- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Targeted Delivery of Chemogenetic Adeno-Associated Viral Vectors to Cortical Sulcus Regions in Macaque Monkeys by Handheld Injections
(*contributed equally to this work, § Technical contact) Published: Vol 13, Iss 23, Dec 5, 2023 DOI: 10.21769/BioProtoc.4897 Views: 1675
Reviewed by: Olga KopachRachael E. HokensonLei GaoVolodymyr Krotov

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Chemogenetic Silencing of Neonatal Spontaneous Activity of Projection Neurons in the Dorsal Striatum of Mice
Bojana Kokinovic [...] Svetlana Molchanova
Oct 20, 2024 1616 Views
Abstract
Recent advancements in chemogenetic tools, such as designer receptors exclusively activated by designer drugs (DREADDs), allow the simultaneous manipulation of activity over a specific, broad brain region in nonhuman primates. However, the introduction of DREADDs into large and complexly shaped cortical sulcus regions of macaque monkeys is technically demanding; previously reported methods are time consuming or do not allow the spatial range of expression to be controlled. In the present report, we describe the procedure for an adeno-associated viral vector (AAV2.1) delivery via handheld injections into the dorsolateral prefrontal cortex (Brodmann’s area 9/46) of macaque monkeys, with reference to pre-scanned anatomical magnetic resonance images. This procedure allows the precise delivery of DREADDs to a specific cortical region.
Key features
• This article describes the procedures for injecting viral vectors encoding functional proteins for chemogenetic manipulation into targeted cortical sulcus regions.
• The protocol requires magnetic resonance imaging for the accurate estimation of the injection sites prior to surgery.
• Viral vector solutions are injected using a handheld syringe under microscopic guidance.
• This protocol allows for the precise introduction of designer receptors exclusively activated by designer drugs (DREADDs) to large and complex cortical regions.
Keywords: MonkeysBackground
Genetic approaches that enable the expression of desired functional proteins in order to manipulate the activity of specific brain regions or cell types have become a central method in systems neuroscience [1, 2]. In particular, chemogenetic tools such as designer receptors exclusively activated by designer drugs (DREADDs) have become a promising means to study the relationship between animal behavior and the neuronal activity of specific cell populations. DREADDs allow the manipulation of activity in specific brain regions in behaving animals following systemic administration of an agonist, without any requirements for special devices or implants [3]. This feature is particularly advantageous for manipulating the brain activity of nonhuman primates such as macaque monkeys, which have relatively large and complexly shaped brains.
The introduction of functional proteins to an intended brain region is typically achieved by the injection of viral vector solutions. To manipulate the neuronal activity of a specific region, these vectors need to be precisely delivered over the entire target region. However, if the target region is large and deep within the brain, such as for the cortical sulcus regions, it is technically demanding to inject viral vectors to cover the entire target region. For example, the introduction of DREADDs into a cortical region surrounding a sulcus [e.g., the dorsolateral prefrontal cortex (dlPFC) or Brodmann’s area 46] requires injection of vectors into a broad area along the banks of the principal sulcus (i.e., from the bottom to the surface; Figure 1). A conventional and typical stereotaxic approach using a manipulator ensures accurate localization of viral delivery. However, completing one injection with this procedure takes several tens of minutes, which makes the total surgical duration very long, with considerable surgical stress to the animal. An alternative approach termed convection-enhanced delivery enables widespread cortical viral delivery via a single injection, which considerably shortens the procedural time and minimizes damage [4]. However, its extent of spatial delivery is uncontrollable, which leads to concerns about off-target expression.
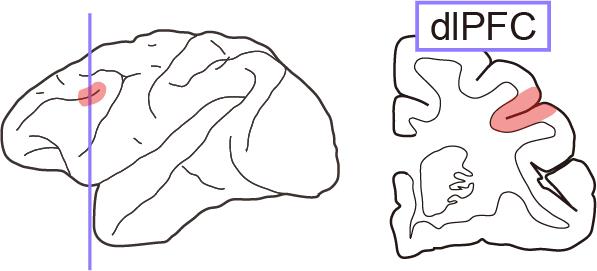
Figure 1. Illustration of the dorsolateral prefrontal cortex (dlPFC). Left, lateral view of the monkey brain. Right, coronal section around the dlPFC (colored area).
In the present report, we describe a procedure for viral vector injection into deep sulcus regions (such as the dlPFC) of macaque monkeys using a handheld injection technique. This procedure enables flexible injection of vectors within 1 min per injection track, each of which has several injection points. This procedure is based on a method described in previous reports [5, 6]. The injections cover 5–10 mm horizontally around the principal sulcus, as we have previously reported [7–9]. Our protocol uses a magnetic resonance (MR) image obtained before surgery to identify the position of the intended injection area, injection tracks and points, and angles for each track. The successful expression of the DREADDs was validated using conventional histological methods.
