- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Habituation of Sugar-Induced Proboscis Extension Reflex and Yeast-Induced Habituation Override in Drosophila melanogaster
Published: Vol 13, Iss 23, Dec 5, 2023 DOI: 10.21769/BioProtoc.4891 Views: 1421
Reviewed by: Nafisa M. JadavjiSurya Jyoti BanerjeeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Assessing Experience-dependent Tuning of Song Preference in Fruit Flies (Drosophila melanogaster)
Xiaodong Li [...] Azusa Kamikouchi
Jul 20, 2018 7473 Views

Caste Transition and Reversion in Harpegnathos saltator Ant Colonies
Comzit Opachaloemphan [...] Hua Yan
Aug 20, 2023 1535 Views

A Method for Studying Social Signal Learning of the Waggle Dance in Honey Bees
Shihao Dong [...] Ken Tan
Aug 20, 2023 1348 Views
Abstract
Habituation, the process by which animals learn to ignore insignificant stimuli, facilitates engagement with salient features of the environment. However, neural mechanisms underlying habituation also allow responses to familiar stimuli to be reinstated when such stimuli become potentially significant. Thus, the habituated state must allow a mechanism for habituation override. The remarkably precise knowledge of cell identity, connectivity, and information coding in Drosophila sensory circuits, as well as the availability of tools to genetically target these cells, makes Drosophila a valuable and important organism for analysis of habituation and habituation-override mechanisms. Studies of olfactory and gustatory habituation in Drosophila suggest that potentiation of GABAergic neurons underlies certain timescales of habituation and have specified some elements of a gustatory habituation-override pathway. More detailed understanding of gustatory habituation and habituation-override mechanisms will benefit from access to robust behavioral assays for (a) the proboscis extension reflex (PER) elicited by a sweet stimulus, (b) exposure paradigms that result in PER habituation, and, most critically, (c) manipulations that result in PER-habituation override. Here, we describe simple protocols for persistent sucrose exposure of tarsal hairs that lead to habituation of proboscis extension and for presentation of a novel appetitive stimuli that reinstate robust PER to habituated flies. This detailed protocol of gustatory habituation provides (a) a simple method to induce habituation by continuous exposure of the flies to sucrose for 10 min without leading to ingestion and (b) a novel method to override habituation by presenting yeast to the proboscis.
Key features
• A protocol for stimulation of Drosophila’s taste (sugar) sensory neurons that induces gustatory habituation without satiation due to ingestion.
• A chemical (yeast) stimulation protocol that rapidly induces habituation override/dishabituation in sugar-habituated Drosophila.
Background
Habituation is a fundamental form of learning that is conserved across species. In habituation, the response to a persistent inconsequential stimulus is reduced, leading to an enhanced ability to attend to more pertinent stimuli. A defining feature of the habituated state is the presence of a latent ability to respond to the familiar stimulus (Thompson and Spencer, 1966; Rankin et al., 2009; Ramaswami, 2014). This latent ability to respond is made evident by the effect of a novel external stimulus, which results in override of habituation, i.e., restoration of a robust response in the habituated animal. This can also be observed when an animal is motivated to attend to the familiar stimulus (Kato et al., 2015). The neural mechanism of habituation has been extensively studied across different species and different modalities, particularly in Aplysia, Drosophila, and mouse, using a variety of different behavioral protocols (Castellucci et al., 1970; Krasne and Edwards, 2002; Engel and Wu, 2009; Ogg et al., 2015). In Aplysia, both homosynaptic depression at the sensory-motor synapse (Castellucci et al., 1970) and potentiation of an inhibitory interneuron synapse (Bristol and Carew, 2005) have been suggested as underlying mechanisms of habituation of the gill-induced siphon withdrawal reflex. Later studies in Drosophila and vertebrates indicate that heterosynaptic potentiation of inhibition can lead to olfactory, auditory, or gustatory habituation (Das et al., 2011; Paranjpe et al., 2012; Ramaswami, 2014; Kato et al., 2015). However, the neural correlates of habituation override in these types of habituation are only just beginning to be investigated (Trisal et al., 2022). Here, we first describe the protocol for the proboscis extension reflex (PER), which has been used previously to study gustatory habituation in Drosophila (Duerr and Quinn, 1982; Le Bourg, 1983; Fois et al., 1991; Engel and Wu, 2009; Paranjpe et al., 2012), and then a protocol to induce override of PER habituation following exposure to a novel sensory stimulus (Paranjpe et al., 2012; Trisal et al., 2022). The novel stimulus used is a non-invasive gustatory stimulus (10% yeast), which the flies have not encountered before, that is presented to the proboscis. Compared to previous publications on PER (Shiraiwa and Carlson, 2007; Li et al., 2022), we provide a simpler and potentially less traumatic method to immobilize the flies. In addition, our protocol (also used by Paranjpe et al., 2012) is unique in that it exposes the flies continuously to 10% sucrose (while preventing ingestion at the same time) to induce more robust PER habituation than in the original studies. Our approach allows a more dynamic range and time to study habituation and dishabituation. While our method requires approximately 15 min for analysis of an individual fly, it requires far fewer flies to reach statistical significance.
