling of aqua regia can result in significant hazards, including the risk of explosions, skin burns, eye damage upon contact, harm to mucous membranes and the upper respiratory tract if inhaled, as well as potential harm to internal organs if ingested. It is crucial to never seal aqua regia in a closed container, as the gases produced can lead to pressure buildup and potentially over-pressurize the vessel. Proper personal protective equipment (PPE) is essential when working with aqua regia.
Rinse the glassware and magnetic stir bars thoroughly with Millipore-filtered water in the chemical fume hood followed by rinsing with endotoxin-free ultrapure water in the Class 2, type A2 biosafety cabinet.
To reduce the possibility of nanoparticle contamination for further in vivo experiments, the nanoparticle syntheses are performed in the biosafety cabinet by following all strict precautions normally adopted during cell culture.
CRITICAL STEP: To avoid nanoparticle contamination, all plasticware used in the synthesis should be endotoxin-free certified. All solvents and other reagents used for nanoparticle preparation in this work were strictly opened inside the biosafety cabinet. All the containers (i.e., conical flasks and dialysis containers) should be rinsed with 70% ethanol and Millipore-filtered water and covered properly with a piece of disinfected parafilm. All reagent solutions are filtered through 0.2 μm Millipore syringe filters in the biosafety cabinet before use unless specified.
Synthesis of 15 nm gold seeds
Add 98 mL of endotoxin-free ultrapure water to the 250 mL glass round-bottom flask.
Add 1 mL of HAuCl4 solution (25 mM) to the round-bottle flask.
Boil the solution under reflux and thorough stirring.
CRITICAL STEP: The thorough and effective mixing is one of the key steps to achieving homogenous nucleation and growth to obtain nanoparticles with high monodispersity.
Add 1 mL of sodium citrate solution (112.2 mM) while stirring.
CRITICAL STEP: The reducing agent should be added quickly to obtain a homogenous nanoparticle growth and high monodispersity.
Stir and boil the solution for 10 min.
Turn off the heat and allow the system to cool to room temperature (23–25 °C) while stirring. The resulting AuNPs should exhibit a ruby-red color.
Characterize the gold seeds.
Measure nanoparticle size and size distribution using dynamic light scattering (DLS) (zetasizer nano zs). The measured hydrodynamic diameter of the nanoparticles is approximately 17 nm. The polydispersity index (PDI) is approximately 0.05.
CRITICAL STEP: The hydrodynamic diameter of the seeds should be 17–18 nm with a relatively low PDI (<0.1) to grow larger nanoparticles with the desired size and quality.
TROUBLESHOOTING: If larger or polydisperse nanoparticle sizes are observed, ensure the solution is boiled before adding the reducing agent. Ensure thorough mixing.
Record UV-Vis-NIR absorption spectra using BioTek Synergy 2 plate reader for concentration measurements (Haiss et al., 2007).
Measure the nanoparticle size and morphology using transmission electron microscopy (JEM-1400 plus electron microscope). The nanoparticle shape should be semi-spherical, and the diameter should be approximately 15 nm.
PAUSE POINT: The seeds can be stored at 4 °C for two weeks. Characterization is required prior to reusing the seeds.
Seed-mediated synthesis of 50 nm AuNPs
Add 95 mL of endotoxin-free ultrapure water to a 250 mL Erlenmeyer flask.
Add 963 μL of HAuCl4 solution (25 mM).
While stirring at room temperature, add 3.71 mL of the as-prepared gold seeds (2.23 nM).
CRITICAL STEP: For gold seeds at different concentrations, use the molar calculation to determine the equivalent volume to be added.
Under thorough mixing, quickly add 963 μL of sodium citrate solution (15 mM) and 963 μL of hydroquinone solution (25 mM).
CRITICAL STEP: These two reducing solutions should be added in quick succession to obtain nanoparticles with high monodispersity.
Stir overnight. Cover the flask with a piece of disinfected parafilm to protect the solution from contamination.
Characterize the nanoparticles.
Measure nanoparticle size and size distribution using DLS (zetasizer nano zs). The measured hydrodynamic diameter of such prepared nanoparticles is approximately 47 nm with a PDI < 0.1.
