- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Expansion and Polarization of Human γδT17 Cells in vitro from Peripheral Blood Mononuclear Cells
Published: Vol 14, Iss 1, Jan 5, 2024 DOI: 10.21769/BioProtoc.4914 Views: 1912
Reviewed by: Vivien J. Coulson-ThomasAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3727 Views
Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2514 Views
Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2354 Views
Abstract
γδ T cells play a critical role in homeostasis and diseases such as infectious diseases and tumors in both mice and humans. They can be categorized into two main functional subsets: IFN-γ-producing γδT1 cells and IL-17-producing γδT17 cells. While CD27 expression segregates these two subsets in mice, little is known about human γδT17 cell differentiation and expansion. Previous studies have identified γδT17 cells in human skin and mucosal tissues, including the oral cavity and colon. However, human γδ T cells from peripheral blood mononuclear cells (PBMCs) primarily produce IFN-γ. In this protocol, we describe a method for in vitro expansion and polarization of human γδT17 cells from PBMCs.
Key Features
• Expansion of γδ T cells from peripheral blood mononuclear cells.
• Human IL-17A-producing γδ T-cell differentiation and expansion using IL-7 and anti-γδTCR.
• Analysis of IL-17A production post γδ T-cell expansion.
Keywords: γδT17 cellBackground
γδ T cells are a group of lymphocytes consisting of a γ and δ chain, being considered as a bridge linking innate and adaptive immunity (Melandri et al., 2018). γδ T cells are mainly found in cutaneous and mucosal tissues such as the skin, gut, and oral mucosa (Cai et al., 2011; Wu et al., 2014; Hovav et al., 2020), but they also circulate in peripheral blood (Davey et al., 2018). γδ T cells are commonly classified based on their TCR chains and cytokine production. In mice, γδ T cells bear different Vγ chains ranging from Vγ1 to Vγ7, according to Heilig and Tonegawa nomenclature (Heilig and Tonegawa, 1986). Murine γδ T cells are heterogenous, secrete different cytokines, and can be divided into two main functional subsets: IFN-γ-producing γδT1 cells and IL-17-producing γδT17 cells (Ribot et al., 2009). Surface markers such as CD27 and CCR6 can be used to define these two subsets (Haas et al., 2009; Ribot et al., 2009). In humans, γδ T cells can be distinguished by the δ chain, including Vδ1, Vδ2, Vδ3, and Vδ5 (Ling et al., 2022). While previous studies have reported methods for in vitro expansion of human γδ T cells (Ness-Schwickerath et al., 2010; Caccamo et al., 2011; Hur et al., 2023), the majority of peripheral blood γδ T cells in humans are Vδ2+ subsets that mainly produce IFN-γ. It is difficult to investigate human γδT17 cells as they are scarce, and little has been done to expand human γδT17 cells in vitro. A previous report from Michel et al. demonstrated that IL-7 promotes the expansion of human IL-17-producing γδ T cells (Michel et al., 2012). In this study, we describe a modified method for in vitro polarization of human γδT17 cells from peripheral blood mononuclear cells (PBMCs) (Chen et al., 2022).