Materials and reagents
Biological material
Drosophila Canton-Special females, aged 3–4 days
Reagents
Sucrose (Fischer Scientific, catalog number: 15925)
Yeast (Sigma-Aldrich, catalog number: YSC2)
Corn flour (Avenue Supermarts Ltd)
Sugar (Avenue Supermarts Ltd)
D-Glucose (Qualigens, catalog number: Q15405)
Yeast extract (Himedia, catalog number: RM027)
Propionic acid (Qualigens, catalog number: Q26955)
Orthophosphoric acid (Qualigens, catalog number: Q29245)
Ethanol (Merck Millipore, catalog number: 100983)
Agar (Qualigens, catalog number: Q21185)
Tego-sept (Sigma-Aldrich, catalog number: M1650000)
Water (double distilled)
Solutions
10% sucrose solution (see Recipes)
2% sucrose solution (see Recipes)
10% yeast solution (see Recipes)
Corn meal media (1 L) (see Recipes)
Recipes
10% sucrose (weight/volume)
Reagent Quantity Sucrose 1 g Water (autoclaved double distilled) 10 mL 2% sucrose (weight/volume)
Reagent Quantity Sucrose 20 mg Water (autoclaved double distilled) 1 mL 10% yeast (weight/volume)
Reagent Quantity Yeast 500 mg Water (autoclaved double distilled) 5 mL Corn meal media (1 L)
Reagent Quantity Corn flour 80 g D-Glucose 20 g Sugar 40 g Agar 8 g Yeast extract 15 g Propionic acid 4 mL Orthophosphoric acid 0.6 mL Tego-sept 1 g (dissolved in 5 mL ethanol)
Laboratory supplies
Coverslips 18 mm × 18 mm (Polar Industrial Corporation, Blue Star)
1 mL syringes with attached needle of size 0.30 mm × 8 mm, gauge 30 G × 5/16 (Hindustan Syringes and Medical Devices Ltd, model: Dispovan U-40)
Synthetic hairbrush (Camlin, catalog number: 2066774)
Transparent nail polish (Lakme Truewear, model: CG012)
Styrofoam box (20 cm × 14 cm × 8.5 cm)
Crushed ice
Styrofoam tray (19 cm × 16 cm × 3 cm)
Glass vial for cold anesthesia (8.5 cm high × 2 cm diameter)
Metal (aluminum) plate (10 cm diameter)
Timer (West Bend, catalog number: 40005X)
Filter paper No. 1 (Indica, Indicators, catalog number: 74039)
Plastic box of dimension 13 cm × 11 cm × 8 cm
Plastic bottle for Drosophila culture 250 mL volume (Bharat Plastic Manufacturing Company, catalog number: FLBT-20)
Absorbent cotton wool (Prabhat Surgical Cotton Private Limited)
Equipment
Stereomicroscope magnification 20× (10× eyepiece, 2× objective), numerical aperture 0.142 (Olympus, model: SZ51)
Micromanipulator (Narishige Japan, model: MM3)
Behavior room or chamber with controlled temperature (21 °C) and humidity (60%)
Software
GraphPad Prism v8.4.2
Procedure
Fly preparation
Raise flies in standard cornmeal media in plastic 250 mL bottles containing 15–20 mL of media at 25 °C temperature, 60% relative humidity, and 12:12 h light/dark cycle. Transfer adult flies every 2–3 days to prevent crowding and sogging of media.
Collect freshly eclosed flies of either sex, which may be 3–4 days old, in fresh media bottles such that each bottle has 20–30 flies. Age flies for 3–4 days at 25 °C, 60% humidity, and 12:12 h light/dark cycle.
Note: The media is not considered fresh if it is dry and starts separating from the walls of the bottle or has low levels of bacterial or fungal growth.