Record UV-Vis-NIR absorption spectra using BioTek Synergy 2 plate reader.
Measure the nanoparticle size and morphology using transmission electron microscopy (JEM-1400 plus electron microscope). The nanoparticle shape should be spherical, and the diameter should be approximately 50 nm.
TROUBLESHOOTING: If polydisperse nanoparticle sizes are observed, ensure the reducing agents are freshly prepared. Ensure thorough mixing.
Concentrate the nanoparticles.
Allocate the nanoparticles in 2 mL endotoxin-free Eppendorf and centrifuge the nanoparticles at 1,300× g for 30 min at room temperature.
Discard the supernatant and collect the nanoparticle pellet in a 2 mL endotoxin-free Eppendorf.
Characterize the nanoparticles after the concentration process.
Dilute the nanoparticles 100-fold using endotoxin-free pure water and perform DLS for size measurement. The measured hydrodynamic diameter of such nanoparticles is approximately 50 nm with a PDI < 0.1.
TROUBLESHOOTING: Larger nanoparticle sizes after centrifugation indicate aggregation during the concentration step. Ensure proper centrifugation time and speed.
Use the diluted nanoparticles to record UV-Vis-NIR absorption spectra for concentration calculation.
PAUSE POINT: The concentrated nanoparticles can be stored at 4 °C for two weeks. Characterization is required before reusing the nanoparticles.
Preparation of polyethylene glycol–antibody conjugates
Thaw the BV11 (anti-JAM-A antibody) on ice, dilute to 0.5 mg/mL in PBS, then dilute in 2 mM borate buffer at pH 8.5 to 0.05 mg/mL.
CAUTION: The borate buffer and PBS should be cooled on ice before starting the experiment. The antibody solution should be kept on ice. Do not filter the antibody solution.
Dissolve OPSS-PEG-SVA in 2 mM borate buffer and quickly add to the antibody solution at a 125:1 molar ratio.
CAUTION: The OPSS-PEG-SVA solution should be kept on ice.
Transfer the solution to a 15 mL Falcon tube, vortex briefly, and shake on ice for 3 h.
Remove the unreacted OPSS-PEG-SVA by dialysis.
Disinfect a plastic container with 70% ethanol, followed by rinsing with Millipore-filtered water and then rinsing with endotoxin-free ultrapure water. Fill the container with 3 L of borate buffer (2 mM, pH 8.5). Cover the container with disinfected parafilm and aluminum film. Cool the buffer to 4 °C.
Immerse a 20 kDa MWCO membrane in the borate buffer for at least 30 min to activate the membrane.
Fill the dialysis bag with the antibody-PEG mixture, followed by dialysis at 4 °C overnight to remove free OPSS-PEG-SVA under mild stirring.
CRITICAL STEP: To ensure the thorough removal of the free OPSS-PEG-SVA, the borate buffer can be replaced after dialysis for 3 h.
Functionalization of AuNPs with polyethylene glycol–antibody conjugates
Collect the thiolated antibodies in a 15 mL Falcon tube and mix the solution with concentrated AuNPs at a 200:1 molar ratio. Shake the nanoparticle–antibody solution for 1 h on ice.
Add mPEG-thiol solution (1,000 Da) at 6 PEG/nm2 to backfill the empty space of AuNPs. Shake the solution for 1 h on ice.
Purify the resulting AuNP-BV11 conjugates by centrifugation (1,300× g, 30 min, 4 °C) with ice-cold borate buffer (2 mM, pH 8.5). Repeat the process three times to remove excess antibodies.
Dilute the nanoparticles 500-fold using borate buffer (2 mM) and characterize the nanoparticles by DLS and UV-Vis-NIR absorption spectra. The measured diameter of these nanoparticles should be approximately 70 nm and the nanoparticle concentration approximately 20 nM. Representative characterization of AuNP and AuNP-BV11 is shown in Figure 1.
PAUSE POINT: AuNP-BV11 can be stored at 4 °C for up to two weeks.
