- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Generation of Mouse Primitive Endoderm Stem Cells
Published: Vol 13, Iss 22, Nov 20, 2023 DOI: 10.21769/BioProtoc.4878 Views: 2445
Reviewed by: Komuraiah MyakalaWei FanAbhijnya Kanugovi

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2226 Views
Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views
Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1453 Views
Abstract
The blastocysts consist of dozens of cells of three distinct lineages: epiblast (Epi), trophoblast (TB), and primitive endoderm (PrE). All embryonic and extraembryonic tissues are derived from Epi, TB, and PrE. Stem cell lines representing preimplantation Epi and TB have been established and are known as embryonic stem cells (ESCs) and trophoblast stem cells (TSCs). Extraembryonic endoderm cells (XENCs) constitute a cell line that has been established from PrE. Although in vivo, PrE gives rise to visceral endoderm (VE), parietal endoderm (PE), and marginal zone endoderm (MZE); XENCs, on blastocyst injection into chimeras, primarily contribute to the distal region of PE. Here, we provide a comprehensive protocol for the establishment of fully potent primitive endoderm stem cell (PrESC) lines. PrESCs are established and maintained on mouse embryonic fibroblast (MEF) feeder cells in a serum-free medium supplemented with fibroblast growth factor 4 (FGF4), heparin, CHIR99021, and platelet-derived growth factor-AA (PDGF-AA). PrESCs co-express markers indicative of pluripotency and endoderm lineage commitment, exhibiting characteristics akin to those of PrE. On transplantation of PrESCs into blastocysts, they demonstrate a high efficiency in contributing to VE, PE, and MZE. PrESCs serve as a valuable model for studying PrE, sharing similarities in gene expression profiles and differentiation potential. PrESCs constitute a pivotal cornerstone for in vitro analysis of early developmental mechanisms and for studies of embryo reconstitution in vitro, particularly in conjunction with ESCs and TSCs.
Key features
• Establishment and maintenance of primitive endoderm stem cell (PrESCs) capable of recapitulating the developmental prowess inherent to PrE.
• Offering a source of PrE lineage for embryo-like organoid reconstitution studies.
Graphical overview

Background
Epiblast (Epi), trophoblast (TB), and primitive endoderm (PrE) are differentiated from the zygote by the late blastocyst stage of preimplantation development. Epi lineage generates all embryonic tissues, extraembryonic mesodermal tissues (i.e., chorion, allantois, yolk sac mesoderm, amniotic mesoderm, and capillaries in the labyrinthine layer of the placenta), and amniotic ectoderm. TB lineage generates major parts of placenta and trophectoderm of parietal yolk sac (PYS). PrE lineage is vital for normal embryonic development and is the origin of the visceral endoderm (VE) layer of the visceral yolk sac (VYS), the parietal endoderm (PE) layer of the PYS, and the marginal zone endoderm (MZE) lining along the edge of the placenta/chorionic plate of the placental disk (Gardner, 1982; Igarashi et al., 2018). The extraembryonic endodermal tissues play a pivotal role in nourishing the embryo by facilitating nutrient provision at the maternal–fetal interface (Jollie, 1990; Lloyd, 1990), especially significant prior to the onset of placental circulation (~E10.5 in mice), anterior–posterior patterning (Brennan et al., 2001), and yolk sac hematopoiesis (Ueno and Weissman, 2010). In mice, the gestation period is 19–21 days, and the initial yolk sac is formed on the fourth day, so embryonic development is uniquely dependent on the yolk sac during the first quarter of development.
Stem cell lines representing preimplantation epiblasts and trophoblasts have been established and are known as embryonic stem cells (ESCs) (Evans and Kaufman, 1981; Nichols et al., 2009) and trophoblast stem cells (TSCs) (Tanaka et al., 1998; Ohinata and Tsukiyama, 2014), respectively. To gain further insight into how the PrE functions during pre- and early post-implantation development, establishment of stem cell lines that fully retain PrE developmental potential has long been awaited. Extraembryonic endoderm cells (XENCs) turned out to be derivatives of the PrE but could only contribute to the distal region of the PE in chimeras (Kunath et al., 2005). Efforts to improve XEN cell culture conditions enabled the generation of XEN-P (extraembryonic endoderm precursor) cells in rats (Debeb et al., 2009; Galat et al., 2009) and pXEN (primitive XEN) cells in mice (Zhong et al., 2018), which express pluripotent makers and endoderm markers simultaneously. XEN-P cells have a slightly improved ability to contribute to cells other than in the distal PE at gastrula stages in chimeras. Further improvement of XENCs was performed by treating them with Nodal or Cripto, a ligand and coreceptor in the nodal signaling, respectively, but the impact of this treatment was again limited (Julio et al., 2011). Therefore, a robust protocol to capture early PrE-derived cells that can generate all derivatives of the PrE in the chimeric conceptus awaits discovery.