Starve flies in an empty glass vial containing wet filter paper for 20–24 h at 25 °C, 60% humidity, and 12:12 h light/dark cycle (Figure 1A).
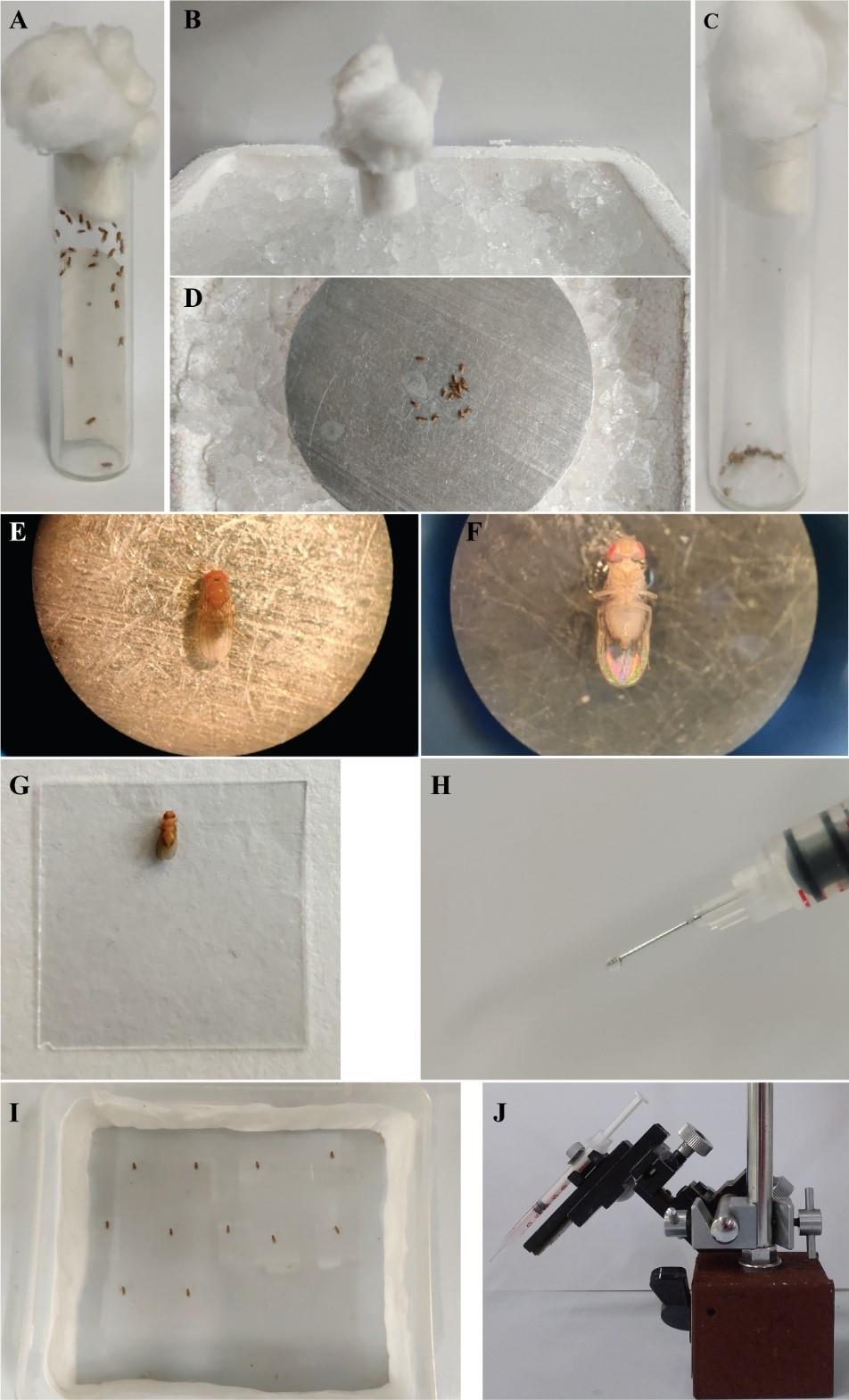
Figure 1. Preparation of flies for proboscis extension reflex assay. (A) Flies are starved in a vial overnight with a wet filter paper. (B) Flies are anesthetized on ice for not more than 1 min. (C) Anesthetized flies in the vial. (D) Anesthetized flies are transferred on a metal plate kept on ice. (E) Single anesthetized fly on the metal plate turned dorsal side up in order to stick to the coverslip using nail polish. (F), (G) Fly stuck on the coverslip with ventral side up. (H) Size of the drop of nail polish on the syringe used to immobilize flies on the coverslip. (I) Flies are kept in a humidified chamber for 1–2 h for recovery. (J) Syringe containing 10% sucrose for habituation, mounted on a micromanipulator.To anesthetize the starved flies, first cool an empty glass vial by placing it in a Styrofoam box filled with ice. After 5 min, transfer the starved flies into the now cold vial. To transfer the flies, tap the vial containing the flies such that they come to the bottom of the vial and quickly invert this vial over the cold empty vial. Tap both the vials together such that all flies are transferred to the cold vial.