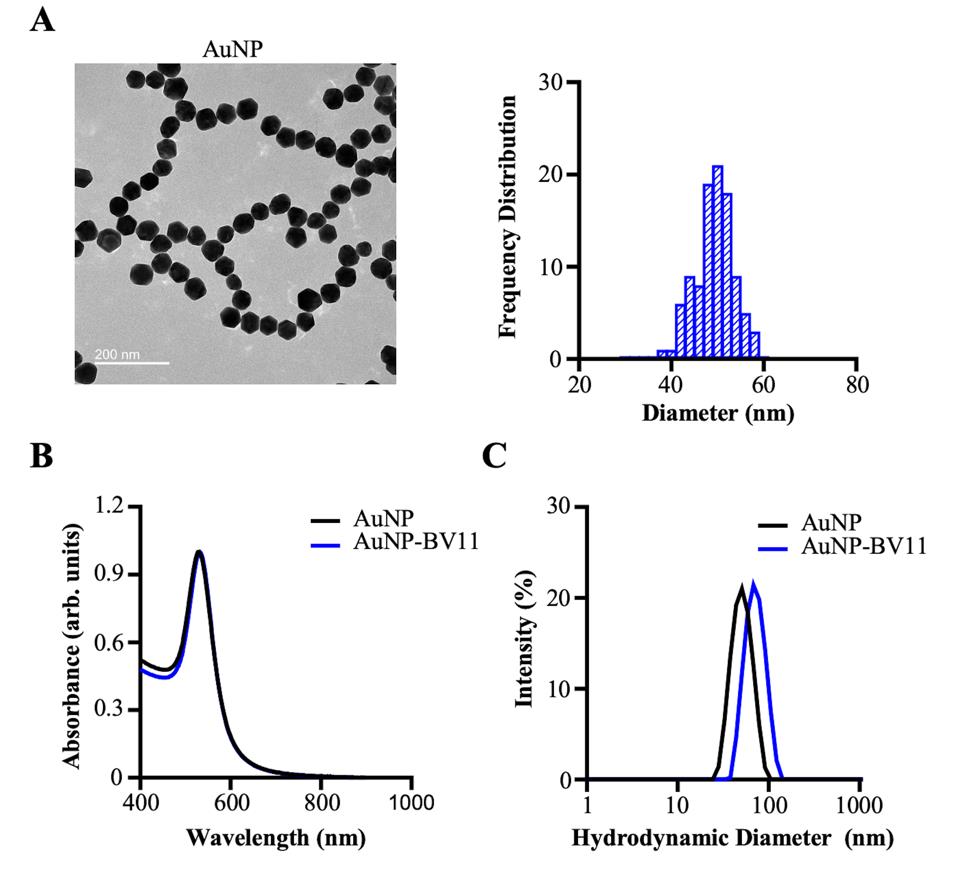
Figure 1. Characterization of the gold nanoparticles (AuNPs). (A) The morphology and size of the AuNP core are characterized by transmission electron microscopy. The size of the nanoparticles (50 ± 4 nm) was measured with ImageJ by manually counting 100 particles. (B) The localized surface plasmon resonance peaks of AuNP and AuNP-BV11 are characterized by UV-Vis-NIR spectroscopy. (C) The hydrodynamic diameter distribution by the relative intensity of AuNP and AuNP-BV11 is characterized by dynamic light scattering. The Z-average for AuNP and AuNP-BV11 was 49 nm and 69 nm, respectively. The data was plotted using GraphPad Prism software. The data was first published in Cai et al. (2023) and presented with modifications.
Glioma cell culture
Culture PS5A1 cells as free-floating neurospheres in DMEM/F12 medium containing B-27 2%, 20 ng/mL EGF, 20 ng/mL FGF2, 20 ng/mL progesterone, and 1% insulin-transferrin-selenium-ethanolamine. Subculture the glioma cells two days before transplantation to reach 70%–80% confluence to use.
Culture 73C glioma cells in DMEM high glucose medium containing 10% FBS and 1% penicillin- streptomycin. Subculture the glioma cells 2 days before transplantation to reach 70%–80% confluence to use.
Before cell transplantation, dissociate the PS5A1 and 73C glioma cells with cell dissociation reagent and Trypsin-EDTA, respectively, and resuspended in HBSS.