Recently, we succeeded in establishing primitive endoderm stem cells (PrESCs) that can fully recapitulate the developmental potential of PrE in mice. PrESCs are established and maintained on MEF feeder cells in a serum-free medium supplemented with FGF4, heparin, CHIR99021, and PDGF-AA. PrESCs co-express markers indicative of pluripotency (i.e., OCT4 and E-Cadherin) and endoderm lineage commitment (i.e., GATA4, GATA6, and SOX17), exhibiting characteristics akin to those of PrE. On transplantation of PrESCs into blastocysts, they demonstrate a high efficiency in contributing to all PrE derivatives, VE, PE, and MZE (Ohinata et al., 2022). In this protocol, we describe the detailed procedure for efficient generation of PrESC lines in mice. PrESCs therefore represent not only a regenerative resource to complement the PrE, but also an intervention tool to elucidate the mechanisms of PrE specification and subsequent pre- and post-implantation development. Importantly, PrESCs can be the final component of stem cell line assemblage required to generate development-competent blastocysts in vitro, in collaboration with ESCs and TSCs. PrESCs will contribute to the opening of a new research field linking pre- and early post-implantation development.
Materials and reagents
Biological materials
Pregnant mouse, 3.5 days post coitum (dpc) (8–20 weeks old) (e.g., CLEA Japan, CD-1). We have confirmed this protocol in CD-1, BALB/c, and B6PWKF1 genetic backgrounds
Mouse embryonic fibroblasts (MEFs) (ATCC, SCRC-1045TM). MEFs can also be derived from DR4 mice [Jackson, Dnmt1tm3Jae Hprt1b-m3 Tg (pPWL512hyg) 1Ems/J (DR4)] that are dissected 14.5 dpc; cells are passaged five times before inactivation by mitomycin C treatment. We routinely use DR4 MEFs, but the widely used CD-1 MEFs can also be used
Human fibroblast growth factor 4 (FGF4) (Sigma, catalog number: F8424)
Human platelet-derived growth factor-AA (PDGF-AA) (R&D, catalog number: 221-AA)
Rabbit anti-OCT4 antibody (MBL, catalog number: PM048)
Goat anti-SOX17 antibody, stock solution: 10 mg/mL in PBS (R&D, catalog number: AF1924)
Donkey anti-rabbit IgG antibody Alexa 488 conjugated (Invitrogen, catalog number: A21206)
Donkey anti-goat IgG antibody Alexa 568 conjugated (Invitrogen, catalog number: A11057)
Reagents
Mitomycin C (MMC) (Sigma, catalog number: M4287)
Fetal bovine serum (FBS) (biosera, catalog number: FB-1380/500)
Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, catalog number: D5796)
β-mercaptoethanol (100×) (Millipore, catalog number: ES-007-E)
Penicillin-streptomycin (100×) (Gibco, catalog number: 15140-122)
Gelatin from porcine skin (Sigma, catalog number: G1890)
TrypLETM Select Enzyme (1×), no phenol red (Gibco, catalog number: 12563-029)
StemFit® AK02N (or StemFit® Basic02, StemFit® Basic03) (Ajinomoto)
Heparin (Sigma, catalog number: H3149)
CHIR99021, GSK3 inhibitor (REPROCELL, catalog number: 04-0004)
CELLBANKER® 1 plus (ZENOGEN PHARMA)
Ethanol (e.g., Nacalai Tesque, catalog number: 14712-34)
Paraformaldehyde (PFA) (TAAB, catalog number: P001)
Tween 20 (Chem Cruz, catalog number: sc-29113)
Normal donkey serum (Sigma, catalog number: D9663)
Hoechst 33342 10 mg/mL solution (Invitrogen, catalog number: H3570)
Solutions
MEF medium (see Recipes)
AK02N medium (see Recipes)
PrESC medium (see Recipes)
100× Mitomycin C stock solution (see Recipes)
0.2% Gelatin (see Recipes)
70% Ethanol (see Recipes)
PBST (see Recipes)
10 N NaOH (see Recipes)
4% PFA/PBST (see Recipes)
Recipes
MEF medium
Reagent Final concentration Quantity DMEM 500 mL FBS 10% 55 mL β-mercaptoethanol 1× 5.6 mL Penicillin-streptomycin 1× 5.6 mL Total n/a 566.2 mL AK02N medium
Reagent Quantity Liquid A 400 mL Liquid B 100 mL Penicillin-streptomycin 5 mL Total 505 mL PrESC medium
Reagent Final concentration Quantity AK02N medium (liquid A+B) 50 mL CHIR99021 10 μM 50 μL (10 mM stock solution) Human FGF4 50 ng/mL 50 μL (50 μg/mL stock solution) Heparin 10 μg/mL 50 μL (50 mg/mL stock solution) Human PDGF-AA 25 ng/mL 50 μL (25 μg/mL stock solution) Total 50 mL 100× Mitomycin C stock solution
Reagent Final concentration Quantity Mitomycin C 0.5 mg/mL 5 mg ddH2O n/a 10 mL Total n/a 10 mL 0.2% Gelatin
Reagent Final concentration Quantity Gelatin 0.2% 1 g ddH2O n/a 500 mL Total n/a 500 mL Sterilize 0.2% gelatin solution by autoclaving.