Materials and reagents
Biological materials
Macaque monkey (5.7 kg; age = 6.0 years old at the beginning of experiments; provided by the National Bio-Resource Project “Japanese Monkeys” of the Ministry of Education, Culture, Sports, Science and Technology, Japan)
Adeno-associated viral vector (e.g., AAV2.1-hSyn-hM4Di-IRES-AcGFP, provided by the Takada Lab, Kyoto University, Japan)
Reagents
Ketamine hydrochloride (Daiichi Sankyo Chemical Pharma, product name: Ketalar for intramuscular injection 500 mg)
Xylazine hydrochloride (Bayer, product name: Seraktar or Rompun)
Sodium cefmetazole (Alfresa Pharma, product name: Cefmetazon for intramuscular injection 0.5 g)
Ketoprofen (Kissei Pharmaceutical, product name: Capisten for intramuscular injection 50 mg)
Lidocaine 2% solution including epinephrine (Sandoz K.K., product name: Xylocaine injection 2% with epinephrine)
Lidocaine spray (Sandoz K.K., product name: Xylocaine pump spray 8%)
Sterilized saline (Otsuka Pharmaceutical, catalog number: 1326)
Povidone-iodine solution (Meiji Seika Pharma, product name: Povidone-iodine 10%)
Propofol (Nichi-Iko, product name: Propofol 1% intravenous injection)
Mannitol (Yoshindo, product name: Mannitol-s injection)
Atropine sulfate (Nipro, product name: Atropine sulfate hydrate 0.5 mg/mL)
Isoflurane [Mylan, product name: Isoflurane inhalation solution (Pfizer)]
Infusion solution (Terumo, catalog number: TP-AB05NR)
Bone wax (TOKYO M.I., catalog number: J901)
Sodium lactate Ringer’s solution (Terumo, product name: Solulact infusion)
Sterilized distilled water (Otsuka Pharmaceutical, catalog number: 1323)
Laboratory supplies
Endotracheal tube (Fuji Systems, catalog number: FR-26)
IV catheter (Terumo, catalog number: SR-FF2419)
25G needle (Terumo, catalog number: NN-2525R)
Surgical tape (Nichiban, catalog number: 21N)
Surgical cotton (Hakujuji, catalog number: 11371)
Disposable electrode for electrocardiogram (Nihon Kohden, catalog number: M-150)
1 mL syringe (Terumo, catalog number: SS-01T)
50 mL syringe (Terumo, catalog number: 5SS-50ESZ)
Valve syringe (Eastsidemed, catalog number: ES-17005)
Micro syringe (Hamilton, model: 1701RN directly connected to a 2 inch PT3 type 30G needle)
Depilatory cream (Center Shoji, product name: Fi-i-mo Epi DX Plus)
Scalpel
No. 11 (Akiyama Medical MFG, catalog number: FS11)
No. 21 (Akiyama Medical MFG, catalog number: FS21)
Suture with needle
Vicryl Plus 3-0 (Ethicon, catalog number: VCP460H)
Vicryl Plus 5-0 (Ethicon, catalog number: VCP303H)
Monosof 2-0 (COVIDIEN, catalog number: SN-628)
Infusion tube (Terumo, catalog number: TK-U200L)
Sterile gown (Nissho Sangyo, catalog number: 24503)
Sterile glove
Overglove (Mölnlycke Health Care, catalog number: 42175)
Underglove (Mölnlycke Health Care, catalog number: 41670)
Sterile drape
1,200 mm × 1,200 mm, no hole (Nissho Sangyo, catalog number: 23203)
900 mm × 900 mm, with hole and tape (Nissho Sangyo, catalog number: 23265)
Ioban antimicrobial incise drape (3M, catalog number: 6661EZ)
Sterile surgical marker (Muranaka Medical Instruments, catalog number: 2730PBX)
Sterile gauze (Hakujuji, catalog number: 14824)
Freer elevator (Roboz, catalog number: RS-8820)
Ruskin rongeur (Biomedical Research Instruments, catalog number: 46-1655)
Knapp scissors (Roboz, catalog number: RS-5965)
Drill bit
Diamond disc, Ø 6–9 mm disc (Biomachinery, custom made)
Twist drill, Ø 2 mm tip (Nakanishi Inc., catalog number: ES-TD-S20)
Equipment
MR imaging scanner (Bruker, model: 7 Tesla 400 mm/SS system)
X-ray computed tomography (CT) scanner (J. Morita, model: 3D Accuitomo170)
Operating microscope (Leica Microsystems GmbH, model: M220)
Patient monitor (Fukuda, model: Bio-Scope AM140)
Shadowless operating lamp (Yamada, model: CRV0404V)
Ventilator (Shin-Ei Industries, Inc., model: A.D.S. 2000)
Vaporizer (Shin-Ei Industries, Inc., model: I-200)
Stereotaxic device (David Kopf Instruments, model: 1530)
Electrolyzed water generator (Omco, model: NDX-70KMW)
Micromanipulator (David Kopf Instruments, model: 2166A)
Micro syringe pump (World Precision Instruments, model: UMP3T-1)
Circulating thermal water system (KIMURAMED, model: T-CARE)
Surgical drill control unit (Nakanishi Inc. model: Primado2)
Software and datasets
PMOD (PMOD Technologies Ltd. ver. 3.6 or above)
Procedure
Planning the surgery using MR and CT images
MR and CT scans (General note 1).
Anesthetize the monkey by intramuscular (i.m.) injection of ketamine (5–10 mg/kg) and xylazine (0.2–0.5 mg/kg) at 5 min after the i.m. injection of atropine sulfate (0.02–0.05 mg/kg) to reduce salivation, which is followed by continuous intravenous (i.v.) infusion of propofol (0.2–0.6 mg/kg/min) to achieve stable anesthesia during the scan.
Monitor the animal’s heart rate and peripheral oxygen saturation (SpO2) until the animal awakens.
Spray lidocaine into the trachea before securing the airway with an endotracheal tube.
Perform an MR imaging on the monkey under anesthesia.
Note: Any MR scan protocol is acceptable as long as the target location is clearly visible. As an example, the scanner, sequences, and parameters used in our institute are as follows: Bruker Biospec 40 cm bore 7T-MRI scanner; three-dimensional fast low angle shot gradient echo sequence; repetition time/echo time = 30/3.7 ms; flip angle = 10°; spatial resolution = 0.5 mm isotropic; field of view = 100 × 100 × 70 mm3; acquisition time = 6 min; fat suppression ON; band width = 347 Hz/Px; acceleration factor = 2.
Perform a CT scan of the head.
Note: We typically take a CT scan as a reference for aligning the MR images to the stereotaxic coordinates. The typical conditions are as follows: 90 kV, 5 mA, and 18.5 s rotation time for Ø 140 mm × 100 mm. The CT image should include the ear and orbit so that it can be virtually aligned to the stereotaxic coordinates. Any other methods that allow such alignment (e.g., MR imaging with a stereotaxic landmark) are also acceptable.
Estimation of injection sites.
Align an MR image to the stereotaxic coordinates using image analysis software (e.g., PMOD). In our typical procedure, we first align the CT image to the stereotaxic coordinates by matching the vertical level of the ear holes and the infraorbital margin (the lower margin of the eye socket), and then fuse the MR image to the stereotaxically aligned CT image (Figure 2).
Note: Any other software that allows the alignment of an MR image to stereotaxic coordinates is acceptable.