Prepare cell suspensions using HBSS solution to achieve a density of 2 × 105 cells/μL.
General preoperative procedure
At the start of surgical procedures and between surgeries, sterilize surgical tools (World Precision Instrument mouse dissecting kit) with a bead sterilizer for 15 min.
Clean the operative table with 70% ethanol and cover it with a sterile drape to create a sterile environment for placing sterile tools.
CRITICAL STEP: Maintain sterility throughout the entire surgical procedure.
For all procedures, anesthetize the mouse with 2%–3% isoflurane (in the air) and keep them on a heating pad at 37 °C.
CAUTION: Start the experiment once the mouse has become unresponsive to painful stimuli, such as a tail pinch. Improperly managed isoflurane can be harmful; prolonged exposure may result in adverse health effects for the operator, including symptoms like dizziness, headaches, vomiting, and nausea. The use of an exhaust system is imperative to effectively remove excess isoflurane during the surgery.
Cover the mice’s eyes with ophthalmic ointment.
Glioma cells transplantation
Subcutaneously inject Buprenorphine (1 mg/kg) into the mice before surgery.
Prepare glass micropipettes with 50 μm tips with a micropipette puller (Figure 2A). Connect the glass micropipette with a nanoinjector and set the injection speed to 30 nL/min.
CRITICAL STEP: Inject slowly to avoid an acute increase of intracranial pressure and facilitate diffusion of the fluid.
Glioma cells injection:
Stabilize the mouse on the small-animal Stereotaxic instrument with ear bars. Check the stability under the dissection scope by lightly probing with a pair of forceps.
CRITICAL STEP: There should be no movement of the skull relative to the ear bars.
Disinfect the scalp with an alcohol prep pad.
Make a midline incision of the scalp using small scissors.
Remove the membranes on top of the skull using a sterile cotton swab.
Place a thin layer of glue (Henkel Loctite 4014 medical device instant adhesive clear) on the skull and the surrounding skin. Wait 5–10 min for the glue to be bonded to the skin and skull.
Using a hand-held drill, make a single burr hole in the skull at the injection site (Figure 2B).
CRITICAL STEP: Use only light motions and avoid direct downward pressure. Stop drilling every 20–30 s to remove bone dust using compressed air.
Fill the glass micropipette with tumor cell suspension. Align the micropipette with the burr hole (Figure 2C).
Lower the micropipette until the tip touches the cortical surface and use this point as zero. Lower the micropipette to the desired depth (0.5 mm below the cortical surface).
Carefully inject 368 nL of PS5A1 glioma cell suspension or 92 nL of 73C glioma cell suspension into the mouse cortex.
After injection, allow 2–5 additional minutes of rest time before starting to withdraw the micropipette.
Seal the burr hole with box wax and apply a layer of body double to the skull for protection (Figure 2D).
House the mouse for three days (73C) to two weeks (PS5A1) to allow tumor growth before starting the experiments.
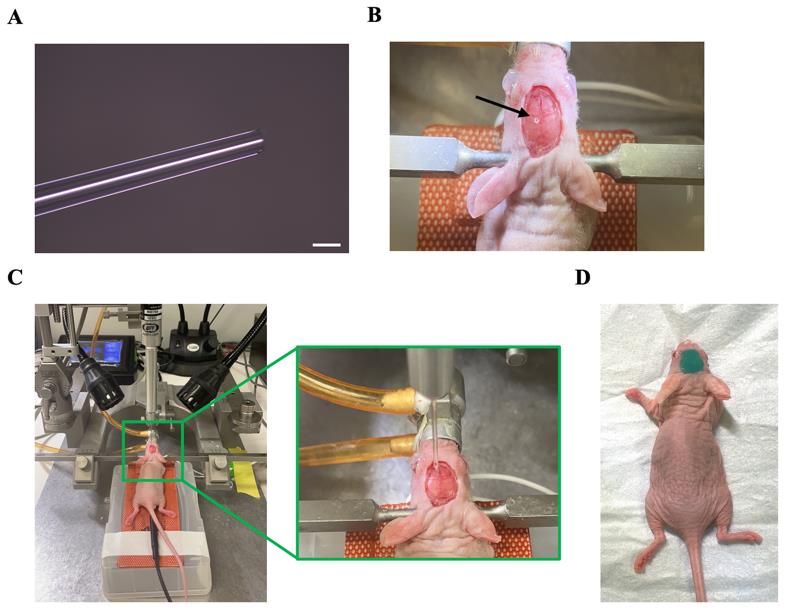
Figure 2. Glioblastoma cell transplantation. (A) Glass micropipette. The scale bar represents 50 μm. (B) Mouse with a burr hole in the skull. The arrow indicates the position of the burr hole. (C) Setup for the glioma cell injection. The zoom-in image illustrates the precise alignment of the glass micropipette with the burr hole. (D) Mouse with body double covered on its head after injection.