70% Ethanol
Reagent Final concentration Quantity Ethanol 70% 350 mL ddH2O n/a 150 mL Total n/a 500 mL PBST
Reagent Final concentration Quantity Tween 20 0.2% 1 mL PBS n/a 500 mL Total n/a 501 mL 10 N NaOH
Reagent Final concentration Quantity NaOH 0.4 g/mL 40 g ddH2O n/a 100 mL Total n/a 100 mL NaOH liberates thermal energy upon dissolution. It is prudent to refrain from dissolving it in its entirety in a single instance. Instead, endeavor to dissolve it incrementally in multiple aliquots before culminating in a final volume of 100 mL. Employ safety spectacles to shield your eyes.
4% PFA/PBST
Reagent Final concentration Quantity PFA 4% 2 g PBST n/a 50 mL 10 N NAOH n/a 10 μL Total n/a 50 mL Incubate at 65 °C and mix several times until the PFA melts.
Equipment
Conical tube (15 mL) (Corning, catalog number: 352196)
Conical tube (50 mL) (Corning, catalog number: 352098)
Tissue culture plate (24 well) (Corning, catalog number: 351147)
Tissue culture plate (12 well) (Corning, catalog number: 351143)
Tissue culture plate (6 well) (Corning, catalog number: 351146)
Culture dish (35 mm) (Thermo Scientific, catalog number: 150460)
Culture dish (150 mm) (Corning, catalog number: 353025)
Petri dish (60 mm) (Corning, catalog number: 351007)
Glass bottom culture dish (35 mm) (IWAKI, catalog number: 3910-035)
Cryotube vial (Thermo Scientific, catalog number: 377224)
P2 micro-pipette (e.g., Gilson, catalog number: F144054M)
P20 micro-pipette (e.g., Gilson, catalog number: F144056M)
P1000 micro-pipette (e.g., Gilson, catalog number: 144059M)
Dissection microscope (e.g., Evident, model: SZX7)
Inverted microscope (e.g., Evident, model: IX71)
Water bath (e.g., TAITEC, model: SJ-07N)
Centrifuge (e.g., TOMY, model: LCX-100)
Hemocytometer (e.g., Biomedical Science, model: BMS-OCC01)
CO2 incubator (e.g., Panasonic, model: MCO-170AICV-PJVH)
Scissors (e.g., Natsume, model: NAPOX B-68T)
Forceps (e.g., Inox-Med, L5, catalog number: 11253-27)
Syringe (5 mL) (e.g., Terumo, model: SS-05LZ)
21-gauge needle (e.g., Terumo, model: NN-2138R)
Freezing container (e.g., Nihon Freezer, model: BICELL)
Ultra-low temperature freezer (-80 °C) (e.g., Panasonic, model: MDF-DU502VHS1-PJ)
Ultra-low temperature freezer (-150 °C) (e.g., Panasonic, model: MDF-1156ATA-PJ)
Confocal microscope (e.g., Evident, model: FV1200)
Procedure
Mitotic inactivation of MEF: mitomycin C (MMC) treatment
Notes:
The size and number of culture dishes used depends on the scale of the experiment. We routinely use 10–20 150 mm dishes. This protocol describes the use of 150 mm dishes.
MMC is toxic, so be sure to wear gloves when performing the experiment.
Culture MEFs in 25 mL of MEF medium per dish (see Recipe 1). Beginning with a confluent layer of MEFs, remove MEF medium from dishes and replace with 5 μg/mL 1× Mitomycin C solution in MEF medium.
Incubate the dishes for 2 h at 37 °C with 5% CO2.
Remove the medium and wash the cells three times with 25 mL of PBS.
Add 10 mL of 0.05% trypsin-EDTA and incubate until the cells come off the dish (3–5 min).
Collect cells in 50 mL conical tubes. Conical tubes are pre-filled with 10 mL of MEF medium to inhibit trypsin.
Centrifuge the tubes at 300× g for 5 min at room temperature and aspirate the supernatant.
Resuspend the cell pellet in 25 mL of MEF medium. Centrifuge the tubes at 300× g for 5 min at room temperature and aspirate the supernatant. Repeat this step once.
Resuspend the cell pellet in CELLBANKER® 1 plus. Count the cells using a hemocytometer, dilute the cells with CELLBANKER® 1 plus to a concentration of 5 × 106 cells/mL, and aliquot 1 mL into each cryotube vial.