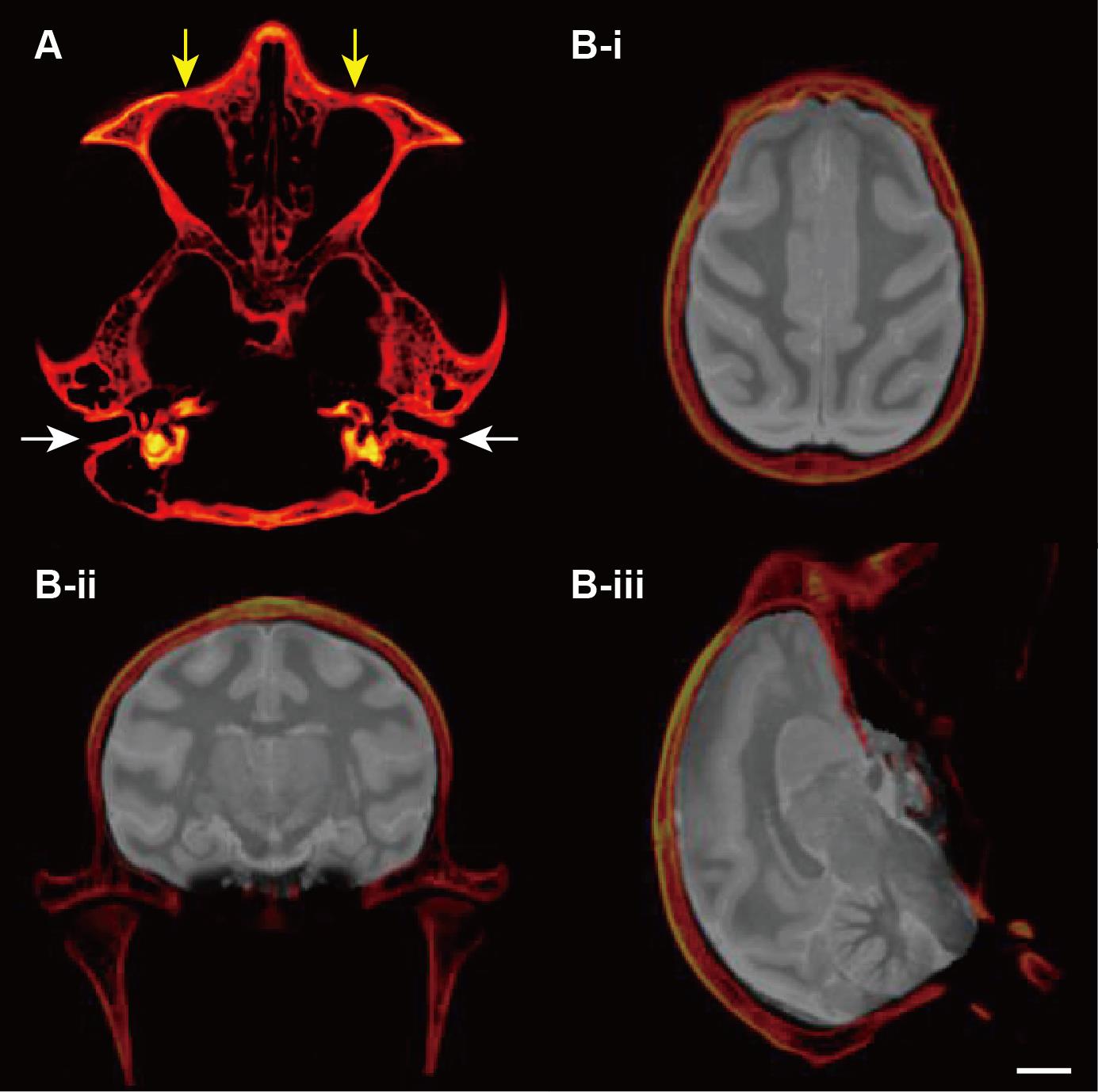
Figure 2. Alignment of the magnetic resonance (MR) image to the stereotaxic coordinates. A. Vertically aligned ear holes (white arrows) and the infraorbital margin (the lower margin of the eye socket) (yellow allows). B. Fused MR and computed tomography (CT) images on the horizontal (i), coronal (ii), and sagittal (iii) planes. Scale bar (white bar below the brain image): 10 mm.Verify the location of the target area based on the stereotaxically aligned MR images. Critical: We typically check the depth of the sulcus from the surface, the distance from the interaural line, and the horizontal angle of the sulcus (which often varies along the anterior–posterior axis and between the left and right hemispheres). Based on the images, we then design the injection tracks and points. For example, in the case of Figure 3, the depth of the cortex (from the bottom to the surface) along the dorsal bank of the principal sulcus is approximately 8 mm, and the angle of the sulcus is 35°. For this case, we designed five injection points along the sulcus (at 1.5 mm intervals) to allow the vector solution to spread throughout the dlPFC (Figure 3, also see Figure 1).
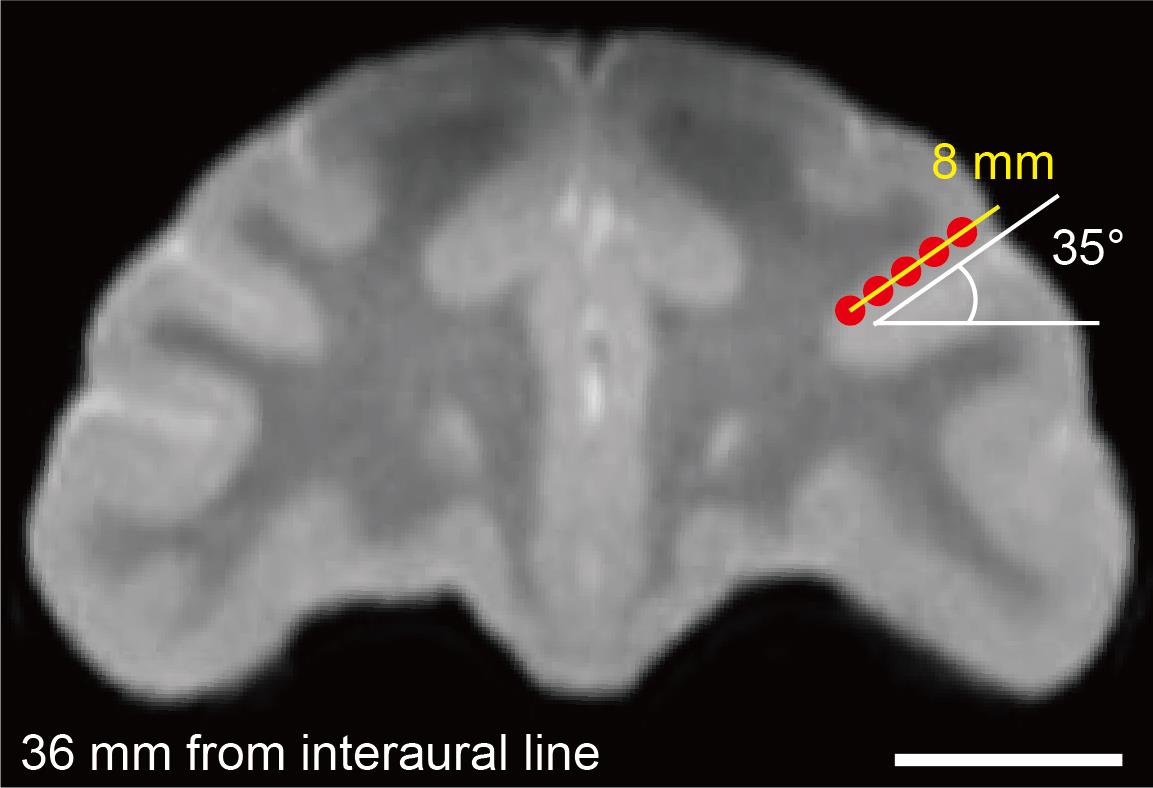
Figure 3. Coordination of injection points on the magnetic resonance image. The red dots and yellow bar represent the intended injection points and injection track, respectively. Scale bar (white bar below the brain image): 10 mm.
Surgical procedures
Preparation for surgery.