Minimal skin opening and laser stimulation of the brain for optoBBTB
Intravenously inject (tail vein) AuNP-BV11 into the GEMMs (18.5 and 37 μg/g in saline for PS5A1 and 73C GEMM, respectively) using PE-20 tubing and butterfly infusion set with 27G × ¾ in needle.
Procedures for optoBBTB:
One hour after the nanoparticle injection, peel the body double to expose the skull. Keep the skull moisturized using saline.
Align the laser beam to the tumor area using the minimum laser power (1%).
Apply one picosecond laser pulse (532 nm, 40 mJ/cm2, 6 mm beam size) through the skull above the tumor area (Figure 3).
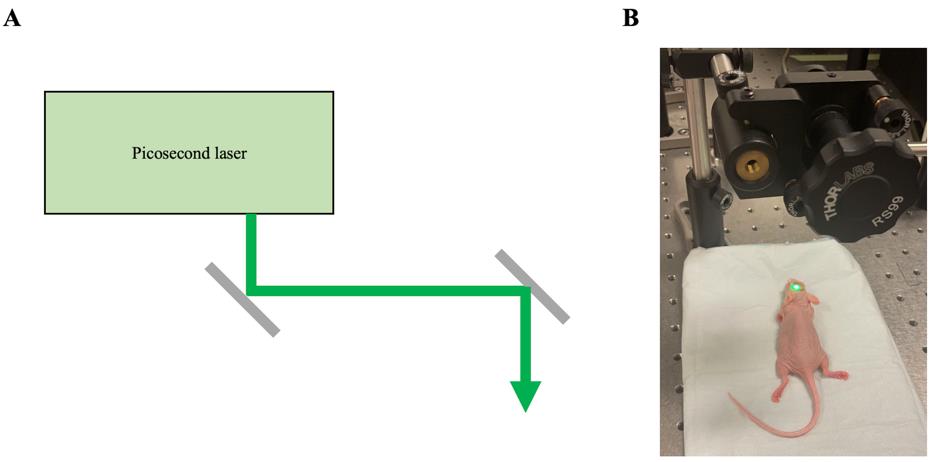
Figure 3. Setup for optoBBTB. (A) Schematic diagram of a picosecond laser for optoBBTB. (B) Photograph capturing a mouse undergoing laser excitation as part of the optoBBTB procedure. The laser was configured in continuous mode to align its position accurately.
Evaluation of the optoBBTB efficiency
Fluorescent imaging of the delivery of Taxol Janelia Fluor® 646 (Taxol646) after optoBBTB (Figure 4A and 4B, left):
Dissolve the Taxol646 in a mixer of Cremophor EL:absolute ethanol (1:1 v/v, as the vehicle) to 6 mg/mL and then dilute to 2 mg/mL with saline.
Immediately after optoBBTB, intravenously inject fluorescent Taxol646 (12.5 mg/kg).
Thirty minutes after the injection, perfuse the mouse with ice-cold PBS (20 mL) followed by 4% PFA (20 mL).
Extract the brain and post-fix the brain with 4% PFA (15 mL) at 4 °C overnight.
Dehydrate the brain with 10 mL of 10%, 20%, and 30% sucrose until it sinks to the bottom of the tube.