Materials and reagents
24-well plate (Falcon, catalog number: 353047)
6-well plate (NEST, catalog number: 703001)
Blood-drawing tubes containing EDTA-K2 (Improve Medical, catalog number: 101680720)
12 × 75 mm plastic tubes (Falcon, catalog number: 352052)
Anti-human γδTCR Ab (Beckman, clone: IMMU510, IM1349)
Human IL-7 (R&D system, catalog number: 207-IL-005/CF)
RPMI-1640 medium (Sigma-Aldrich, catalog number: R8758-500ML)
2-Mercaptoethanol (Gibco, catalog number: 21985023)
Sterile PBS (Sangon Biotech, catalog number: E607008-0500)
Fetal bovine serum (FBS) (Atlanta Biologicals, catalog number: S11150)
Penicillin-Streptomycin liquid (100×) (Solarbio, catalog number: P1400)
Trypan Blue (STEMCELL Technologies, catalog number: 07050)
Lympholyte® cell separation media (Ficoll) (Cedarlane Laboratories, catalog number: CL5020)
Anti-human IL-17A (BioLegend, catalog number: 512306, referred to as anti-human IL-17 later in this protocol)
Anti-human CD3 (BioLegend, catalog number: 300328)
Anti-human γδTCR (Miltenyi Biotec, catalog number: 130-113-508)
Anti-human CCR6 (BioLegend, catalog number: 353412)
Viability dye (Invitrogen, catalog number: 65-0865-14)
GolgiPlug (Brefeldin A solution 1,000×) (BioLegend, catalog number: 420601)
Phorbol 12-myristate 13-acetate (PMA) (Millipore Sigma, catalog number: P8139)
Ionomycin (Millipore Sigma, catalog number: I0634)
Human TruStain FcXTM (BioLegend, catalog number: 422302)
Fixation buffer (BioLegend, catalog number: 420801)
Intracellular staining perm 10× wash buffer (BioLegend, catalog number: 421002, referred to as wash buffer later in this protocol)
Equipment
CO2 incubator (Thermo Fisher Scientific, model number: 3543 or 3111)
Centrifuge (Beckman Coulter, model: Allegra® X-15R, catalog number: 392934)
Laminar flow hood (Scitech Equipments Ltd., model: EVL-5S, catalog number: ZX0907-04)
Flow cytometry (BD Bioscience, model: FACSCantoTM II, catalog number: 338962)
Pipettes (multi-channel, Eppendorf)
Software and Datasets
FlowJo v10.8.1 (BD Biosciences)
Procedure
Recipes for preparation
Make complete RPMI-1640 medium: RPMI-1640 medium + 10% FBS + 1% Penicillin-Streptomycin liquid + 2-Mercaptoethanol (0.1 mL/L).
Make anti-human γδTCR solution for coating: 0.1 mg of human γδTCR Ab (clone: IMMU510, IM1349) + 500 μL of PBS = 0.2 μg/μL human γδTCR Ab.
Make working stock of viability dye: 1 μL of viability dye + 49 μL of PBS (Viability dye:PBS = 1:49).
Make 1× wash buffer: 1 mL of 10× wash buffer + 9 mL of H2O (10× wash buffer:H2O = 1:9).
PBMC isolation from peripheral blood (see Note 1)
This section provides PBMCs for polarization of human γδ T cells in Procedure C.
Draw peripheral blood from healthy donors and collect approximately 15–20 mL of blood with EDTA-K2-containing tubes in accordance with institutional ethics and safety protocols.
Dilute blood samples with an equal volume of RPMI-1640 medium.
Carefully layer the diluted blood suspension (5 mL) over 5 mL of Ficoll in a 15 mL conical tube.
Centrifuge at 931× g for 15 min at room temperature without brakes.
Carefully transfer the mononuclear cell layer to a new 15 mL conical tube [the mononuclear cell layer is the white layer between topside plasma layer and Ficoll layer (Figure 1A); the colors from top to down are light yellow, white, clear, and red]. The white layer must be collected.
Fill the conical tube with complete RPMI-1640 medium to 10 mL.
Mix gently by hand (up and down mixing) and centrifuge at 524× g for 10 min at 4 °C.
Carefully remove and discard completely the supernatant by pipetting. The cells are at the bottom of the 15 mL conical tube.
Wash the cells with 5 mL of complete RPMI-1640 medium. Specifically, resuspend the cells in 5 mL of complete RPMI-1640 medium and centrifuge at 524× g for 10 min at 4 °C. Then, completely remove the supernatant by pipetting.
Resuspend the cell pellet in 5 mL of complete RPMI-1640 medium and count the cell numbers directly with a hemocytometer under a microscope or using Trypan blue staining (see Note 2).
In vitro polarization
This section provides polarization steps for human γδ T cells.
Coat a 24-well plate with PBS containing anti-human γδTCR Ab. Please note that plates need to be coated one day before cell isolation and polarization. Add 245 μL of PBS per well and then add 5 μL of 0.2 μg/μL anti-human γδTCR Ab per well to achieve a final concentration of 1 μg anti-human γδTCR Ab/250 μL of PBS. Incubate overnight (18–24 h) in a 37 °C incubator with 5% CO2.
Aspirate and discard the coating antibody solution from the 24-well plate on the second day (see Note 3).