Freeze the cells in a -80 °C ultra-low temperature freezer by using a BICELL. By using BICELL, the temperature of cryotubes can be gradually lowered.
Mouse mating to obtain blastocysts
Both male and female mice are sexually mature at eight weeks, being able to mate and give birth. One male should be kept in each cage for mating. In the evening, select females in estrus with large vulvae and keep them with one male and one or two females per cage.
The next morning, check female mice. If they have mated, their vagina is filled with the copulatory plug. The noon of the plug confirmation day is defined as 0.5 dpc.
Separate plugged females from the male and keep them in an individual cage until being used for dissection.
Preparation of MEF feeders
Day -1. One day before starting the derivation, coat 24-well plate with 0.2% (weight/vol) gelatin (see Recipe 5) (200 μL/well) for 15 min at room temperature. Aspirate the gelatin solution before seeding the feeder cells (Figure 2A).
Rapidly melt a frozen vial of mitotically inactivated MEFs in a 37 °C water bath until thawed and transfer the cells into a 15 mL centrifuge tube containing 5 mL of MEF medium. Centrifuge the tube at 300× g for 5 min at room temperature and aspirate the supernatant.
Resuspend the cell pellet in MEF medium. Plate at a density of 5 × 106 cells per plate of a 24-well plate, 12-well plate, 6-well plate, or six 35 mm dishes in MEF medium, and then incubate the plates overnight at 37 °C with 5% CO2.
Recovery of blastocyst-stage embryos from uterus
Notes:
Use scissors and forceps that have been sterilized in an autoclave.
StemFit® AK02N comprises liquids A, B, and C. Liquid C remains unutilized in the establishment of PrESCs. Liquids A and B are entirely identical to StemFit® Basic02. StemFit® Basic03 serves as a viable substitute for AK02N. StemFit® AK02N, Basic02, and AK03N are exclusively accessible within Japan, whereas StemFit® Basic03 boasts global availability.
Day 0. Thoroughly clean an experiment bench and a dissection microscope with 70% ethanol.
Euthanize a pregnant mouse at 3.5 dpc, dissect out the uterus, and place it on a clean paper towel or filter paper. Follow the facility’s rules for euthanasia. Remove fat tissues and cut away the ovary and oviducts (Figure 1A). Place the uterus in a fresh 2 mL drop of AK02N medium (see Recipe 2) in a 60 mm Petri dish.
Under a dissection microscope, with a 5 mL syringe, take 5 mL of the AK02N medium and attach a needle (21 gauge). While securing the uterine horn with forceps, stick the needle and flush the uterine horn with ~0.5 mL of AK02N medium in the direction of the ovary to the vagina and remove the uterine horn from the dish. Repeat the same process for the other uterine horn (Figure 1B).
Under higher magnification, find the flushed blastocysts in the drop and transfer them to a new drop of AK02N medium by using a P20 micro-pipette.
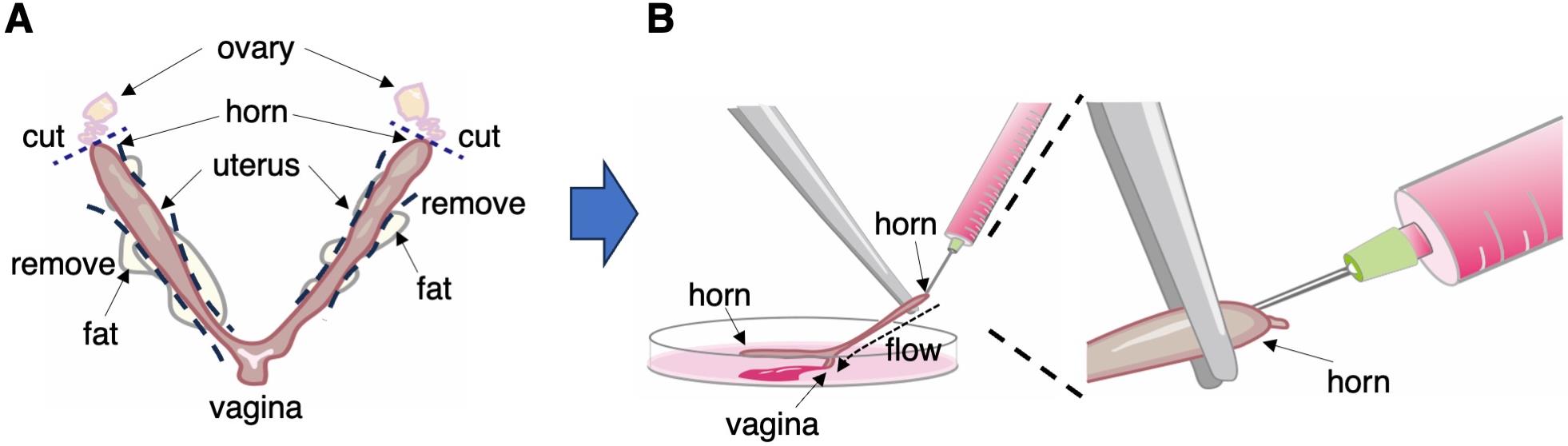
Figure 1. Recovery of blastocyst-stage embryos from uterus
PrESC derivation
Notes:
Steps E1–E5 can be executed under ambient conditions; however, it is imperative to manipulate the culture plate lid with utmost care and minimal frequency to avert potential contamination by microorganisms or particulate matter. Avoid prolonged exposure of the plate lid. Consider the advantageous inclusion of a dissection microscope within a sterile workbench.