Gently tap the cold vial on a rubber pad to bring the flies down to the bottom of the vial and place the vial back in ice for one more minute until all flies are anesthetized (Figure 1B, 1C).
Place a metal plate on a Styrofoam tray filled with ice and wipe it with filter paper to remove condensed water. Gently transfer the anesthetized flies onto the metal plate kept on ice. Make sure the plate is dry by wiping it repeatedly before or after transferring flies onto it, whenever water condenses over it. Keep only the female flies and discard all male flies. Male and female flies are distinguished based on the standard sexually dimorphic traits like male leg sex combs, male genitalia, and female abdomen pigmentation. Using a fine synthetic hairbrush, turn the flies’ dorsal side up (Figure 1D, 1E). It is important to note that the total amount of time flies spend on ice, including both in the vial and on the metal plate, should not be more than 5 min, as it can affect their response.
Take a small amount of transparent nail polish (~1 μL) using the needle of a 1 mL syringe (Figure 1H) and place the drop on the coverslip. Gently place the coverslip on the fly such that the nail polish touches the posterior part of the head and anterior part of the thorax. This is done so that the head does not move while performing PER. It is also important to use only a small drop of nail polish on the coverslip so that it does not reach the proboscis or the legs while fixing the fly (Figure 1F, 1G). Small bubbles may appear occasionally in the nail polish but that does not affect the immobilization of the fly on the coverslip. Note that all these steps requiring flies to be anesthetized are performed on the metal plate.
Place the fly fixed on the coverslip (in a ventral side up direction so that the proboscis and legs are exposed for stimulation) in a box for 1–2 h for recovery at 25 °C. The box is lined with wet filter paper as the coverslips with flies are at the base, to maintain humidity inside (Figure 1I). Flies are transferred manually by gently placing the coverslip with the immobilized flies in the box. The number of flies placed in the box depends on its size. A box with the dimensions mentioned in the current study allows a maximum of 15–20 flies so that crowding and therefore overlap can be avoided. The minimum number of flies can be determined by the user.
Habituation of the sucrose-induced PER
Fill three 1 mL syringes: one with 2% sucrose solution, one with 10% sucrose solution, and the third with double-distilled water. Fix the 1 mL syringe with 10% sucrose solution on a micromanipulator in the slot provided (Figure 1I).
Gently pick the coverslip with the immobilized fly and place it under the stereoscope. Using the syringe containing double-distilled water, touch the proboscis or tarsus of the fly with water, as shown in Video 1. Allow the fly to drink water through proboscis until it stops and is sated. Flies are sated after being presented with water 1–2 times. If the fly does not respond to water, consider the fly for the experiment and test the naïve response to sucrose as explained in step B3.
Note: The occasional fly may not stop drinking water even after being presented with water three or more times; discard such flies.
Video 1. Water satiation. Initially, the fly is presented with water. If the fly extends its proboscis, water is offered to satiate it until there is no further response to water.Immediately after feeding water to the fly, carefully touch the distal tips of the foreleg tarsi with a sucrose drop with the syringe containing 2% sucrose, while preventing any contact with the proboscis. Record whether there is a proboscis extension in response or not. Repeat this step five times with a gap of 10 s between each two trials (Video 2).
Video 2. Proboscis extension reflex (PER) in response to 2% sucrose. Video in real time showing the tarsi of the forelegs being stimulated with 2% sucrose five times, with 10 s of inter-trial interval, to quantify the PER response before and after exposure to habituating stimulus.Using the knobs attached to the micromanipulator to move the syringe in XY and Z plane, adjust the angle and height of the syringe containing 10% sucrose fixed on the micromanipulator, such that the sucrose drop hanging from the syringe is out of reach from the extended proboscis and touches only the distal tips of the forelegs (Video 3). Keep the syringe in this position, touching the tarsi, for 10 min, set on a timer. If the size of the sucrose drop becomes smaller due to evaporation, carefully press the plunger of the syringe and readjust the size. Flies are exposed to 10% sucrose immediately after step B3.