Anesthetize the monkey with i.m. injection of ketamine (5–10 mg/kg) and xylazine (0.2–0.5 mg/kg) after the i.m. injection of atropine sulfate (0.02–0.05 mg/kg) to reduce salivation.
Note: The animal injected with an appropriate dose of ketamine and xylazine may be immobilized within 10 min after the injection, and the effects of ketamine and xylazine can continue for 30–40 min. Therefore, the next two steps should be done within that timeframe to maintain immobilization.
Move the animal to the preparation room.
Maintain immobilization with anesthesia using isoflurane (2%–3%) through a mask until the airway is secure.
Remove the hair around the head (first coarsely with hair clippers and then with depilatory cream).
Secure vascular access from the right foot vein using an IV catheter.
Spray lidocaine into the trachea before securing the airway with an endotracheal tube.
Start the anesthesia with isoflurane (1%–3%) through the secured airway and maintain it throughout the surgery.
Note: The surgery can be performed with the continuous use of a mask. We recommend the use of an endotracheal tube to secure the airway, which allows fine-tuning of anesthetic control by reducing the dead space.
Monitor the animal’s electrocardiogram, SpO2, end-tidal CO2, body temperature, and respiratory rate throughout the surgery.
Note: The surgery should be suspended immediately and appropriate measures taken if the following are noted: body temperature > 40 °C or < 35 °C, heart rate >150 beats/min or < 60 beats/min, SpO2 < 95%, and end-tidal CO2 > 50 mmH2O or < 20 mmH2O.
Maintain body temperature at 36–38 °C using a circulating thermal water system throughout and after surgery until the animal awakens.
Fix the monkey’s head to the stereotaxic device and check whether the head is leveled.
Note: The vertical level of the ear holes and the infraorbital margin should be matched with the alignment of the MR image aligned to stereotaxic coordinates.
After fixation, close the monkey’s eyes and cover them with surgical cotton for protection.
Connect the infusion tube to the vascular access and provide an i.v. injection of infusion solution (sodium lactate Ringer’s solution, approximately 10 mL/kg/h) during the surgery.
Administer prophylactic antibiotics (sodium cefmetazole, 25–50 mg/kg, i.m.).
Clean the monkey’s head by brushing with hypochlorite water (produced using the electrolyzed water generator). Dry the head and wipe with disinfectant (povidone-iodine) solution and sterile gauze.
Note: When cleaning with the sterile gauze, the operator should wear sterile gloves.
The following surgical procedures should be performed under sterile operating conditions.
From the beginning of surgery until before the craniotomy:
Put on a sterile gown and sterile gloves (undergloves and overgloves).
Note: Two people wearing sterile equipment are required for the handheld injections in the flow protocol—one to hold the syringe and one to push the plunger. Additionally, a third person is required to handle the viral vector.
Prepare sterile surgical instruments on sterile drapes.
Mark the incision line with the sterile surgical marker, which is typically approximately 10 mm from the tip of the forehead to the lambdoid suture at the midline of the head.
Cover the monkey with the Ioban antimicrobial incise drape and the sterile drape (900 mm × 900 mm, with a 90 mm hole), with the head exposed.
Begin the i.v. injection of hypotensive agent (i.e., 15%–20% mannitol, 4 mL/kg for 30 min).
Note: Glycerol is also acceptable (recommended dose: 8 mL/kg for 30 min, i.v. injection).
Subcutaneously inject the lidocaine 2% solution including epinephrine along the intended incision line to induce an analgesic effect and prevent bleeding.
Incise the skin and subcutaneous tissue with the scalpel (No. 21) and exfoliate the muscles with the elevator to expose the skull.
Spread and secure the incised skin and exfoliated muscles on both sides using suture thread (Vicryl Plus 3-0) to maintain the operative field.
Note: Cover the spread tissues (such as skin, subcutaneous tissue, and muscle) with damp gauze to keep them moist throughout the surgery. Keep the gauze damp by adding saline using the valve syringe.
Craniotomy, dura incision, and viral vector injections
Mark the contour of the intended cranial extraction site with the sterile surgical marker (Figure 4).
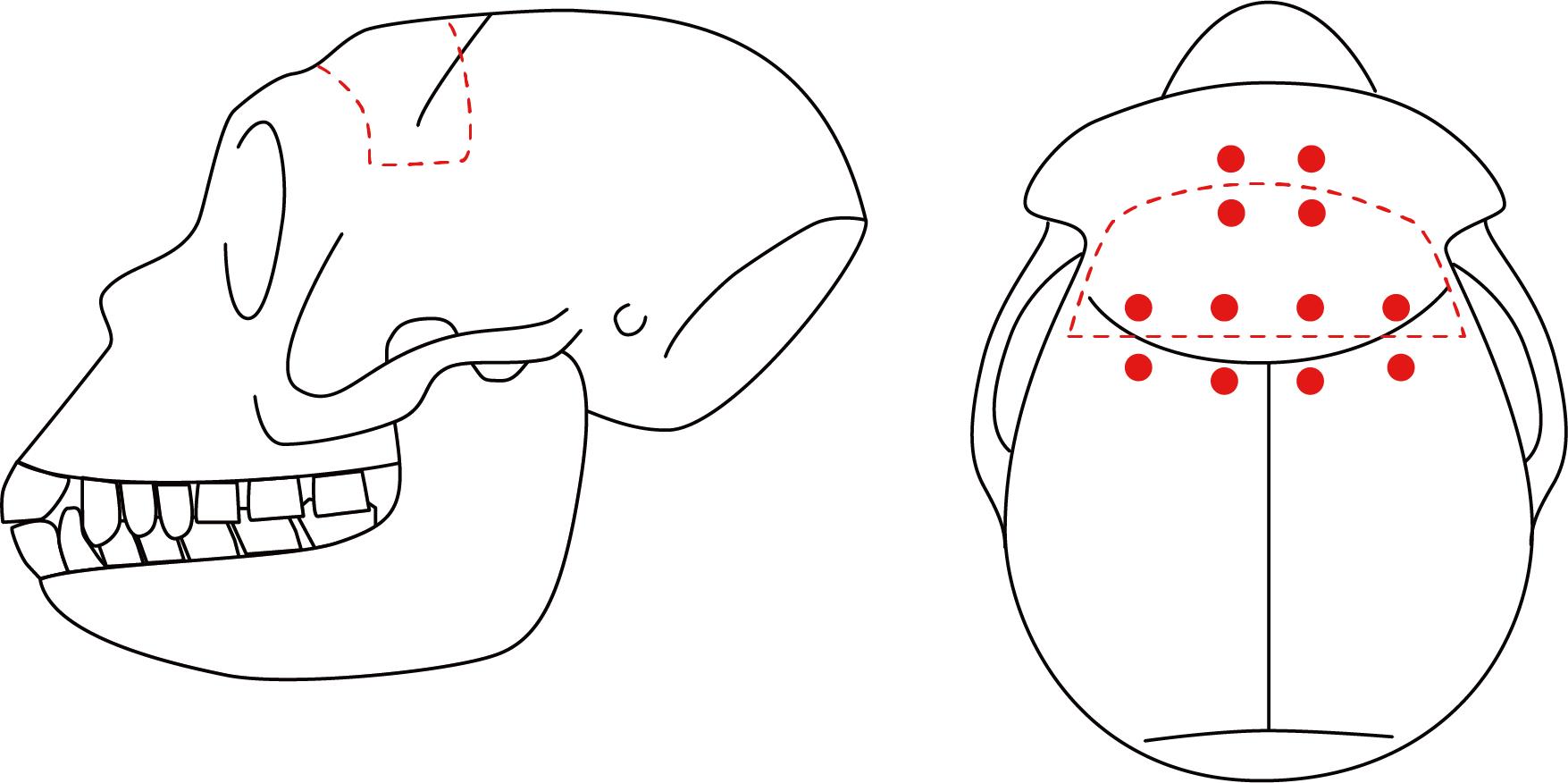
Figure 4. Contour of the intended cranial extraction site. Illustration of the intended cranial extraction site (red dashed line) on the monkey’s head from the lateral (left) and top (right) views. Red dots indicate the location of the suture holes to be made.Craniotomy. Cut the edge of the intended area with the disc drill and extract the bone flap from the skull using the elevator.