StemFit® Basic03 medium by Ajinomoto serves as a viable substitute for AK02N. StemFit® AK02N, Basic02, and AK03N are exclusively accessible within Japan, whereas StemFit® Basic03 boasts global availability.
The survival rate of MMC-treated MEFs following freeze-thaw exhibits variability; it is imperative to ensure comprehensive coverage of the well’s bottom by MEFs. Employing MEFs with an insufficient density may precipitate differentiation into PrESCs.
The establishment of PrESCs should be precluded by the utilization of N2B27/Neurobasal/DMEMF12 (1:1) medium, a conventional choice in stem cell culture, as a replacement for the specialized AK02N.
The utilization of serum-containing media in lieu of AK02N will result in failure to establish PrESCs, accompanied by the loss of pluripotency marker expression and the emergence of XENC-like cells.
Day 0. Thoroughly clean an experiment bench and a dissection microscope with 70% ethanol.
The day before starting the derivation, aspirate the medium on the MEF feeders and replace it with 500 μL of PrESC medium (see Recipe 3) in the prepared 24-well plate (Figure 2A).
Under a dissection microscope, using P2 micro-pipette, place one blastocyst per each MEF-covered well in the plate and then incubate the plates at 37 °C with 5% CO2.
Day 5. Observe the plate under an inverted microscope. The blastocysts should have started to form an outgrowth (Figure 2B). Carefully aspirate the medium, change it with 500 μL of fresh PrESC medium, and return the plate to the incubator.
Day 7, passage 1. Observe the plate under an inverted microscope. The blastocysts should have formed an outgrowth. Carefully aspirate the medium and add 200 μL of TrypLETM Select to each well. Incubate the plate at 37 °C for 5 min.
Dissociate the outgrowth by pipetting into single cells using a P1000 pipette and transfer the cells into a 15 mL centrifuge tube containing 2 mL of AK02N medium. Centrifuge the tube at 300× g for 5 min at room temperature and carefully aspirate the supernatant as much as possible. Excessive carryover of TrypLETM Select adversely affects MEF after passage. Since the pellet after centrifugation is very small and fragile, gently aspirate and leave approximately 50 μL of medium together with the pellet.
Resuspend the cell pellet in 500 μL of PrESC medium, passage into one MEF-covered well in the prepared 24-well plate, and then incubate the plates overnight at 37 °C with 5% CO2.
Day 12. Observe the plate under an inverted microscope. Small colonies should be forming (Figure 2B and 2C). Carefully aspirate the medium and change it with 500 μL of fresh PrESC medium. When using StemFit® Basic03 medium, the morphology of the colony after the first passaging may be indistinct and difficult to see, but after the second passaging a well-defined colony becomes visible.