Isolate PBMCs from healthy donors’ peripheral blood (Procedure B) and dilute PBMC prepared in Procedure B with complete RPMI-1640 medium to an appropriate concentration.
Seed PBMCs (1–4 million per well in 1 mL of complete RPMI-1640 medium containing 20 ng/mL human IL-7) into the 24-well plate, which has been pre-coated with anti-human γδTCR Ab.
Check the status of cells every day under a microscope to exclude contamination. Healthy cells will appear bright (see Note 4). If cells are contaminated with bacteria, the medium will appear cloudy. Sudden drops in the pH of the culture medium (the color of the culture medium turns yellow) are also signs of contamination.
According to the cell density in the plate, transfer and passage the cells to a new 6-well plate (not coated with anti-human γδTCR Ab) on the third to fourth day using complete RPMI-1640 medium containing 20 ng/mL human IL-7. Transfer and passage the cells before two-thirds of plate confluency (see Note 5). Please note that γδ T cells are suspension cells, and passage of cells can be performed by aspirating 1 mL of cells medium by pipetting into a new well in a 6-well plate and adding 2 mL of complete RPMI-1640 medium containing 20 ng/mL human IL-7 into this new well.
Check the status of cells every day under a microscope to exclude any contaminations and make sure the cells appear healthy (bright).
Passage the cells using complete RPMI-1640 medium containing 20 ng/mL human IL-7 when necessary.
Culture the cells for 2–3 weeks (approximately 4–5 passages).
Collect the cells by aspirating 1 mL of cells medium by pipetting and perform intracellular cytokine staining of IL-17 with the cells (Procedure D)
Data acquisition and analysis
This procedure provides an analysis of IL-17 production from human γδ T cells after polarization.
Stimulate approximately 100,000 cells that have been polarized in Procedure C with PMA (final concentration: 50 ng/mL) and ionomycin (final concentration: 1 μg/mL) in the presence of GolgiPlug (final concentration: 1× in the medium) in a total 250 μL of complete RPMI-1640 medium per well using 24-well plate.
Incubate for 4–6 h in a 37 °C incubator with 5% CO2.
Collect the cell suspension (T cells are non-adherent cells) into a 12 × 75 mm plastic tube by pipetting from the wells in the 24-well plate and wash the wells with 1 mL of PBS (also collect by pipetting).
Centrifuge at 524× g for 5 min at 4 °C and discard the supernatant.
Add human TruStain FcXTM (Fc receptor blocking solution, 5 μL/test) and incubate at 4 °C for 10 min.
Add commercial antibodies for surface staining, including working stock of viability dye (5 μL/test), anti-human CD3 (5 μL/test), anti-human γδTCR (3 μL/test), and anti-human CCR6 (5 μL/test) according to the instructions. Incubate at 4 °C for 30 min.
Wash the cells with 1 mL of PBS and centrifuge at 524× g (1,500 rpm) for 5 min at 4 °C.
Completely discard the supernatant by pipetting.
Add 400 μL of fixation buffer per tube and vortex.
Fix the cells at room temperature for 20 min in the dark.
Wash the cells with 1 mL of 1× wash buffer and centrifuge at 524× g for 5 min at 4 °C.
Discard the supernatant and wash the cells with 1 mL of 1× wash buffer again.
Centrifuge at 524× g for 5 min at 4 °C and discard the supernatant.
Add anti-human IL-17 antibody (5 μL/test) and incubate at 4 °C overnight (see Note 6).
Wash the cells with 1 mL of 1× wash buffer and centrifuge at 524× g for 5 min at 4 °C.
Discard the supernatant and resuspend the cells.
Acquire the cells on FACSCantoTM II flow cytometer (see Note 7).
Analyze data with FlowJo software (see Notes 8 and 9, Figures 1B, C, and 2).