Note: When targeting both sides, a wide bilateral craniotomy is acceptable. However, when cutting the midline, be very careful not to damage the superior sagittal sinus. Tilt the disc slightly from vertical so that the brain is not compressed when the bone is put back in place.
Smooth the edge of the opened skull (especially on the cerebral side) using the Ruskin rongeur.
Stop any bleeding on the incision surface of the skull using bone wax.
Make suture holes in the skull and bone flap with the drill to allow for the return of the bone flap into place (Figure 4, General note 2).
Note: The holes are drilled first to prevent bone fragments from entering under the dura mater during the drilling process.
Cut the dura with the scalpel (No. 11) and Knapp scissors so that the operator can see the landmarks of the brain surface, such as the arcuate and principal sulci for Brodmann’s area 46 (Figure 5).
Note: The incised dura mater should be moved aside (the dura flap folded back) for later suturing. After cutting the dura, particular care should be taken to keep the brain surface and dura mater moist with sterile saline.
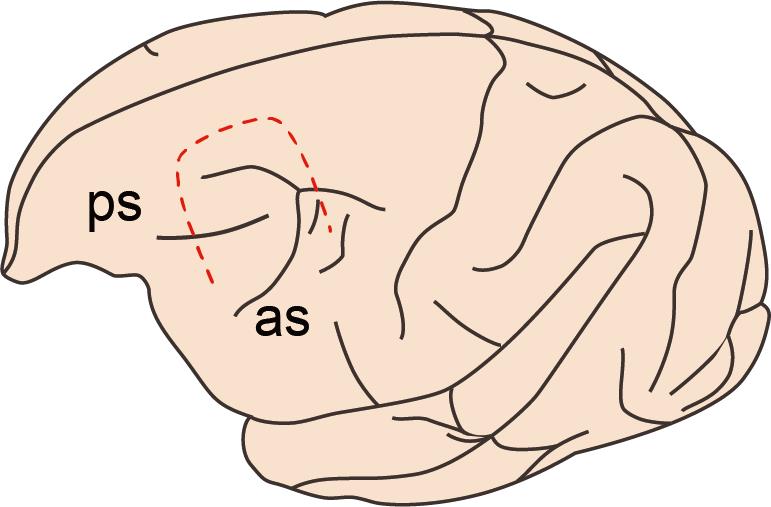
Figure 5. Incision line of the dura mater. Illustration of the intended incision area of the dura mater (red dashed line). The dura is incised to visualize the rostral tip of the ascending limb of the arcuate sulcus (as) and the caudal tip of the principal sulcus (ps).Prepare the viral vector solution.
Note: We typically use adeno-associated virus vectors expressing DREADDs under the control of general or specific promoters, with marker proteins or a tag sequence (e.g., AAV2.1-hSyn-hM4Di-IRES-AcGFP, as used in our previous studies [10–12]). For more details on the high-efficacy viral vector used in our recent work, see also [13].
Fill the injection syringe (10 μL Hamilton syringe with PT3 type 30 G needle) with the vector solution to be used.
Note: A PT2 needle or 25 μL micro syringe is also acceptable. Before filling the syringe with the vector solution, wash it first with ethanol and then with distilled water.
Perform the injection. One operator should manually hold the syringe and insert the needle to the intended depth under the guidance of an operating microscope. The other operator then pushes the plunger (Figure 6). Critical: The needle that we use is marked at 3 and 7 mm from the tip so that we can easily recognize the depth (Figure 7). For one hemisphere, perform nine penetrations surrounding the principal sulcus (Figure 8), with three to five injections at different depths (1 μL per injection) per penetration (see also Figure 3). It takes approximately 2 s to perform one injection. In total, 35–44 μL of vector solution is injected into one hemisphere.
Note: To avoid movement of the syringe during injection, the hands holding the syringe should be placed on the stereotaxic frame and the skull and supported by the other hand also placed on the stereotaxic frame.