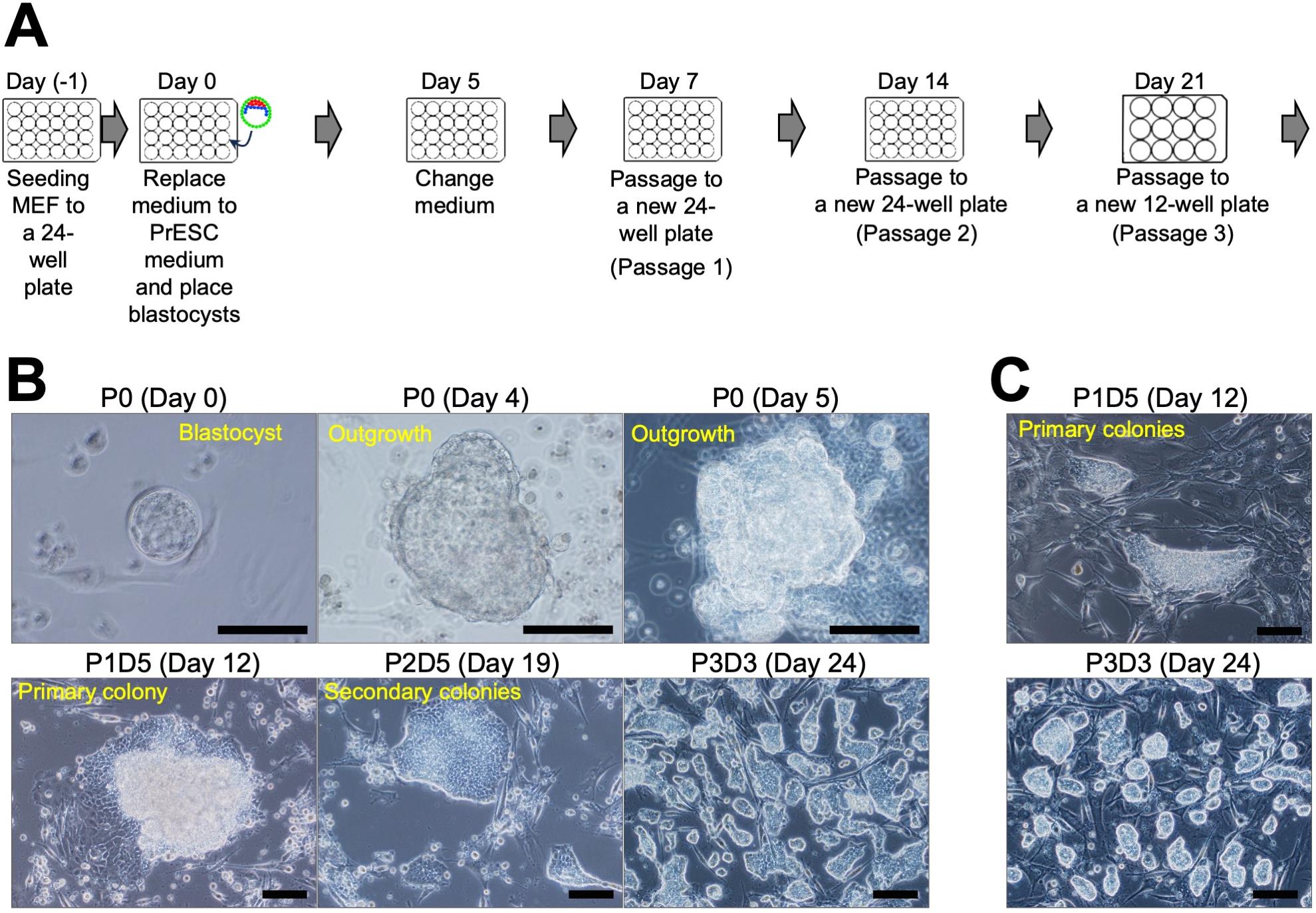
Figure 2. Primitive endoderm stem cell (PrESC) derivation from mouse blastocyst. A. Schedule for PrESC derivation. B. Outgrowth or colony morphology at each time point. C. Derivation of PrESCs in Basic03 medium. Scale bar, 100 μm.Day 14, passage 2. Carefully aspirate the medium and add 200 μL of TrypLETM Select to each well. Incubate the plate at 37 °C for 5 min.
Dissociate the cells by pipetting into single cells by using a P200 pipette and transfer the cells into a 15 mL centrifuge tube containing 2 mL of AK02N medium. Centrifuge the tube at 300× g for 5 min at room temperature and aspirate the supernatant.
Resuspend the cell pellet in 1 mL of PrESC medium, passage into one MEF-covered well in the prepared 12-well plate, and then incubate the plate at 37 °C with 5% CO2.
Day 19. Observe the plate under an inverted microscope. PrESC colonies should be forming. Carefully aspirate the medium and change it with 1 mL of fresh PrESC medium.
Day 21, passage 3. Carefully aspirate the medium and add 200 μL of TrypLETM Select to each well. Incubate the plate at 37 °C for 5 min.
Dissociate the cells by pipetting into single cells by using a P1000 pipette and transfer the cells into a 15 mL centrifuge tube containing 2 mL of AK02N medium. Centrifuge the tube at 300× g for 5 min at room temperature and aspirate the supernatant.
Resuspend the cell pellet in 2 mL of PrESC medium, passage into MEF-covered wells in the prepared 6-well plate, and then incubate the plate at 37 °C with 5% CO2.
After PrESCs reach approximately 70% confluency, passage into MEF-covered wells at 1:5 to 1:10 dilution. Change medium every other day.
Cryopreservation
The viable rate of PrESCs after freeze-thaw is low, and cryopreservation must be at a higher density than for ESCs or TSCs. PrESCs have grown to 70% confluency in a 35 mm dish; aspirate the medium and add 1 mL of TrypLETM Select to each well. Incubate the plate at 37 °C for 5 min.
Dissociate the cells by pipetting into single cells by using a P1000 pipette and transfer the cells into a 15 mL centrifuge tube containing 5 mL of AK02N medium. Centrifuge the tube at 300× g for 5 min at room temperature and aspirate the supernatant.