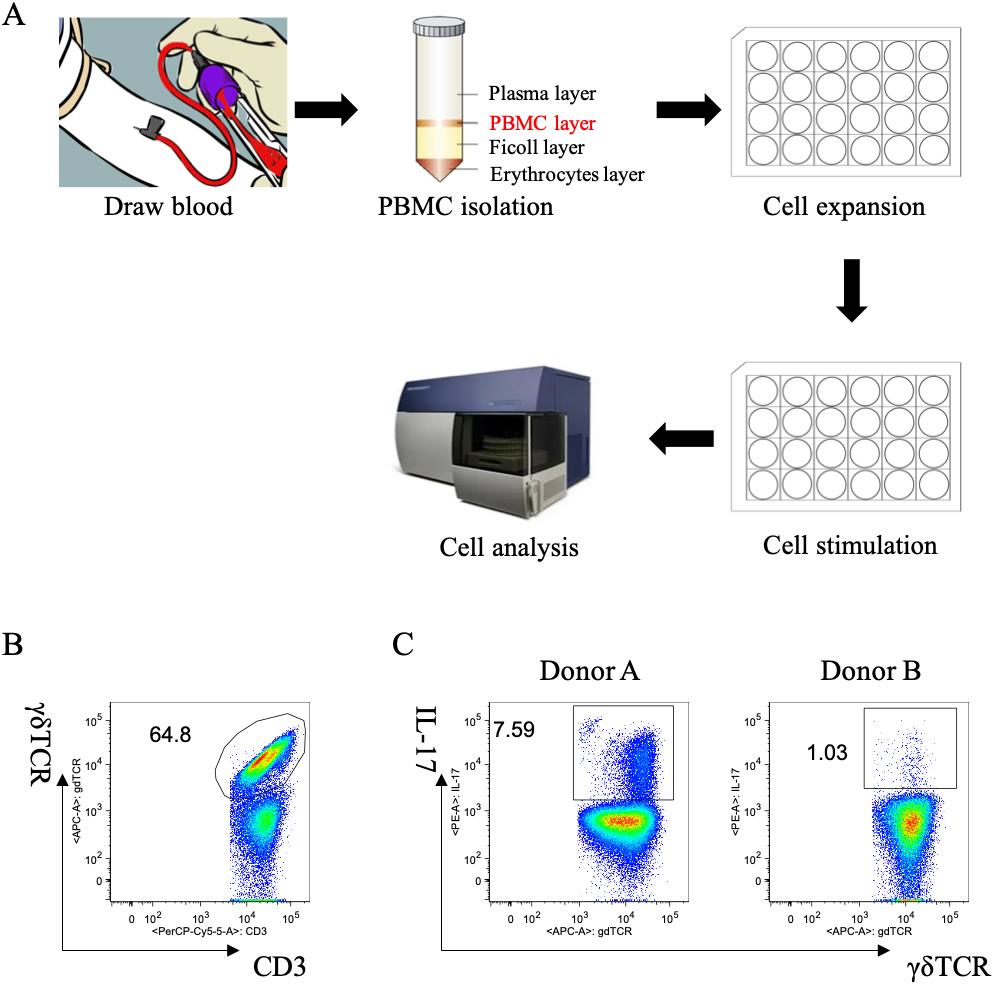
Figure 1. Human γδT17 expansion in vitro from peripheral blood mononuclear cells (PBMCs). A. Overall workflow of this protocol. B. Percentage of human γδ T cells after expansion. Cells were gated on CD3+ cells. C. Production of IL-17 by human γδ T cells after polarization from two different donors. Cells were gated on γδ TCR+ cells.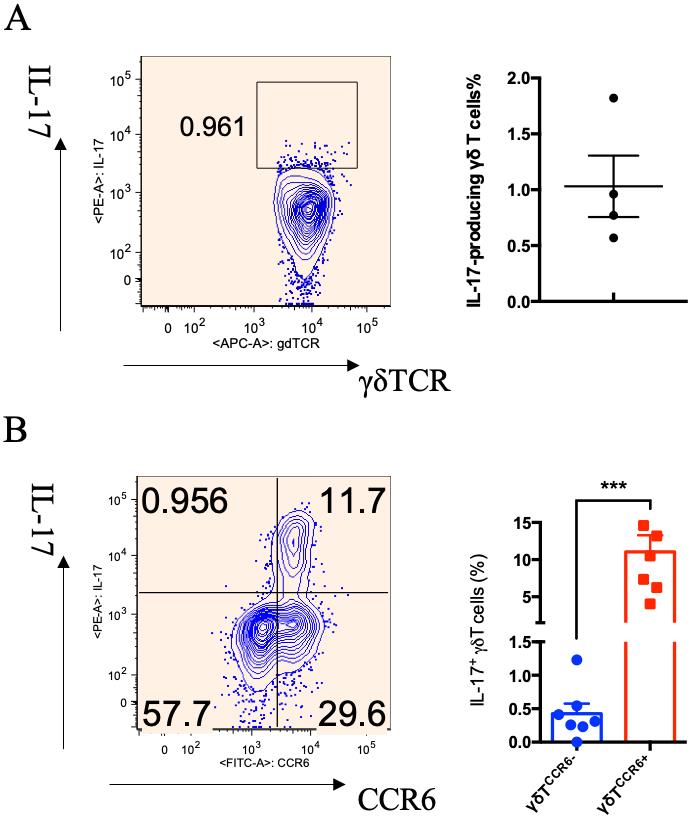
Figure 2. Analysis of IL-17 production in γδ T cells from peripheral blood mononuclear cells (PBMCs) before and after polarization. A. Representative dot plot and summarized data of IL-17 production in primary γδ T cells from PBMCs. B. Representative dot plot and summarized data of IL-17 production in polarized γδ T cells (Chen et al., 2022). Each dot represents one individual donor, and student t-test was used for statistical analysis; *** p < 0.001.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article(s):
Chen et al. (2022). Differential metabolic requirement governed by transcription factor c-Maf dictates innate γδT17 effector functionality in mice and humans. Science Advances (Figure 6, panel A).
Notes
A 24-well plate should be coated with PBS containing anti-human γδTCR Ab on the day before PBMC isolation; details can be found in the first step of Procedure C.
Count the cell numbers using a hemocytometer under a microscope directly or use Trypan blue staining. Either method is suitable since the cells are mostly viable. When counting cells directly, healthy cells appear bright; when counting cells using Trypan blue staining, live cells remain unstained and bright while dead cells are stained with the blue dye. If 10 μL of cell suspension is mixed with 90 μL of Trypan blue staining solution, the dilution factor is 10 when calculating the total cell numbers.
There are no washing steps after discarding the coating solution.
Healthy cells under the microscope appear bright, as shown in Figure 3.