CRITICAL STEP: The brain should be well-fixed with PFA before cryopreservation with sucrose because sucrose solutions above 10% are hypertonic and will cause water to flow out of cells and tissue shrinkage if tissues are not fully fixed.
Snap-freeze the brain on dry ice.
PAUSE POINT: The brains can be wrapped in aluminum foil and stored at -20 °C.
Put two drops of O.C.T. into a plastic cryomold. Place the brain in the center and pour O.C.T. to embed the brain. Place the mold at -20 °C to freeze.
Slice the brain into 20 μm thick slices using a cryostat.
PAUSE POINT: The slides can be stored in a slide storage box at -20 °C.
Stain the brain slices with Hoechst staining (1:2,000 in PBS) at room temperature for 10 min, followed by rinsing three times with PBS (5 min for each wash).
Blot excess PBS from the non-sample surface of the glass slide.
Slowly add 150 μL of the mounting medium on the glass slide and cover the glass slide with a cover slip.
CRITICAL STEP: Avoid creating bubbles when adding the mounting medium and lower the coverslip to its place.
Cure the slides at room temperature for at least 1 h before imaging.
Evaluation of the treatment efficiency of optoBBTB and Paclitaxel (Taxol) delivery in PS5A1 GEMM (Figure 4A, middle, right):
At 14 dpi, randomly divide the mice into four groups: (1) vehicle control; (2) free Taxol control (12.5 mg/kg); (3) optoBBTB+vehicle; (4) optoBBTB+Taxol (12.5 mg/kg). Prepare five mice for each group.
Perform optoBBTB as described above.
Immediately after optoBBTB, intravenously inject vehicle or Taxol into the mice.
Repeat the treatment every four days for three times. Sacrifice the mice at 42 dpi.
Perfuse the mice with PBS, extract the brains, and image the tumor size by fluorescent imaging.
To analyze the survival, use the same treatment groups with seven mice in each group. The tumor-bearing mice should be euthanized if they develop weight loss (>20%), loss of grooming, seizures, or focal motor deficits according to the approved animal protocol.
Evaluation of the treatment efficiency of optoBBTB and Paclitaxel (Taxol) delivery in 73C GEMM (Figure 4B, middle, right):
At 4 dpi, randomly divide the mice into four groups: (1) vehicle control; (2) free Taxol control (12.5 mg/kg); (3) optoBBTB+vehicle; (4) optoBBTB+Taxol (12.5 mg/kg). Prepare five mice for each group.
Perform optoBBTB as described.
Immediately after optoBBTB, intravenously inject vehicle or Taxol into the mice.
Repeat the treatment every four days for three times. Sacrifice the mice at 15 dpi.
Perfuse the mice with PBS, extract the brains, and image the tumor size by fluorescent imaging.
To analyze the survival, use the same treatment groups with seven mice in each group. The tumor-bearing mice should be euthanized if they develop weight loss (>20%), loss of grooming, seizures, or focal motor deficits according to the approved animal protocol.