Video 3. Exposure to habituating stimulus. The tarsi of the fly are exposed to 10% sucrose for 10 min in order to habituate the PER response. The video is representative, showing 12 s from the 10 min exposure. The syringe containing 10% sucrose is fixed on a micromanipulator such that only tarsi are stimulated whereas the proboscis cannot reach the stimulus.After 10 min, move aside the 10% sucrose syringe and wash the tarsal tips with double-distilled water immediately after sucrose exposure (Video 4).
Video 4. Leg wash after exposure to 10% sucrose. The tarsi are washed with double-distilled water to clear 10% sucrose that may be sticking to the legs.Repeat step B3 immediately after leg wash to record the habituated response. Record all data. In a habituated fly, the number of times the proboscis is extended in response to 2% sucrose should decrease (Video 5).
Video 5. Response after sucrose exposure. After exposure to 10% sucrose for 10 min, tarsal stimulation with 2% sucrose does not lead to PER response, thus exhibiting habituation.Dishabituation using yeast
Touch the proboscis of the habituated fly with 10% yeast solution using a 1 mL syringe and quickly remove the syringe in order to prevent or minimize potential consumption of yeast (Video 6). Repeat this over a period of 1 min, set on a timer, with a gap of approximately 10 s such that the proboscis is stimulated 5–6 times with yeast.
Video 6. Dishabituation/habituation override induced by 10% yeast. 10% yeast is presented five times to the proboscis in order to dishabituate the fly. Yeast is quickly removed in order to prevent ingestion.Leave the fly for 1 min without any stimulus so that it gets time to groom itself in order to remove any sticking yeast.
Record the proboscis extension response to 2% sucrose as described previously, in yeast-exposed flies. The flies should show increased (dishabituated) response.
The procedure to induce habituation to sucrose and its override using 10% yeast is summarized in Figure 2.
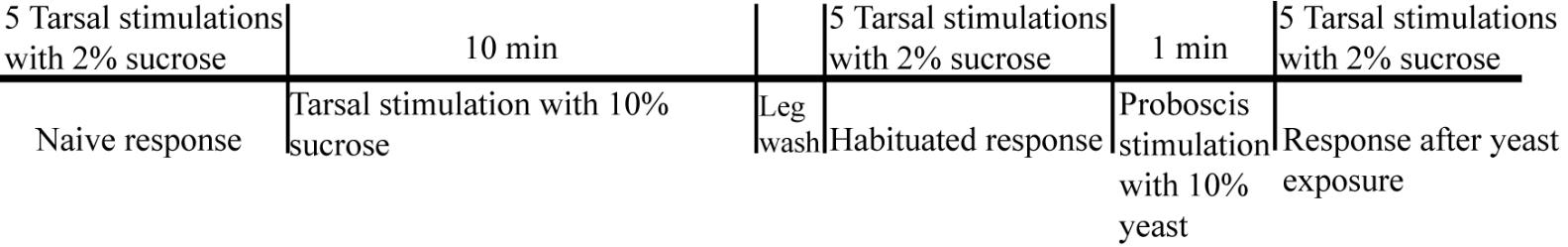
Figure 2. Line diagram representing the protocol. Figure shows the various key steps in the protocol to habituate flies to sucrose and override it by presenting 10% yeast to proboscis. The figure also shows the time period of the time-bound steps.
Data analysis
For each fly, calculate the percentage of proboscis extensions in response to five tarsal stimulations using a simple formula as = [Total observed number of proboscis extension/5] × 100.
A total of 25–30 flies are required to achieve statistical significance. Therefore, 2–3 bottles containing 20–30 flies each may be required to get 25–30 female flies, which are tested in the experiment. Since data is paired, all the conditions have the same number of flies. PER response of the first five tarsal stimulations is considered. More stimulations are avoided to prevent any decrease in response due to habituation. Plot these PER response data observed during different conditions in all the flies tested as bar graphs representing the mean percentage PER with SEM error bars and individual fly data points overlaid on it using GraphPad Prism v8.4.2.
Note: Compare the %PER response observed with 2% sucrose during naïve, habituated, and dishabituated conditions only. PER response during 10 min exposure to 10% sucrose is not considered for data analysis.
The data is not normally distributed due to its discrete nature and therefore no normality test is required. The Friedman test, a non-parametric test, followed by Dunn’s post-hoc test is used to evaluate differences across naïve, habituated, and dishabituated responses. To perform Friedman test in GraphPad Prism software, carry out the following steps:
In the Result section, Select New Analysis.