Figure 3. Morphology of the cells under a microscope. Different densities of the cells are shown: low density (A) and high density (B).Passage the cells according to the cell density; two-thirds of the plate confluency is considered a target density, as shown in Figure 3B.
Incubate anti-human IL-17 according to the manufacturer’s instructions. We usually incubate anti-human IL-17 Ab overnight.
The analysis of IL-17 production in γδ T cells (gating strategy) is as follows: 1) gating live cells (viability dye negative), 2) gating CD3-positive cells, 3) gating CD3-positive and γδTCR-positive cells (Figure 1B), 4) gating IL-17-positive cells on γδ T cells (Figure 1C).
The percentage of γδ T cells increased to 64.8% of CD3+ cells after expansion (Figure 1B). However, the percentage of IL-17-producing γδ T cells varies depending on different donors. As shown in Figure 1C after polarization, the percentages of IL-17+ γδ T cells were 7.59% and 1.03% in Donor A and Donor B, respectively.
The percentage of γδ T cells in CD3+ T cells post polarization is approximately a 10-fold increase. Primary γδ T cells from PBMCs produce scarce IL-17 (Figure 2A). After polarization, IL-17+ γδ T cells are predominantly CCR6+ γδ T cells, and the percentage of IL-17+ γδ T cells in the total γδ T-cell population is approximately 10% on average (Figure 2B) (Chen et al., 2022).
Acknowledgments
This work was supported by the NIH R01AI128818 and R01CA213990. Xu Chen was supported by the Shanghai Pujiang Program (23PJD047) and on-job postdoctoral program.
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
All experimental procedures have been approved by the Ethics Committee of the Shanghai Ninth People’s Hospital, Shanghai, China.
References
Caccamo, N., La Mendola, C., Orlando, V., Meraviglia, S., Todaro, M., Stassi, G., Sireci, G., Fournié, J. J. and Dieli, F. (2011). Differentiation, phenotype, and function of interleukin-17–producing human Vγ9Vδ2 T cells. Blood 118(1): 129–138.