Resuspend the cell pellet in 1 mL of CELLBANKER® 1 plus cryoprotective reagent and transfer into two cryotubes (500 μL/tube). Use of cryoprotective reagents that do not contain serum may significantly further reduce cell viability after thawing.
Freeze the cells in a -80 °C ultra-low temperature freezer by using a BICELL. By using BICELL, the temperature of cryotubes can be gradually lowered.
For long-term storage, store the tubes after freezing in a -150 °C ultra-low temperature freezer.
Checking gene expression of PrESCs markers
PrESCs co-express pluripotency and endodermal markers (Ohinata, Endo et al., 2022). Check the gene expression by immunofluorescence staining for OCT4 (a pluripotency marker) and SOX17 (an endoderm marker) (Figure 3).
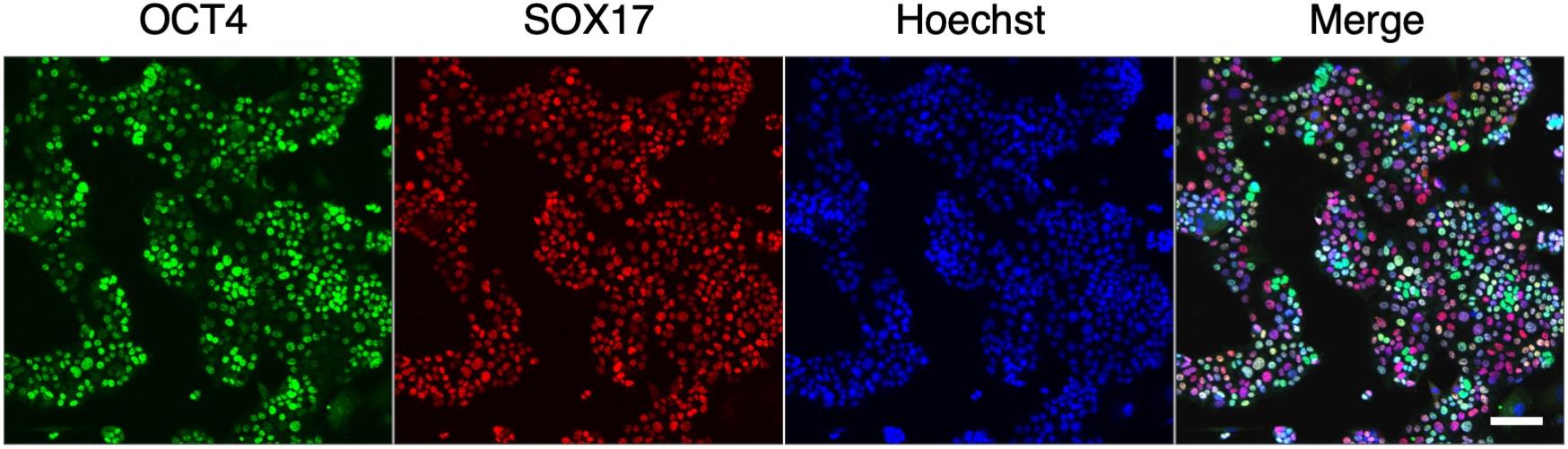
Figure 3. Co-expression of a pluripotent marker (OCT4) and an endoderm marker (SOX17) in primitive endoderm stem cell (PrESCs). Scale bar, 100 μm.Culture PrESCs in a MEF-covered 35 mm glass-bottom cell culture dish.
When PrESCs reach desired cell density (usually approximately 70% confluency recommended), aspirate medium, wash twice with 2 mL of PBS, and fix with 2 mL of 4% PFA/PBST at room temperature for 30 min.
Wash cells three times in 2 mL of PBST (see Recipe 7) at room temperature for 15 min.
Block cells in 2 mL of 0.5% normal donkey serum (NDS)/PBST at room temperature for 30 min.
React cells with 1 mL of primary antibody solution [rabbit anti-OCT4 (1:1,000), goat anti-SOX17 (1:1,000, 10 mg/mL stock solution) in 0.5% NDS/PBST] at room temperature for 30 min.
Wash cells three times in 2 mL of PBST at room temperature for 15 min.
React cells with 1 mL of secondary antibody solution [donkey anti-rabbit IgG Alexa 488 (1:1,000), donkey anti-goat IgG Alexa 568 (1:1,000), Hoechst 33342 (5 μg/mL) in PBST] at room temperature for 30 min.
Wash cells three times in 2 mL of PBST at room temperature for 15 min.
Observe cells with an inverted confocal microscope.
Validation of protocol
This protocol was used in the following paper:
Ohinata, Y. et al. (2022). Establishment of mouse stem cells that can recapitulate the developmental potential of primitive endoderm. Science 375(6580): 574-578.