Under Column Analysis section, select One way ANOVA (non-parametric or mixed) and tick the columns that need to be analyzed.
A new window opens. In the tab Under the Experimental Design, select Each row represents matched or repeated measures, data.
Select No. use nonparametric test under the option Assume Gaussian Distribution of residuals option.
Under the Multiple Comparison tab, select compare the mean of each column with every other column. In case of comparing PER responses between fed and starved flies (Figure 3A1) and between sucrose and water (Figure 3A2), Mann Whitney U test is used. Follow the steps from 3a to 3d, with the exception of under Column Analysis, where you should select the t tests (and nonparametric tests) option.
If the fly extends the proboscis to 2% sucrose less than three out of five trials while testing for the naïve response, discard the fly.
Trisal et al. (2022). A Drosophila circuit for habituation override. Journal of Neuroscience (Figure 2, panel B).
Bristol, A. S. and Carew, T. J. (2005). Differential role of inhibition in habituation of two independent afferent pathways to a common motor output. Learn. Mem. 12(1): 52–60.
- Castellucci, V., Pinsker, H., Kupfermann, I. and Kandel, E. R. (1970). Neuronal Mechanisms of Habituation and Dishabituation of the Gill-Withdrawal Reflex in Aplysia. Science 167(3926): 1745–1748.
- Das, S., Sadanandappa, M. K., Dervan, A., Larkin, A., Lee, J. A., Sudhakaran, I. P., Priya, R., Heidari, R., Holohan, E. E., Pimentel, A., et al. (2011). Plasticity of local GABAergic interneurons drives olfactory habituation. Proc. Natl. Acad. Sci. U.S.A. 108(36): e1106411108.
- Duerr, J. S. and Quinn, W. G. (1982). Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc. Natl. Acad. Sci. U. S. A. 79(11): 3646–3650.
- Engel, J. E. and Wu, C. F. (2009). Neurogenetic approaches to habituation and dishabituation in Drosophila. Neurobiol. Learn. Mem. 92(2): 166–175.
- Fois, C., Médioni, J. and Le Bourg, E. (1991). Habituation of the proboscis extension response as a function of age in Drosophila melanogaster. Gerontology 37(4): 187–192.
- Kato, H. K., Gillet, S. N. and Isaacson, J. S. (2015). Flexible Sensory Representations in Auditory Cortex Driven by Behavioral Relevance. Neuron 88(5): 1027–1039.
- Krasne, F. B. and Edwards, D. H. (2002). Modulation of the Crayfish Escape Reflex--Physiology and Neuroethology. Integr. Comp. Biol. 42(4): 705–715.
- Le Bourg, E. (1983). Aging and habituation of the tarsal response in Drosophila melanogaster. Gerontology 29(6): 388–393.
- Li, Q., Yang, L. and Montell, C. (2022). Drosophila proboscis extension response and GCaMP imaging for assaying food appeal based on grittiness. STAR protocols 3(4): 101806.
- Ogg, M. C., Bendahamane, M. and Fletcher, M. L. (2015). Habituation of glomerular responses in the olfactory bulb following prolonged odor stimulation reflects reduced peripheral input. Front. Mol. Neurosci. 8: 53.
- Paranjpe, P., Rodrigues, V., VijayRaghavan, K. and Ramaswami, M. (2012). Gustatory habituation in Drosophila relies on rutabaga (adenylate cyclase)-dependent plasticity of GABAergic inhibitory neurons. Learn. Mem. 19(12): 627–635.
- Ramaswami, M. (2014). Network plasticity in adaptive filtering and behavioral habituation. Neuron 82(6): 1216–1229.
- Rankin, C. H., Abrams, T., Barry, R. J., Bhatnagar, S., Clayton, D. F., Colombo, J., Coppola, G., Geyer, M. A., Glanzman, D. L., Marsland, S., et al. (2009). Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 92(2): 135–138.
- Shiraiwa, T. and Carlson, J. R. (2007). Proboscis Extension Response (PER) Assay in Drosophila. J. Vis. Exp. (3): 193.
- Thompson, R. F. and Spencer, W. A. (1966). Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 73(1): 16–43.
- Trisal, S., Aranha, M., Chodankar, A., VijayRaghavan, K. and Ramaswami, M. (2022). A Drosophila Circuit for Habituation Override. J. Neurosci. 42(14): 2930–2941.