- Cai, Y., Shen, X., Ding, C., Qi, C., Li, K., Li, X., Jala, V. R., Zhang, H. g., Wang, T., Zheng, J., et al. (2011). Pivotal Role of Dermal IL-17-Producing γδ T Cells in Skin Inflammation. Immunity 35(4): 596–610.
- Chen, X., Cai, Y., Hu, X., Ding, C., He, L., Zhang, X., Chen, F. and Yan, J. (2022). Differential metabolic requirement governed by transcription factor c-Maf dictates innate γδT17 effector functionality in mice and humans. Sci. Adv. 8(21): eabm9120.
- Davey, M. S., Willcox, C. R., Hunter, S., Kasatskaya, S. A., Remmerswaal, E. B. M., Salim, M., Mohammed, F., Bemelman, F. J., Chudakov, D. M., Oo, Y. H., et al. (2018). The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9- subsets. Nat. Commun. 9(1): e1038/s41467–018–04076–0.
- Haas, J. D., González, F. H. M., Schmitz, S., Chennupati, V., Föhse, L., Kremmer, E., Förster, R. and Prinz, I. (2009). CCR6 and NK1.1 distinguish between IL-17A and IFN-γ-producing γδ effector T cells. Eur. J. Immunol. 39(12): 3488–3497.
- Heilig, J. S. and Tonegawa, S. (1986). Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322(6082): 836–840.
- Hovav, A. H., Wilharm, A., Barel, O. and Prinz, I. (2020). Development and Function of gammadeltaT Cells in the Oral Mucosa. J. Dent. Res. 99(5): 498–505.
- Hur, G., Choi, H., Lee, Y., Sohn, H. J., Kim, S. Y. and Kim, T. G. (2023). In Vitro Expansion of Vδ1+ T Cells from Cord Blood by Using Artificial Antigen-Presenting Cells and Anti-CD3 Antibody. Vaccines 11(2): 406.
- Ling, S., You, Z., Li, Y., Zhang, J., Zhao, S., He, Y. and Chen, X. (2022). The role of γδ T17 cells in cardiovascular disease. J. Leukoc. Biol. 112(6): 1649–1661.
- Melandri, D., Zlatareva, I., Chaleil, R. A. G., Dart, R. J., Chancellor, A., Nussbaumer, O., Polyakova, O., Roberts, N. A., Wesch, D., Kabelitz, D., et al. (2018). The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 19(12): 1352–1365.
- Michel, M. L., Pang, D. J., Haque, S. F. Y., Potocnik, A. J., Pennington, D. J. and Hayday, A. C. (2012). Interleukin 7 (IL-7) selectively promotes mouse and human IL-17–producing γδ cells. Proc. Natl. Acad. Sci. U.S.A. 109(43): 17549–17554.
- Ness-Schwickerath, K. J., Jin, C. and Morita, C. T. (2010). Cytokine Requirements for the Differentiation and Expansion of IL-17A– and IL-22–Producing Human Vγ2Vδ2 T Cells. J. Immunol. 184(12): 7268–7280.
- Ribot, J. C., deBarros, A., Pang, D. J., Neves, J. F., Peperzak, V., Roberts, S. J., Girardi, M., Borst, J., Hayday, A. C., Pennington, D. J., et al. (2009). CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17–producing γδ T cell subsets. Nat. Immunol. 10(4): 427–436.
- Wu, P., Wu, D., Ni, C., Ye, J., Chen, W., Hu, G., Wang, Z., Wang, C., Zhang, Z., Xia, W., et al. (2014). δγT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40(5): 785–800.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chen, X., Hu, X., Chen, F. and Yan, J. (2024). Expansion and Polarization of Human γδT17 Cells in vitro from Peripheral Blood Mononuclear Cells. Bio-protocol 14(1): e4914. DOI: 10.21769/BioProtoc.4914.
- Chen, X., Cai, Y., Hu, X., Ding, C., He, L., Zhang, X., Chen, F. and Yan, J. (2022). Differential metabolic requirement governed by transcription factor c-Maf dictates innate γδT17 effector functionality in mice and humans. Sci. Adv. 8(21): eabm9120.
Category
Immunology > Immune cell differentiation > T cell
Immunology > Immune cell isolation > Lymphocyte
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