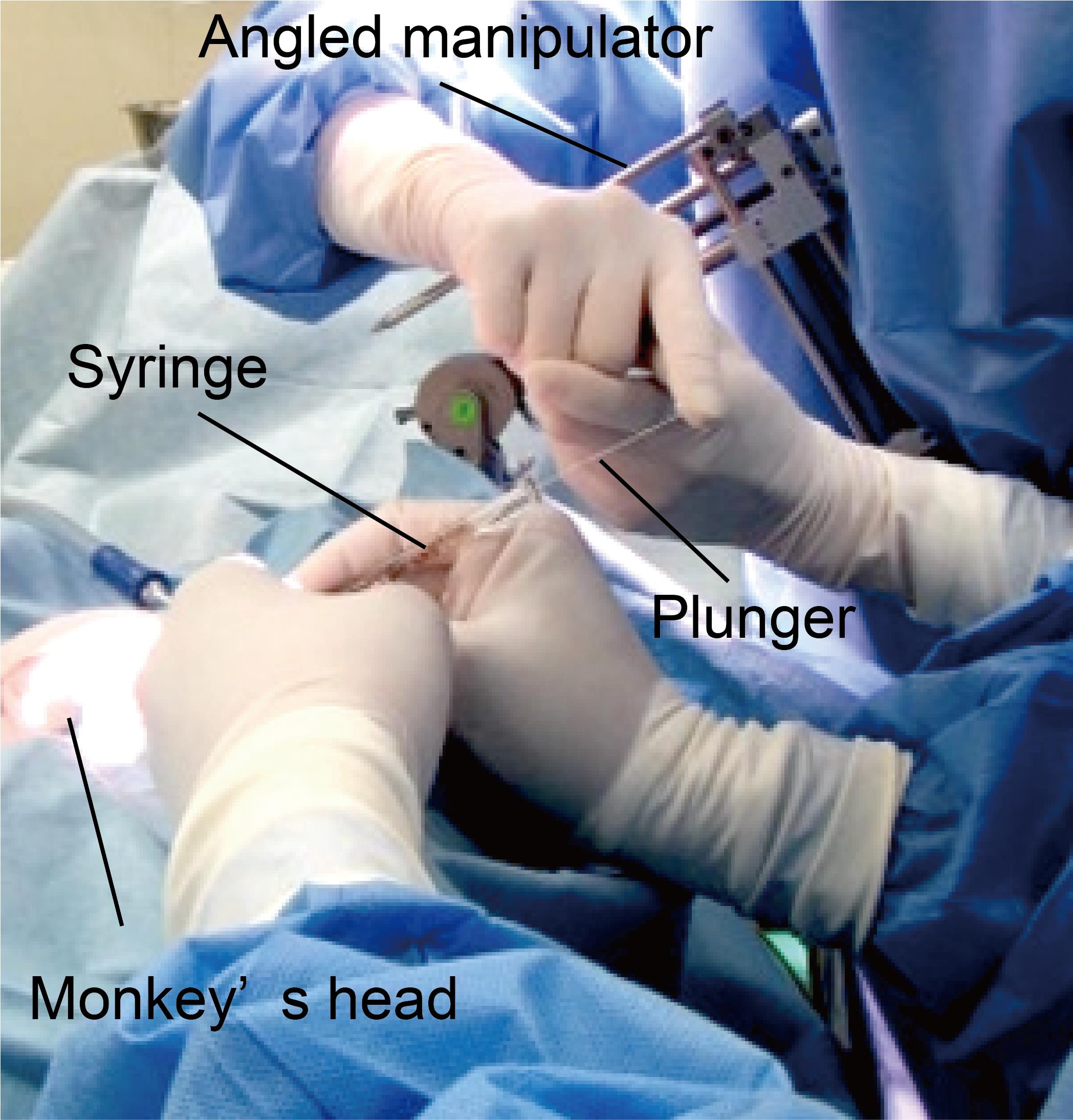
Figure 6. Handheld injection. The needle is inserted into the intended area by one person (front). A second person (back) presses the plunger to expel approximately 1 µL of solution. The angle of the syringe is visually calibrated to be parallel to the manipulator, which is tilted to the angle of the sulcus.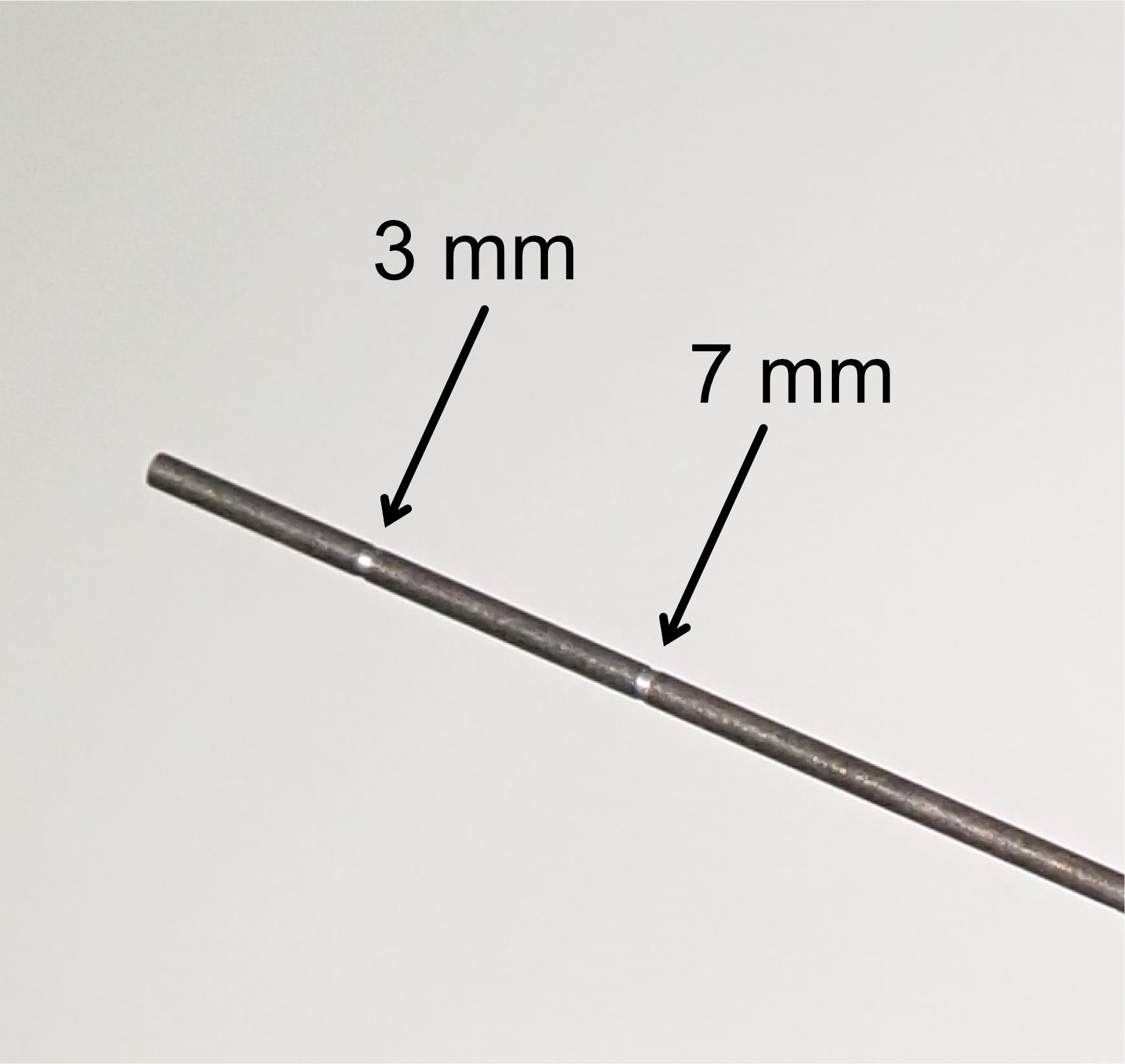
Figure 7. Customized needle with marker for injection. Arrows indicate markers at 3 and 7 mm from the needle tip.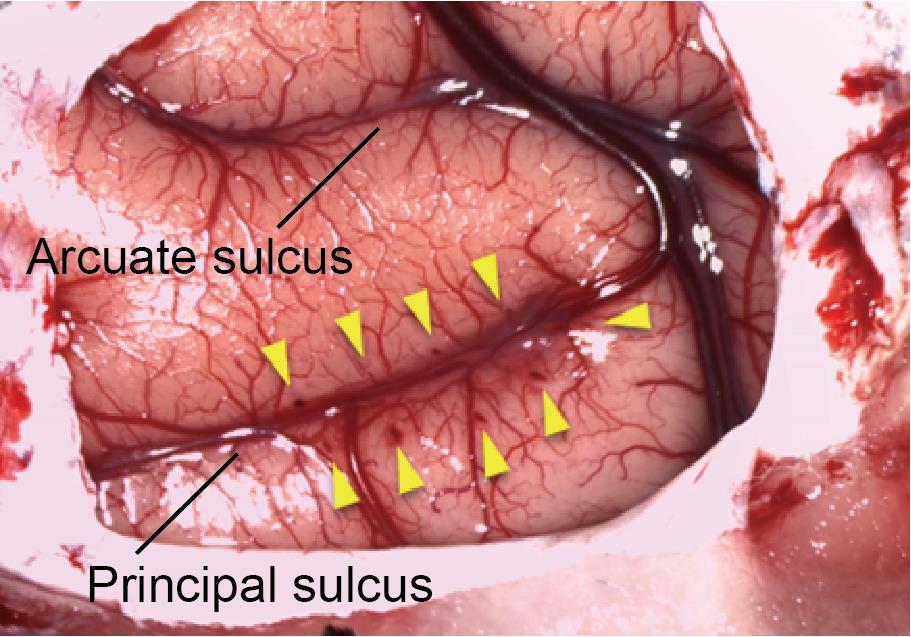
Figure 8. Injection area. Arrows indicate injection tracks along the principal sulcus.