Raw data of naïve PER response to 2% sucrose, habituated response after 10 min of exposure to 10% sucrose, and PER response after presentation of 10% yeast solution, tested in 30 Canton S flies, is shown in Table 1; a representative bar plot of the data with individual data points is shown in Figure 3B. This protocol has previously been shown to mediate and override habituation (Figure 2B in Trisal et al., 2022). Analysis of PER response in fed and starved, age- and density-matched CS flies to 2% sucrose shows that PER is specifically induced by sucrose (Figure 3A1). In addition, water sated flies do not respond to water, whereas PER is observed when stimulated with sucrose, further confirming that PER is specific to sucrose (Figure 3A2).
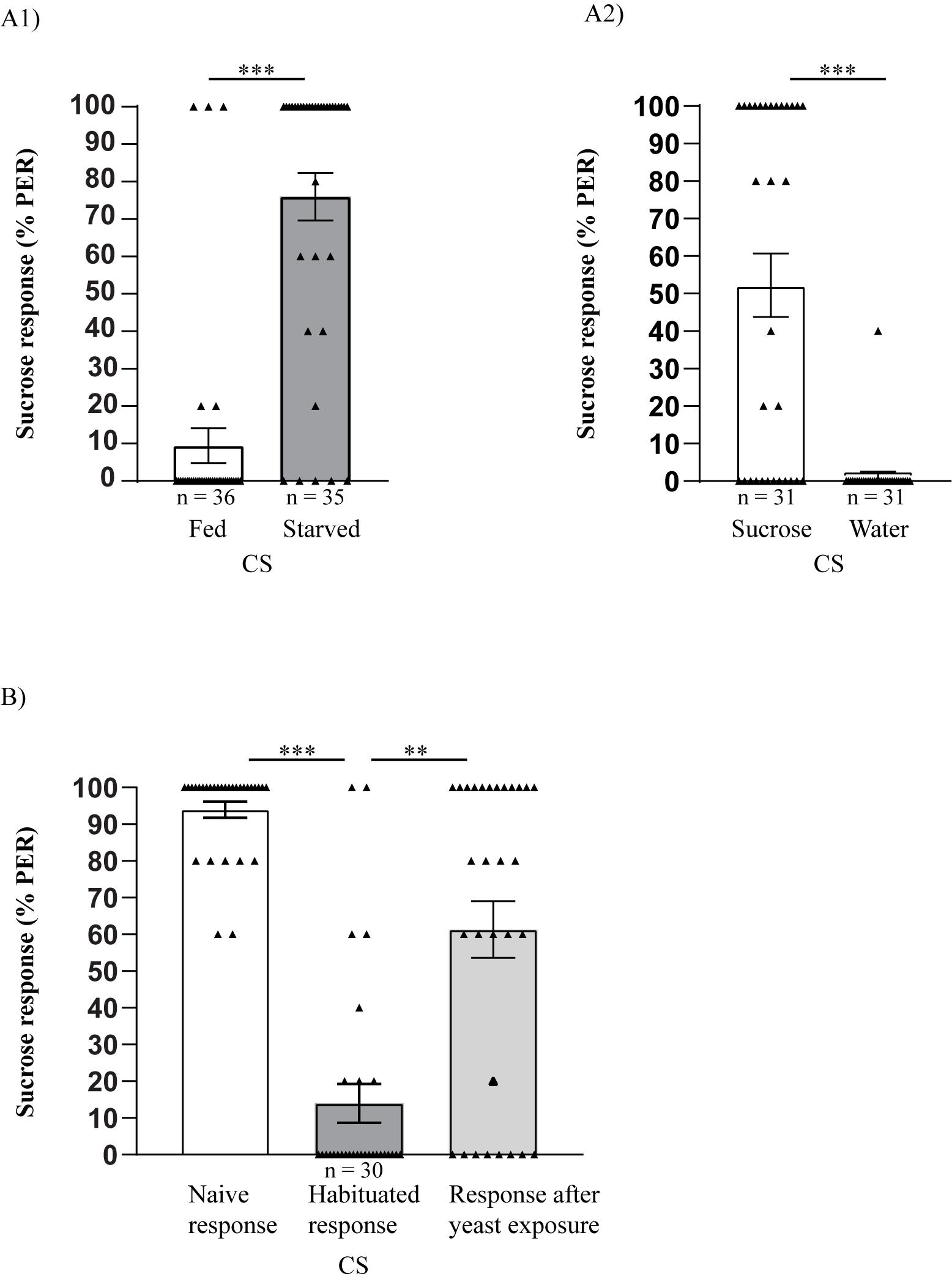
Figure 3. Habituation override using a novel yeast stimulus. (A1) Control experiment shows proboscis extension reflex (PER) response in fed and starved flies. PER response is specific to 2% sucrose, as fed flies show significantly lower response compared to starved flies (Mann Whitney U stat = 159, ***p < 0.0001). (A2) Comparison of PER response to sucrose and water in water sated flies. Flies extend their proboscis to 2% sucrose, which is not observed when water is further presented, confirming that PER is specific to sucrose (Mann Whitney U stat = 194.5, ***p < 0.0001. (B) Presentation of 2% sucrose solution to the tarsal hairs of Canton S flies generates a proboscis extension response. PER response shows a significant decrease upon exposure to 10% sucrose for 10 min, which can be overridden significantly upon presentation of 10% yeast solution (Friedman statistic = 42.70, ***p < 0.0001, **p = 0.0072, Dunn’s multiple comparison post-hoc test). Triangles represent individual data point whereas bars represent mean ± SEM (error bars).