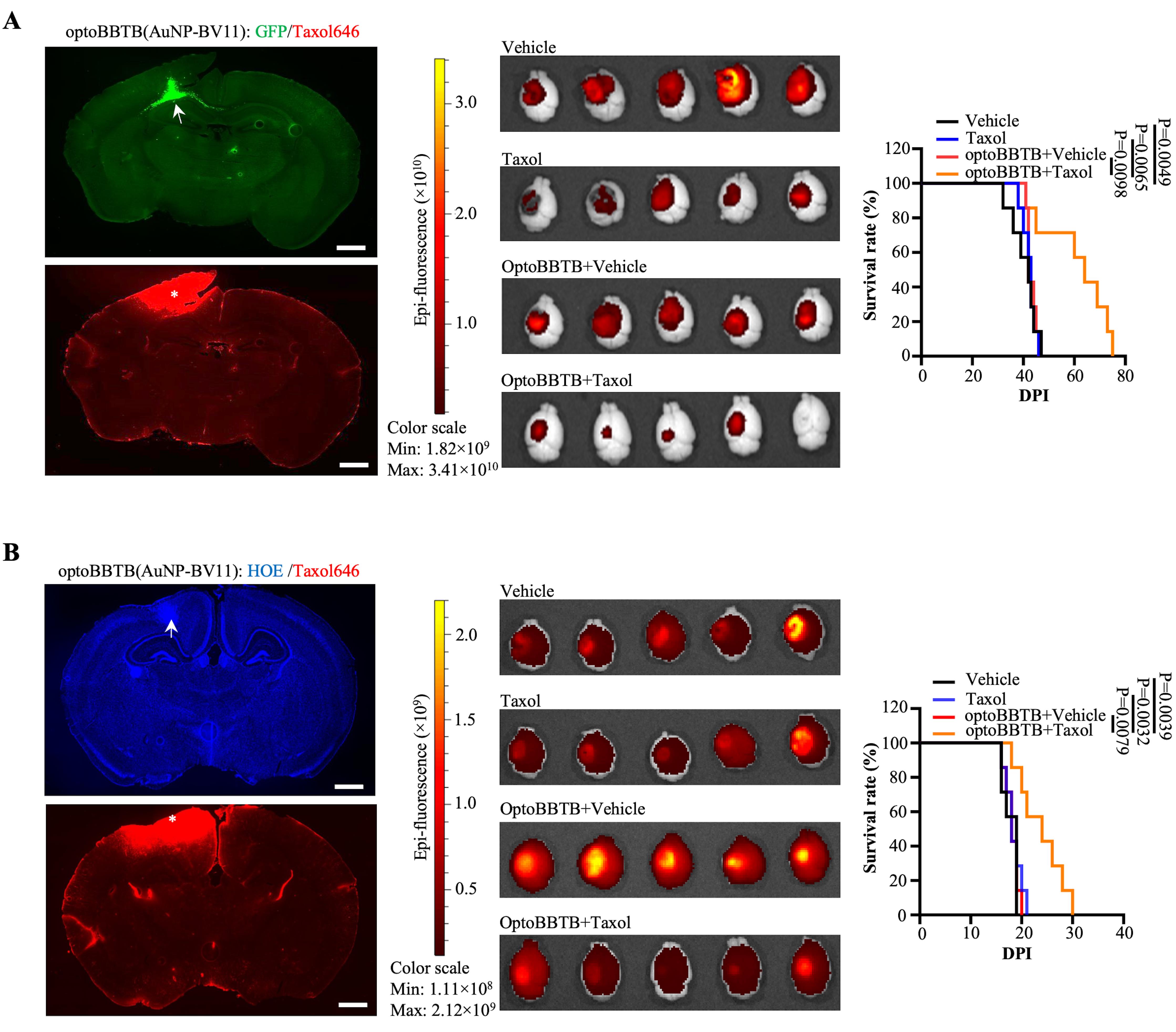
Figure 4. Evaluation of Taxol delivery after optoBBTB. (A) Left: OptoBBTB facilitates the delivery of Taxol646 in PS5A1 GEMM. The tumor is indicated by GFP fluorescent (arrows), and BBB opening is characterized by Taxol646 leakage (asterisks). The scale bar represents 1 mm. Middle: Tumor size imaging by GFP fluorescent. Right: Kaplan-Meier survival analysis. N = 7 mice in each group. (B) Left: OptoBBTB facilitates the delivery of Taxol646 in 73C GEMM. The tumor is indicated by high density Hoechst staining (arrows), and BBB opening is characterized by Taxol646 leakage (asterisks). The scale bar represents 1 mm. Middle: Tumor size imaging by GFP fluorescent. Right: Kaplan-Meier survival analysis. N = 7 mice in each group. Statistical analyses were performed using GraphPad Prism software. The data was first published in Cai et al. (2023).
Validation of protocol
This protocol was validated in Cai et al., Nature Communications (2023), DOI: https://doi.org/10.1038/s41467-023-40579-1.
Acknowledgments
This research was partially supported by Cancer Prevention and Research Institute of Texas (CPRIT) grants RP190278 and RP210236, Department of Defense grant W81XWH-21-1-0219, and American Heart Association grant 19CSLOI34770004, National Institute of Health grant RF1NS110499, National Science Foundation grant 2123971, and funds from a Eugene McDermott Professorship to Z.Q. Illustration figures were created with Biorender.com. Figure 1 and Figure 4 were first published in Cai et al. (2023) and modified for this publication.