Closing the head.
Suture the dura with the fusible sutures (Vicryl Plus 5-0) before checking for cerebrospinal fluid (CSF) leakage.
Note: Sutures should be evenly spaced at approximately 2 mm to prevent CSF leakage. If CSF leakage is noted, continue suturing until no leakage occurs.
Suture the bone flap back in place with the fusible sutures (Vicryl Plus 3-0) (General note 2).
Suture the fascia and subcutaneous tissue with the fusible sutures (Vicryl Plus 3-0) and then suture the skin with the nylon sutures (Monosof 2-0).
Administer analgesia (ketoprofen, 1–2 mg/kg, i.m.).
Upon awakening, return the monkey to its home cage.
Note: To prevent being harmed by other monkeys, it is preferable to keep the monkey alone until the sutures are removed.
Post-surgical procedures
Perioperative management
Administer prophylactic antibiotics by i.m. injections (sodium cefmetazole, 25–50 mg/kg/day) and analgesics (ketoprofen, 1–2 mg/kg/day) for seven days.
Remove the sutures when the skin has healed (after ≥ two weeks), after which experiments can be initiated.
Validation of protocol
The successful expression of DREADDs was confirmed by post-mortem histological inspection (Figure 9) in each study using our previously reported protocol [7–9]. We also confirmed DREADDs expression in vivo by conducting positron emission tomography (PET) scans with a radiolabeled DREADDs ligand at 45 days after the surgery [8, 9].
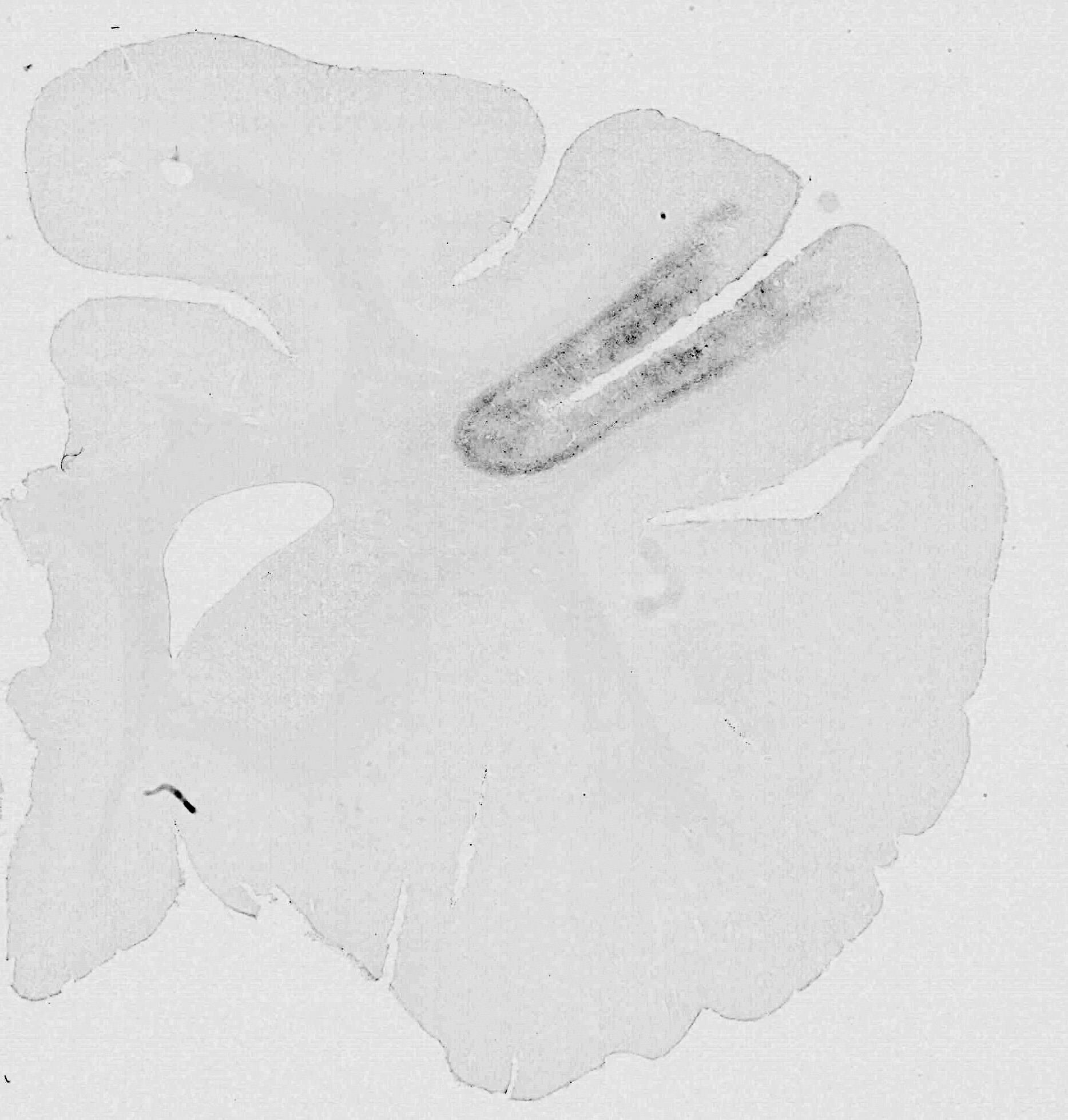
Figure 9. Histological section. 3,3’-diaminobenzidine-stained section showing immunoreactivity against a reporter protein (AcGFP).