Table 1. Average %PER to 2% sucrose in 30 naïve, habituated, or dishabituated individual flies calculated from five stimulations
| Naive response (% PER) | Habituated response (% PER) | Response after yeast exposure (% PER) |
| 100 | 0 | 80 |
| 80 | 20 | 0 |
| 100 | 0 | 80 |
| 100 | 20 | 0 |
| 100 | 0 | 80 |
| 60 | 0 | 100 |
| 100 | 0 | 60 |
| 100 | 60 | 100 |
| 100 | 0 | 20 |
| 100 | 0 | 100 |
| 80 | 0 | 0 |
| 100 | 100 | 100 |
| 100 | 0 | 100 |
| 100 | 20 | 100 |
| 100 | 40 | 80 |
| 100 | 0 | 0 |
| 100 | 0 | 100 |
| 80 | 0 | 60 |
| 100 | 0 | 100 |
| 100 | 0 | 100 |
| 100 | 100 | 100 |
| 80 | 0 | 60 |
| 60 | 0 | 0 |
| 100 | 60 | 100 |
| 100 | 0 | 100 |
| 80 | 0 | 60 |
| 100 | 0 | 0 |
| 100 | 0 | 0 |
| 100 | 0 | 60 |
| 100 | 0 | 0 |
Validation of protocol
This protocol or parts of it has been used and validated in the following research article(s):
General notes and troubleshooting
Mounted flies must be healthy and undamaged and capable of robust PER before they can be used for experiments. In protocol section B, step 3, we therefore discard any flies that respond less than three times to five sequential tarsal stimulations with 2% sucrose. If a large fraction of the flies in the batch do not respond to 2% sucrose or fail to habituate, make sure the flies are starved for 20–24 h, are not crowded, and are not kept on ice for more than 5 min. While dishabituating the flies using 10% yeast as in protocol section C, step 1, make sure flies do not consume significant amounts of yeast. In protocol section B, step 2, discard flies that do not stop consuming water, as such flies are likely damaged in some way.
Acknowledgments
We thank Marcia Aranha, Camilla Roselli, Ankita Chodankar and members of Ramaswami and VijayRaghavan labs for advice, support and useful discussions, particularly Pushkar Paranjpe for early help setting up the PER assay. We thank Spraha Bhandari for volunteering to test and confirm that this protocol was sufficient for a new researcher to successfully observe and record PER, its habituation to sucrose, and yeast-induced override. This protocol was previously described in Paranjpe et al. (2012) and Trisal et al. (2022). The work was supported by a Wellcome Trust Investigator Award and a Science Foundation Ireland Investigator Programme Grant to MR, by core support from the National Centre for Biological Sciences (Tata Institute of Fundamental Research) to KVR and by a CSIR postgraduate fellowship and a Biocon-Trinity Scholarship to ST.
Competing interests
The authors declare no competing financial interests.
References
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Trisal, S., VijayRaghavan, K. and Ramaswami, M. (2023). Habituation of Sugar-Induced Proboscis Extension Reflex and Yeast-Induced Habituation Override in Drosophila melanogaster. Bio-protocol 13(23): e4891. DOI: 10.21769/BioProtoc.4891.
- Trisal, S., Aranha, M., Chodankar, A., VijayRaghavan, K. and Ramaswami, M. (2022). A Drosophila Circuit for Habituation Override. J. Neurosci. 42(14): 2930–2941.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link