Acknowledgments
This work was supported by JSPS KAKENHI under Grant Number 18H05366 and 19H05757 to Y.O.
This protocol was derived from the original work of Ohinata et al. (2022).
Competing interests
RIKEN has a patent pending (JP2019-118733) on the method for derivation and maintenance of PrESCs. Y.O. and H.K. are the inventors of the patent. There is no conflict of interest relevant to this study.
Ethical considerations
All animal experiments conformed to our guidelines for the care and use of laboratory animals and were approved by the Institutional Committee for Laboratory Animal Experimentation (RIKEN Center for Integrative Medical Sciences AEY2023-019(2) and Chiba University A5-002).
References
Brennan, J., Lu, C. C., Norris, D. P., Rodriguez, T. A., Beddington, R. S. P. and Robertson, E. J. (2001). Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411(6840): 965–969.
- Debeb, B. G., Galat, V., Epple-Farmer, J., Iannaccone, S., Woodward, W. A., Bader, M., Iannaccone, P. and Binas, B. (2009). Isolation of Oct4-expressing extraembryonic endoderm precursor cell lines. PLoS One 4(9): e7216.
- Evans, M. J. and Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292(5819): 154–156.
- Galat, V., Binas, B., Iannaccone, S., Postovit, L. M., Debeb, B. G. and Iannaccone, P. (2009). Developmental Potential of Rat Extraembryonic Stem Cells. Stem Cells Dev. 18(9): 1309–1318.
- Gardner, R. L. (1982). Investigation of cell lineage and differentiation in the extraembryonic endoderm of the mouse embryo. Development 68(1): 175–198.
- Igarashi, H., Uemura, M., Hiramatsu, R., Hiramatsu, R., Segami, S., Pattarapanawan, M., Hirate, Y., Yoshimura, Y., Hashimoto, H., Higashiyama, H., et al. (2018). Sox17 is essential for proper formation of the marginal zone of extraembryonic endoderm adjacent to a developing mouse placental disk†. Biol. Reprod. 99(3): 578–589.
- Jollie, W. P. (1990). Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology 41(4): 361–381.
- Julio, M. d., Alvarez, M. J., Galli, A., Chu, J., Price, S. M., Califano, A. and Shen, M. M. (2011). Regulation of extra-embryonic endoderm stem cell differentiation by Nodal and Cripto signaling. J. Cell Sci. 124(18): e1–e1.
- Kunath, T., Arnaud, D., Uy, G. D., Okamoto, I., Chureau, C., Yamanaka, Y., Heard, E., Gardner, R. L., Avner, P., Rossant, J., et al. (2005). Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 132(7): 1649–1661.
- Lloyd, J. B. (1990). Cell physiology of the rat visceral yolk sac: A study of pinocytosis and lysosome function. Teratology 41(4): 383–393.
- Nichols, J., Silva, J., Roode, M. and Smith, A. (2009). Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136(19): 3215–3222.
- Ohinata, Y., Endo, T. A., Sugishita, H., Watanabe, T., Iizuka, Y., Kawamoto, Y., Saraya, A., Kumon, M., Koseki, Y., Kondo, T., et al. (2022). Establishment of mouse stem cells that can recapitulate the developmental potential of primitive endoderm. Science 375(6580): 574–578.
- Ohinata, Y. and Tsukiyama, T. (2014). Establishment of Trophoblast Stem Cells under Defined Culture Conditions in Mice. PLoS One 9(9): e107308.
- Tanaka, S., Kunath, T., Hadjantonakis, A. K., Nagy, A. and Rossant, J. (1998). Promotion of trophoblast stem cell proliferation by FGF4. Science 282(5396): 2072–2075.
- Ueno, H. and Weissman, I. L. (2010). The origin and fate of yolk sac hematopoiesis: application of chimera analyses to developmental studies. Int. J. Dev. Biol. 54: 1019–1031.
- Zhong, Y., Choi, T., Kim, M., Jung, K. H., Chai, Y. G. and Binas, B. (2018). Isolation of primitive mouse extraembryonic endoderm (pXEN) stem cell lines. Stem Cell Res. 30: 100–112.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ohinata, Y., Saraya, A. and Koseki, H. (2023). Generation of Mouse Primitive Endoderm Stem Cells. Bio-protocol 13(22): e4878. DOI: 10.21769/BioProtoc.4878.
- Ohinata, Y., Endo, T. A., Sugishita, H., Watanabe, T., Iizuka, Y., Kawamoto, Y., Saraya, A., Kumon, M., Koseki, Y., Kondo, T., et al. (2022). Establishment of mouse stem cells that can recapitulate the developmental potential of primitive endoderm. Science 375(6580): 574–578.
Category
Stem Cell > Embryonic stem cell > Maintenance and differentiation
Stem Cell > Germ cell > In vitro culture
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