Competing interests
All authors declare no competing interests.
Ethical considerations
The animal ethics guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of Texas at Dallas were strictly followed, ensuring that the mouse was euthanized upon reaching either when its brain tumor surpassed the predetermined maximum volume of 1 cm3 or when its weight loss exceeded 20%.
References
- Belykh, E., Shaffer, K. V., Lin, C., Byvaltsev, V. A., Preul, M. C. and Chen, L. (2020). Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 10: e00739. doi: 10.3389/fonc.2020.00739
- Brunner, J., Ragupathy, S. and Borchard, G. (2021). Target specific tight junction modulators. Adv. Drug Delivery Rev. 171: 266–288. doi: 10.1016/j.addr.2021.02.008
- Cai, Q., Li, X., Xiong, H., Fan, H., Gao, X., Vemireddy, V., Margolis, R., Li, J., Ge, X., Giannotta, M., et al. (2023). Optical blood-brain-tumor barrier modulation expands therapeutic options for glioblastoma treatment. Nat. Commun. 14: 4934. doi: 10.1038/s41467-023-40579-1
- Gao, X., Zhang, Z., Mashimo, T., Shen, B., Nyagilo, J., Wang, H., Wang, Y., Liu, Z., Mulgaonkar, A., Hu, X. L., et al. (2020). Gliomas Interact with Non-glioma Brain Cells via Extracellular Vesicles. Cell Rep. 30(8): 2489–2500.e2485. doi: 10.1016/j.celrep.2020.01.089
- Haiss, W., Thanh, N. T. K., Aveyard, J. and Fernig, D. G. (2007). Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 79(11): 4215–4221. doi: 10.1021/ac0702084
- Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. and Peer, D. (2018). Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9(1): e1038/s41467–018–03705–y. doi: 10.1038/s41467-018-03705-y
- Sarkaria, J. N., Hu, L. S., Parney, I. F., Pafundi, D. H., Brinkmann, D. H., Laack, N. N., Giannini, C., Burns, T. C., Kizilbash, S. H., Laramy, J. K., et al. (2018). Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 20(2): 184–191. doi: 10.1093/neuonc/nox175
- Singh, D. K., Kollipara, R. K., Vemireddy, V., Yang, X. L., Sun, Y., Regmi, N., Klingler, S., Hatanpaa, K. J., Raisanen, J., Cho, S. K., et al. (2017). Oncogenes Activate an Autonomous Transcriptional Regulatory Circuit That Drives Glioblastoma. Cell Rep. 18(4): 961–976. doi: 10.1016/j.celrep.2016.12.064
- Straehla, J. P., Hajal, C., Safford, H. C., Offeddu, G. S., Boehnke, N., Dacoba, T. G., Wyckoff, J., Kamm, R. D. and Hammond, P. T. (2022). A predictive microfluidic model of human glioblastoma to assess trafficking of blood–brain barrier-penetrant nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 119(23): e2118697119. doi: 10.1073/pnas.2118697119
- Stupp, R., Mason, W. P., van den Bent, M. J., Weller, M., Fisher, B., Taphoorn, M. J. B., Belanger, K., Brandes, A. A., Marosi, C., Bogdahn, U., et al. (2005). Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 352(10): 987–996. doi: 10.1056/NEJMoa043330
- van Tellingen, O., Yetkin-Arik, B., de Gooijer, M., Wesseling, P., Wurdinger, T. and de Vries, H. (2015). Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resistance Updates 19: 1–12. doi: 10.1016/j.drup.2015.02.002
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Cai, Q., Fan, H., Li, X., Giannotta, M., Bachoo, R. and Qin, Z. (2024). Optical Modulation of the Blood-Brain Barrier for Glioblastoma Treatment. Bio-protocol 14(2): e4920. DOI: 10.21769/BioProtoc.4920.
Category
Neuroscience > Basic technology > Optogenetics
Cancer Biology > General technique > Cancer therapy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