We have also observed robust chemogenetic effects on behavior (i.e., working memory impairment) following the administration of a DREADD agonist (deschloroclozapine) [7–9].
General notes and troubleshooting
General notes
MRI and CT scans are performed on the same day. Following estimation of the injection sites based on MRI, the surgery is performed on a later day (typically ≥ one week later).
Insert an elevator or flat instrument between the skull and the dura mater to prevent injury of the dura mater and brain when making suture holes and when suturing to restore the bone flap.
Acknowledgments
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/ Japan Society for the Promotion of Science (JSPS) KAKENHI grant numbers JP21K07268, JP22H05521 (to KO), JP19K08138, JP23H02405 (to YN), and JP20H05955 (to TM); JST PRESTO grant number JPMJPR22S3 (to KO); and AMED grant number JP18dm0307007 (to TM). The two Japanese monkeys used in the original work (Oyama et al., 2021) were provided by the National Bio-Resource Project “Japanese Monkeys” of MEXT, Japan. We thank Jun Kamei, Ryuji Yamaguchi, Yuichi Matsuda, Yoshio Sugii, Takashi Okauchi, Rie Yoshida, Risa Suma, and Tomomi Kokufuta for their technical assistance. We also thank Mark A. G. Eldridge and Richard C. Saunders for providing us with tremendous guidance and advice on surgical techniques. This protocol was adapted from our previous works [7–9].
Competing interests
The authors declare no competing interests.
Ethical considerations
All experimental procedures involving animals were carried out in accordance with the Guide for the Care and Use of Nonhuman Primates in Neuroscience Research (The Japan Neuroscience Society; https://www.jnss.org/en/animal_primates) and were approved by the Animal Ethics Committee of the National Institutes for Quantum Science and Technology.
References
- Deisseroth, K. (2011). Optogenetics. Nat. Methods 8(1): 26–29. doi: 10.1038/nmeth.f.324
- Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. and Roth, B. L. (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U. S. A. 104(12): 5163–5168. doi: 10.1073/pnas.0700293104
- Roth, B. L. (2016). DREADDs for Neuroscientists. Neuron 89(4): 683–694. doi: 10.1016/j.neuron.2016.01.040
- Khateeb, K., Griggs, D. J., Sabes, P. N. and Yazdan-Shahmorad, A. (2019). Convection Enhanced Delivery of Optogenetic Adeno-associated Viral Vector to the Cortex of Rhesus Macaque Under Guidance of Online MRI Images. J. Vis. Exp. (147): e3791/59232-v. doi: 10.3791/59232-v
- Lerchner, W., Corgiat, B., Der Minassian, V., Saunders, R. C. and Richmond, B. J. (2014). Injection parameters and virus dependent choice of promoters to improve neuron targeting in the nonhuman primate brain. Gene Ther. 21(3): 233–241. doi: 10.1038/gt.2013.75
- Eldridge, M. A. G., Lerchner, W., Saunders, R. C., Kaneko, H., Krausz, K. W., Gonzalez, F. J., Ji, B., Higuchi, M., Minamimoto, T., Richmond, B. J., et al. (2016). Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat. Neurosci. 19(1): 37–39. doi: 10.1038/nn.4192
- Oyama, K., Hori, Y., Nagai, Y., Miyakawa, N., Mimura, K., Hirabayashi, T., Inoue, K. i., Takada, M., Higuchi, M., Minamimoto, T., et al. (2022). Chronic Behavioral Manipulation via Orally Delivered Chemogenetic Actuator in Macaques. J. Neurosci. 42(12): 2552–2561. doi: 10.1523/jneurosci.1657-21.2021
- Oyama, K., Hori, Y., Nagai, Y., Miyakawa, N., Mimura, K., Hirabayashi, T., Inoue, K. i., Suhara, T., Takada, M., Higuchi, M., et al. (2021). Chemogenetic dissection of the primate prefronto-subcortical pathways for working memory and decision-making. Sci. Adv. 7(26): eabg4246. doi: 10.1126/sciadv.abg4246
- Nagai, Y., Miyakawa, N., Takuwa, H., Hori, Y., Oyama, K., Ji, B., Takahashi, M., Huang, X. P., Slocum, S. T., DiBerto, J. F., et al. (2020). Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 23(9): 1157–1167. doi: 10.1038/s41593-020-0661-3
- Miyakawa, N., Nagai, Y., Hori, Y., Mimura, K., Orihara, A., Oyama, K., Matsuo, T., Inoue, K. i., Suzuki, T., Hirabayashi, T., et al. (2023). Chemogenetic attenuation of cortical seizures in nonhuman primates. Nat. Commun. 14(1): e1038/s41467-023-36642-6. doi: 10.1038/s41467-023-36642-6
- Hori, Y., Mimura, K., Nagai, Y., Fujimoto, A., Oyama, K., Kikuchi, E., Inoue, K. i., Takada, M., Suhara, T., Richmond, B. J., et al. (2021). Single caudate neurons encode temporally discounted value for formulating motivation for action. eLife 10: e61248. doi: 10.7554/elife.61248
- Hirabayashi, T., Nagai, Y., Hori, Y., Inoue, K. i., Aoki, I., Takada, M., Suhara, T., Higuchi, M. and Minamimoto, T. (2021). Chemogenetic sensory fMRI reveals behaviorally relevant bidirectional changes in primate somatosensory network. Neuron 109(20): 3312–3322.e5. doi: 10.1016/j.neuron.2021.08.032
- Kimura, K., Nagai, Y., Hatanaka, G., Fang, Y., Tanabe, S., Zheng, A., Fujiwara, M., Nakano, M., Hori, Y., Takeuchi, R. F., et al. (2023). A mosaic adeno-associated virus vector as a versatile tool that exhibits high levels of transgene expression and neuron specificity in primate brain. Nat. Commun. 14(1): e1038/s41467-023-40436-1. doi: 10.1038/s41467-023-40436-1
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Oyama, K., Nagai, Y. and Minamimoto, T. (2023). Targeted Delivery of Chemogenetic Adeno-Associated Viral Vectors to Cortical Sulcus Regions in Macaque Monkeys by Handheld Injections. Bio-protocol 13(23): e4897. DOI: 10.21769/BioProtoc.4897.
Category
Neuroscience > Basic technology > Chemogenetics
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









